Hepatitis C: New Treatments in the Pipeline - CD8 T cells - The Body
Hepatitis C: New Treatments in the Pipeline - CD8 T cells - The Body
Hepatitis C: New Treatments in the Pipeline - CD8 T cells - The Body
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
30<br />
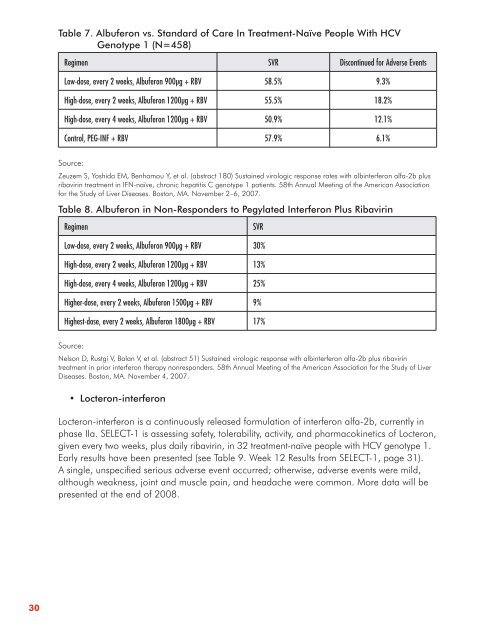
Table 7 . Albuferon vs . Standard of Care In Treatment-Naïve People With HCV<br />
Genotype 1 (N=458)<br />
Regimen SVR Discont<strong>in</strong>ued for Adverse Events<br />
Low-dose, every 2 weeks, Albuferon 900μg + RBV 58.5% 9.3%<br />
High-dose, every 2 weeks, Albuferon 1200μg + RBV 55.5% 18.2%<br />
High-dose, every 4 weeks, Albuferon 1200μg + RBV 50.9% 12.1%<br />
Control, PEG-INF + RBV 57.9% 6.1%<br />
Source:<br />
Zeuzem S, Yoshida EM, Benhamou Y, et al. (abstract 180) Susta<strong>in</strong>ed virologic response rates with alb<strong>in</strong>terferon alfa-2b plus<br />
ribavir<strong>in</strong> treatment <strong>in</strong> IFN-naïve, chronic hepatitis C genotype 1 patients. 58th Annual Meet<strong>in</strong>g of <strong>the</strong> American Association<br />
for <strong>the</strong> Study of Liver Diseases. Boston, MA. November 2–6, 2007.<br />
Table 8 . Albuferon <strong>in</strong> Non-Responders to Pegylated Interferon Plus Ribavir<strong>in</strong><br />
Regimen SVR<br />
Low-dose, every 2 weeks, Albuferon 900μg + RBV 30%<br />
High-dose, every 2 weeks, Albuferon 1200μg + RBV 13%<br />
High-dose, every 4 weeks, Albuferon 1200μg + RBV 25%<br />
Higher-dose, every 2 weeks, Albuferon 1500μg + RBV 9%<br />
Highest-dose, every 2 weeks, Albuferon 1800μg + RBV 17%<br />
Source:<br />
Nelson D, Rustgi V, Balan V, et al. (abstract 51) Susta<strong>in</strong>ed virologic response with alb<strong>in</strong>terferon alfa-2b plus ribavir<strong>in</strong><br />
treatment <strong>in</strong> prior <strong>in</strong>terferon <strong>the</strong>rapy nonresponders. 58th Annual Meet<strong>in</strong>g of <strong>the</strong> American Association for <strong>the</strong> Study of Liver<br />
Diseases. Boston, MA. November 4, 2007.<br />
• Locteron-<strong>in</strong>terferon<br />
Locteron-<strong>in</strong>terferon is a cont<strong>in</strong>uously released formulation of <strong>in</strong>terferon alfa-2b, currently <strong>in</strong><br />
phase IIa. SELECT-1 is assess<strong>in</strong>g safety, tolerability, activity, and pharmacok<strong>in</strong>etics of Locteron,<br />
given every two weeks, plus daily ribavir<strong>in</strong>, <strong>in</strong> 32 treatment-naïve people with HCV genotype 1.<br />
Early results have been presented (see Table 9. Week 12 Results from SELECT-1, page 31).<br />
A s<strong>in</strong>gle, unspecified serious adverse event occurred; o<strong>the</strong>rwise, adverse events were mild,<br />
although weakness, jo<strong>in</strong>t and muscle pa<strong>in</strong>, and headache were common. More data will be<br />
presented at <strong>the</strong> end of 2008.