7.2 Evidence of a Chemical Reaction
7.2 Evidence of a Chemical Reaction
7.2 Evidence of a Chemical Reaction
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
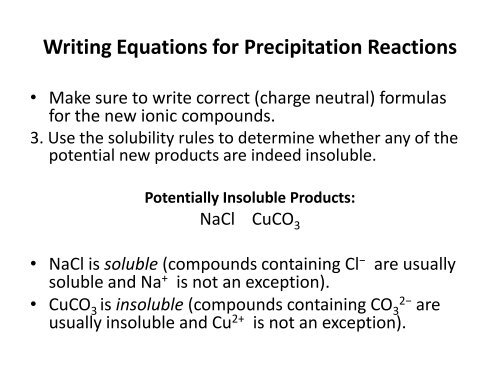
Writing Equations for Precipitation <strong>Reaction</strong>s<br />
• Make sure to write correct (charge neutral) formulas<br />
for the new ionic compounds.<br />
3. Use the solubility rules to determine whether any <strong>of</strong> the<br />
potential new products are indeed insoluble.<br />
Potentially Insoluble Products:<br />
NaCl CuCO 3<br />
• NaCl is soluble (compounds containing Cl − are usually<br />
soluble and Na + is not an exception).<br />
• CuCO 3 is insoluble (compounds containing CO 3 2− are<br />
usually insoluble and Cu 2+ is not an exception).