Strategy For Limiting Risks Human Health Draft of ... - ECHA - Europa
Strategy For Limiting Risks Human Health Draft of ... - ECHA - Europa
Strategy For Limiting Risks Human Health Draft of ... - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
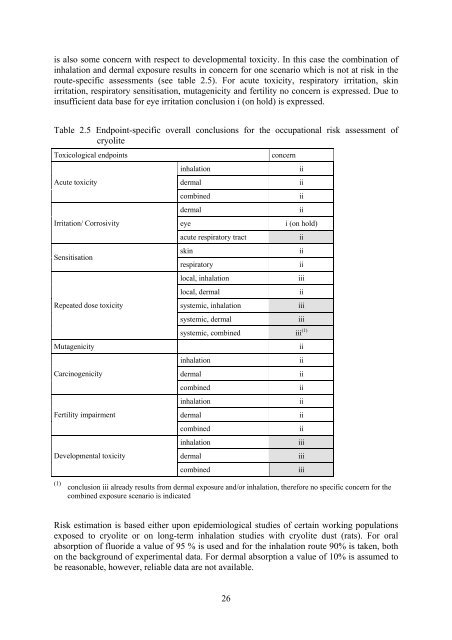
is also some concern with respect to developmental toxicity. In this case the combination <strong>of</strong><br />
inhalation and dermal exposure results in concern for one scenario which is not at risk in the<br />
route-specific assessments (see table 2.5). <strong>For</strong> acute toxicity, respiratory irritation, skin<br />
irritation, respiratory sensitisation, mutagenicity and fertility no concern is expressed. Due to<br />
insufficient data base for eye irritation conclusion i (on hold) is expressed.<br />
Table 2.5 Endpoint-specific overall conclusions for the occupational risk assessment <strong>of</strong><br />
cryolite<br />
Toxicological endpoints concern<br />
Acute toxicity<br />
Irritation/ Corrosivity<br />
Sensitisation<br />
Repeated dose toxicity<br />
inhalation ii<br />
dermal ii<br />
combined ii<br />
dermal ii<br />
eye i (on hold)<br />
acute respiratory tract ii<br />
skin ii<br />
respiratory ii<br />
local, inhalation iii<br />
local, dermal ii<br />
systemic, inhalation iii<br />
systemic, dermal iii<br />
systemic, combined iii (1)<br />
Mutagenicity ii<br />
Carcinogenicity<br />
Fertility impairment<br />
Developmental toxicity<br />
inhalation ii<br />
dermal ii<br />
combined ii<br />
inhalation ii<br />
dermal ii<br />
combined ii<br />
inhalation iii<br />
dermal iii<br />
combined iii<br />
(1) conclusion iii already results from dermal exposure and/or inhalation, therefore no specific concern for the<br />
combined exposure scenario is indicated<br />
Risk estimation is based either upon epidemiological studies <strong>of</strong> certain working populations<br />
exposed to cryolite or on long-term inhalation studies with cryolite dust (rats). <strong>For</strong> oral<br />
absorption <strong>of</strong> fluoride a value <strong>of</strong> 95 % is used and for the inhalation route 90% is taken, both<br />
on the background <strong>of</strong> experimental data. <strong>For</strong> dermal absorption a value <strong>of</strong> 10% is assumed to<br />
be reasonable, however, reliable data are not available.<br />
26