Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Copyright © Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc.<br />
Name Date Class<br />
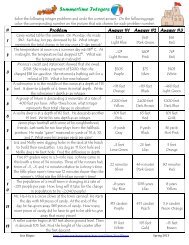
Directions: List the three assumptions of kinetic theory.<br />
1.<br />
2.<br />
3.<br />
1<br />
<strong>Study</strong> Guide<br />
Kinetic Theory<br />
Chapter<br />
16<br />
Directions: Define each phenomenon from the chapter, and describe what the particles do to cause<br />
that phenomenon. You may sketch what the particles are doing, if you wish.<br />
Phenomenon Definition Descriptions and Diagrams<br />
of what the Molecules are<br />
Doing, Additional Notes<br />
4. Thermal Energy<br />
4a. Kinetic Energy<br />
4b. Potential Energy<br />
5. Average Kinetic<br />
Energy<br />
6. Solid State<br />
7. Melting Point<br />
7a. Heat of Fusion<br />
8. Liquids Flow<br />
9. Gas State<br />
9a. Evaporation<br />
10. Boiling Point<br />
10a. Heat of<br />
Vaporization<br />
11. Diffusion<br />
12. Plasma State<br />
13. Thermal Expansion<br />
14. Water’s Strange<br />
Expansion<br />
15. Melting Amorphous<br />
Solids<br />
Kinetic Theory 57