MUSA - Alberta Pharmacy Students' Association
MUSA - Alberta Pharmacy Students' Association
MUSA - Alberta Pharmacy Students' Association
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
REVIEW<br />
with specific functions. 9 Primarily two<br />
types of mammalian stem cells are used in<br />
myocardial regeneration: embryonic stem<br />
cells found in the blastocyst during early<br />
embryogenesis, and adult stem cells found<br />
in adult tissues acting as progenitor cells.<br />
ES cells are pluripotent and can potentially<br />
give rise to a number of cell types, they<br />
are vital to tissue regeneration therapy,<br />
regardless of the field of research. Skeletal<br />
myoblasts, on the other hand, are committed<br />
progenitor cells of skeletal muscle; they<br />
are resistant to ischemia and highly<br />
proliferative. 10 These myoblasts, harvested<br />
from neonatal and adult animals, have<br />
been shown to differentiate into skeletal<br />
myotubes and improve left ventricular<br />
function following an infarct. 10 These<br />
results have been obtained in autologous,<br />
syngeneic, allogenic, and xenogenic<br />
transplants – largely in mice and rats, but<br />
also in swine and canine subjects. 10<br />
Adult bone marrow derived stem cells have<br />
also been studied: there are hematopoietic<br />
stem cells, endothelial progenitor cells,<br />
and mesenchymal stem cells in adult bone<br />
marrow. Research has shown that treatment<br />
with mesenchymal stem cells (MSCs –<br />
precursors to muscle, bone, tendons, and<br />
ligaments) improves myocardial function<br />
by limiting ventricular remodeling. 11 It<br />
was known that intramyocardial injection<br />
of Akt-MSCs (mesenchymal stem cells<br />
overexpressing the survival gene Akt)<br />
restored cardiac function after only 72<br />
hours. 11 Gnecchi et al. hypothesized that,<br />
because such a rapid recovery could not<br />
be due to differentiation of the donor cells,<br />
regeneration was accomplished through the<br />
action of factors provided by the MSCs. 12<br />
It was thought that these factors acted in<br />
a paracrine fashion to rescue the damaged<br />
heart tissue. Gnecchi et al. found that it<br />
is possible, in an animal model, to use<br />
mesenchymal stem cells to alleviate acute<br />
MI by injecting a cell-free supernatant<br />
that had been recovered from cultures of<br />
mesenchymal stem cells. 12<br />
Adult CD34 + cells, easily obtained from<br />
peripheral blood, can trans-differentiate<br />
into cardiomyocytes in vivo at the site of<br />
injury in mice – yet this is still a work in<br />
progress. 13 Lastly, some sources suggest that<br />
there are small populations of “resident<br />
cardiac stem cells” endogenous to the heart<br />
that may serve a minor role in repair. 14<br />
While researchers clearly have many types<br />
of stem cells at their disposal for use in<br />
cardiovascular therapies, only ES-derived<br />
cardiomyocytes and skeletal myoblasts<br />
have been able to achieve a proper level<br />
of cell survival for complete myocardial<br />
regeneration. 7<br />
14<br />
Skeletal Myoblast<br />
Transplantation<br />
Studies on skeletal myoblasts began with<br />
work of Chiu et al., dating back to 1995.<br />
His team studied the ability to repair<br />
injured myocardium in the presence of<br />
skeletal muscle cells, called “satellite<br />
cells.” 15 Each skeletal muscle fiber contains<br />
a few myogenic satellite cells, which are<br />
normally undifferentiated and quiescent.<br />
Injury activates these cells, causing them to<br />
enter mitosis and restore the functionality<br />
of the fiber. 16 Chiu et al. hypothesized that<br />
satellite cells, when implanted into injured<br />
myocardium and influenced by the cardiac<br />
environment, would undergo “milieudependent<br />
differentiation.” 15 Chiu et al.<br />
conducted two experiments: one in which<br />
the histological outcome of implanting<br />
skeletal satellite cells into acutely damaged<br />
myocardium was observed, and the other<br />
in which the presence of satellite cells at<br />
the site of implantation was confirmed. 15<br />
Satellite cells were isolated from samples<br />
obtained from the tibialis anterior muscle of<br />
adult dogs, and then labeled with tritiated<br />
thymidine. Following which, the cells<br />
were grown in vitro for either 10 days or 3<br />
weeks and implanted into the cryoinjured<br />
myocardium of the same animal. A catheter<br />
was used to implant the cells into the<br />
injured left ventricular free wall, which was<br />
acutely damaged by liquid nitrogen. Implant<br />
sites were evaluated radiographically to<br />
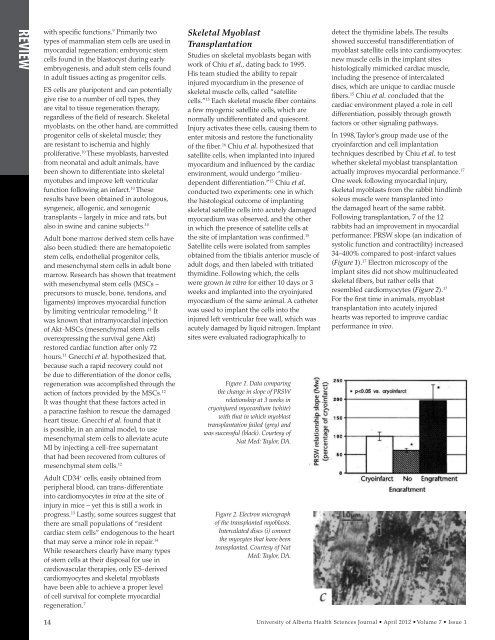
Figure 1. Data comparing<br />
the change in slope of PRSW<br />
relationship at 3 weeks in<br />
cryoinjured myocardium (white)<br />
with that in which myoblast<br />
transplantation failed (grey) and<br />
was successful (black). Courtesy of<br />
Nat Med: Taylor, DA.<br />
Figure 2. Electron micrograph<br />
of the transplanted myoblasts.<br />
Intercalated discs (i) connect<br />
the myocytes that have been<br />
transplanted. Courtesy of Nat<br />
Med: Taylor, DA.<br />
detect the thymidine labels. The results<br />
showed successful transdifferentiation of<br />
myoblast satellite cells into cardiomyocytes:<br />
new muscle cells in the implant sites<br />
histologically mimicked cardiac muscle,<br />
including the presence of intercalated<br />
discs, which are unique to cardiac muscle<br />
fibers. 15 Chiu et al. concluded that the<br />
cardiac environment played a role in cell<br />
differentiation, possibly through growth<br />
factors or other signaling pathways.<br />
In 1998, Taylor’s group made use of the<br />
cryoinfarction and cell implantation<br />
techniques described by Chiu et al. to test<br />
whether skeletal myoblast transplantation<br />
actually improves myocardial performance. 17<br />
One week following myocardial injury,<br />
skeletal myoblasts from the rabbit hindlimb<br />
soleus muscle were transplanted into<br />
the damaged heart of the same rabbit.<br />
Following transplantation, 7 of the 12<br />
rabbits had an improvement in myocardial<br />
performance: PRSW slope (an indication of<br />
systolic function and contractility) increased<br />
34–400% compared to post-infarct values<br />
(Figure 1). 17 Electron microscopy of the<br />
implant sites did not show multinucleated<br />
skeletal fibers, but rather cells that<br />
resembled cardiomyocytes (Figure 2). 17<br />
For the first time in animals, myoblast<br />
transplantation into acutely injured<br />
hearts was reported to improve cardiac<br />
performance in vivo.<br />
University of <strong>Alberta</strong> Health Sciences Journal • April 2012 • Volume 7 • Issue 1