7.2 Evidence of a Chemical Reaction
7.2 Evidence of a Chemical Reaction
7.2 Evidence of a Chemical Reaction
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
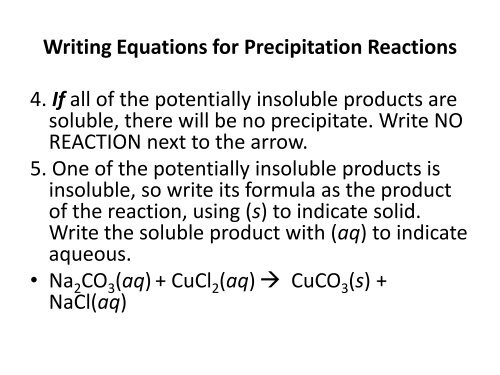
Writing Equations for Precipitation <strong>Reaction</strong>s<br />
4. If all <strong>of</strong> the potentially insoluble products are<br />
soluble, there will be no precipitate. Write NO<br />
REACTION next to the arrow.<br />
5. One <strong>of</strong> the potentially insoluble products is<br />
insoluble, so write its formula as the product<br />
<strong>of</strong> the reaction, using (s) to indicate solid.<br />
Write the soluble product with (aq) to indicate<br />
aqueous.<br />
• Na 2CO 3(aq) + CuCl 2(aq) CuCO 3(s) +<br />
NaCl(aq)