Gradient conditions
Gradient conditions
Gradient conditions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
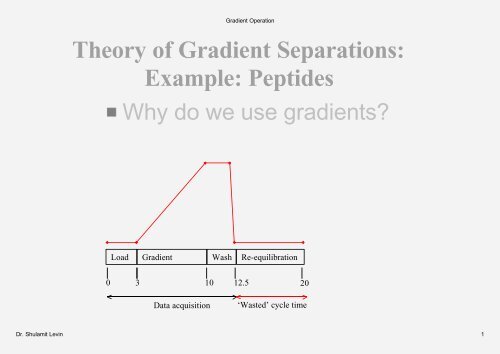
Theory of <strong>Gradient</strong> Separations:<br />
Load<br />
Example: Peptides<br />
Why do we use gradients?<br />
<strong>Gradient</strong> Wash Re-equilibration<br />
0 3 10 12.5<br />
<strong>Gradient</strong> Operation<br />
Data acquisition ‘Wasted’ cycle time<br />
Dr. Shulamit Levin 1<br />
20
Theory of <strong>Gradient</strong> Separations:<br />
Example: Peptides<br />
Why do we use gradients?<br />
Load<br />
<strong>Gradient</strong> Wash Re-equilibration<br />
0 3 10 12.5<br />
20<br />
Data acquisition ‘Wasted’ cycle time<br />
Why do we use gradients?<br />
The retention (k) of peptides have a steep dependence<br />
on the % organic in the mobile phase<br />
Neue<br />
ln (k)<br />
6<br />
4<br />
2<br />
0<br />
0<br />
Small Molecule<br />
- The steeper the slope of the line the shallower the<br />
gradient must be to achieve maximum resolution<br />
0.2<br />
Peptide<br />
0.4<br />
Protein<br />
0.6<br />
Volume Fraction Acetonitrile<br />
0.8<br />
1<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
Why do we use gradients?...<br />
...because...<br />
Properties of analytes<br />
Retention (k) of the solutes has a steep<br />
dependence on the % organic in the<br />
mobile phase<br />
Wide range of differing hydrophobicities<br />
of the analytes<br />
Introduction<br />
Outline<br />
Theory of <strong>Gradient</strong> Separations of Peptides<br />
Optimization of Separations to...<br />
achieve maximum resolution<br />
maximize throughput<br />
maximize reproducibility<br />
Assay Reproducibility<br />
Conclusion<br />
Dr. Shulamit Levin 1-4<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation
Column dead<br />
time<br />
Calculation of Isocratic<br />
Mobile Phase<br />
k-Dependence on Mobile Phase Composition<br />
in Reversed-Phase HPLC:<br />
<strong>Gradient</strong> Retention Times:<br />
( )<br />
t = t + t + G 0 d 1<br />
b ×S ×ln b×S ×t ×k(Φ ) + 1<br />
0 0<br />
System delay<br />
time<br />
ln( k ) = ln( k ) - S . Φ<br />
0<br />
<strong>Gradient</strong> slope<br />
Resolution Equations<br />
Slope of lnk vs. organic<br />
-Factors Factors Influencing Resolution for an Isocratic<br />
Separation RR s<br />
t N a - 1 k<br />
~ × ×<br />
w 4 a k +1<br />
Rs =<br />
}<br />
}<br />
Efficiency Selectivity<br />
k at gradient starting<br />
composition<br />
s = Resolution<br />
t = Retention time<br />
w = Peak width<br />
N = Plate Count<br />
a = Selectivity Factor<br />
k = Retention Factor<br />
-Factors Influencing Resolution for a <strong>Gradient</strong> Separation<br />
Rs =<br />
}<br />
}<br />
Efficiency Selectivity<br />
-Factors are similar, however...<br />
}<br />
Retention<br />
t N 1<br />
~ × ln a ×<br />
w 4 Bct0 + 1<br />
}<br />
Retention<br />
B = Slope of of ln ln (k) (k) with<br />
solvent composition (an<br />
analyte dependent<br />
property)<br />
c = <strong>Gradient</strong> Slope<br />
t 0<br />
0=<br />
= Time of of elution for an an<br />
unretained peak<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
Basic Resolution Equation<br />
-Factors Factors Influencing Resolution for an<br />
Isocratic Separation<br />
t<br />
Rs =<br />
w<br />
N<br />
~ ×<br />
4<br />
}<br />
α − 1 k<br />
×<br />
α k +1<br />
}<br />
Rs= Resolution Efficiency Selectivity<br />
N = Plate Count<br />
t = Retention Time<br />
w = Average peak width<br />
α = Selectivity Factor<br />
k = Retention Factor<br />
{<br />
w1<br />
}<br />
t<br />
{<br />
Retention<br />
Analyte Retention as a Function of <strong>Gradient</strong><br />
Slope<br />
kk (retention) for each<br />
analyte changes<br />
independently as the<br />
gradient slope changes.<br />
Thus, the resolution<br />
between peaks<br />
changes.<br />
ln k<br />
peak 8<br />
peak 9<br />
w2<br />
peak 10<br />
peak 11<br />
% Acetonitrile<br />
Dr. Shulamit Levin 5-8<br />
Carmody<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Flow Rate; F<br />
Part II II - Factors influencing selectivity<br />
Concentration and Type of Modifier<br />
Temperature<br />
Chemistry and Pore Size of the Packing Material<br />
Part III - Factors influencing reproducibility<br />
Column<br />
HPLC system<br />
© 1998 Waters Corporation<br />
What Factors Influence <strong>Gradient</strong> Slope?<br />
c = %B/minute =<br />
Two ways to change the slope<br />
change the percent organic (D ( %) of the mobile<br />
phase across a specified gradient run time.<br />
change the gradient run time (t (tg<br />
g ) while keeping<br />
the % organic of the mobile phase constant.<br />
%<br />
tg<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
Rs (Resolution)<br />
Factors Influencing Resolution in<br />
<strong>Gradient</strong> RP-HPLC Separations...<br />
Part I - Factors influencing efficiency and<br />
retention<br />
<strong>Gradient</strong> Slope; c (%B/min.) - increase in organic<br />
concentration per unit time<br />
t N 1<br />
Rs = ∼ × ln α ×<br />
w 4 Bct0 + 1<br />
}<br />
}<br />
}<br />
Efficiency Selectivity Retention<br />
Selectivity<br />
Retention<br />
Principle of <strong>Gradient</strong> Separations<br />
Neue<br />
approaching<br />
isocratic<br />
<strong>conditions</strong><br />
General slope of the gradient<br />
© 1998 Waters Corporation<br />
0 0.01 0.1 1 10<br />
Dr. Shulamit Levin 9-12<br />
© 1998 Waters Corporation
0.400<br />
0.300<br />
AU 0.200<br />
0.100<br />
0.000<br />
0.300<br />
0.200<br />
AU AU<br />
0.100<br />
0.000<br />
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c (%B/min.) - varied by changing the % organic across across<br />
a specified gradient run time . . All All other other variables are are kept constant.<br />
Rs =<br />
t N<br />
∼ × lnα<br />
w 4<br />
}<br />
Efficiency Selectivity<br />
×<br />
}<br />
1<br />
Bct0 + 1<br />
}<br />
Retention<br />
tg = gradient<br />
run time<br />
1<br />
%<br />
B. . t0 + 1<br />
Resolution as a Function of <strong>Gradient</strong> Slope<br />
30.00 35.00 40.00<br />
Minutes<br />
0.62%/min (0-28%)<br />
30.00 30.00 35.00 35.00<br />
Minutes Minutes<br />
40.00 40.00<br />
Alden<br />
9<br />
10<br />
8 11<br />
0.66%/min<br />
9<br />
10<br />
8 11<br />
Rs (btw. 8 and 9) = 2.0<br />
Rs (btw. 9 and 10) = 5.4<br />
Rs (btw. 10 and 11) = 6.9<br />
Rs (btw. 8 and 9) = 2.3<br />
Rs (btw. 9 and 10) = 6.0<br />
Rs (btw. 10 and 11) = 6.8<br />
0.600<br />
0.500<br />
0.400<br />
0.300<br />
AU<br />
0.200<br />
0.100<br />
tg<br />
Conditions<br />
9<br />
0.71%/min (0-35%)<br />
© 1998 Waters Corporation<br />
-Column: Symmetry300, C 18,<br />
5 µm, 3.9 x 150 mm<br />
- Sample: Tryptic digests of<br />
bovine cytochrome c<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- Detection: 214 nm<br />
- Flow rate: 0.75 mL/min.<br />
- Temperature: 35 ºC<br />
Rs (btw. 8 and 9) = 1.7<br />
10<br />
Rs (btw. 9 and 10) = 4.7<br />
Rs (btw. 10 and 11) = 6.8<br />
8 11<br />
0.000<br />
30.00 35.00<br />
Minutes<br />
40.00<br />
<strong>Gradient</strong> Operation<br />
Resolution as a Function of<br />
<strong>Gradient</strong> Slope<br />
-Slope of the gradient = 0.66%/min<br />
0.400<br />
0.300<br />
AU 0.200<br />
0.100<br />
0.000<br />
0.00 20.00 40.00<br />
Minutes<br />
Alden<br />
8<br />
9<br />
10<br />
11<br />
Conditions<br />
-Column: Symmetry300, C 18 ,<br />
5 µm, 3.9 x 150 mm<br />
- Sample: Tryptic digests of bovine<br />
cytochrome c<br />
- Injection: 20µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in<br />
acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Flow rate: 0.75 mL/min.<br />
- Temperature: 35 ºC<br />
- Detection: 214 nm<br />
© 1998 Waters Corporation<br />
Analyte Retention as a Function of <strong>Gradient</strong><br />
Slope<br />
kk (retention) for each<br />
analyte changes<br />
independently as as the<br />
gradient slope changes.<br />
Thus, the the resolution<br />
between peaks changes.<br />
Carmody<br />
ln k<br />
peak 8<br />
peak 9<br />
peak 10<br />
peak 11<br />
% Acetonitrile<br />
Dr. Shulamit Levin 13-16<br />
© 1998 Waters Corporation
0.2000<br />
0.1500<br />
0.1000<br />
AU AU<br />
0.1000 0.1000<br />
AU AU<br />
0.2000 0.2000<br />
0.1500 0.1500<br />
0.0500 0.0500<br />
0.0000 0.0000<br />
-0.0500 -0.0500<br />
0.0500<br />
0.0000<br />
-0.0500 -0.0500<br />
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c (%B/min.) - - varied by by changing the the<br />
gradient run time. time.<br />
All other variables are kept constant.<br />
t N<br />
Rs = ∼ × lnα<br />
w 4<br />
}<br />
×<br />
}<br />
Efficiency Selectivity<br />
1<br />
%<br />
B. . t0 + 1<br />
tg<br />
}<br />
Retention<br />
<strong>Gradient</strong> Modifications:<br />
Initial ACN% in Peptide Separations<br />
0.00 0.00<br />
0-28% in 75 min.,<br />
(0.37%/min)<br />
20.00 20.00<br />
40.00 40.00<br />
Minutes Minutes<br />
6-28% in 60 min.,<br />
9<br />
(0.37%/min)<br />
1<br />
3<br />
5<br />
4<br />
2<br />
5<br />
1 4<br />
3<br />
2<br />
6<br />
7<br />
8<br />
60.00 60.00<br />
80.00 80.00<br />
0.00 0.00 20.00 20.00 40.00 40.00 60.00 60.00 80.00 80.00<br />
Minutes Minutes<br />
6<br />
7<br />
8<br />
9<br />
10<br />
11<br />
10<br />
11<br />
r.t. = 85 min.<br />
r.t. = 70 min.<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> SLOPE is<br />
more important if peaks<br />
elute during gradient (not<br />
in initial condition)<br />
-By changing the initial<br />
mobile phase <strong>conditions</strong>,<br />
but keeping the gradient<br />
slope the same, the run<br />
time can effectively be<br />
shortened without a loss<br />
in resolution.<br />
<strong>Gradient</strong> Operation<br />
Resolution as a Function of <strong>Gradient</strong> Duration<br />
0.1200<br />
0.1000<br />
0.0800<br />
AU 0.0600<br />
0.0400<br />
0.0200<br />
0.0000<br />
0.00 5.00 10.00 15.00<br />
Minutes<br />
0.0800 0.0800<br />
0.0600 0.0600<br />
0.0400 0.0400<br />
AU AU<br />
0.0200 0.0200<br />
0.0000 0.0000<br />
0.00 0.00 10.00 10.00 20.00<br />
Minutes Minutes<br />
0.0600<br />
0.0400<br />
AU<br />
0.0200<br />
0.0000<br />
0.0500 0.0500<br />
0.0400 0.0400<br />
0.0300 0.0300<br />
AU AU<br />
0.0200 0.0200<br />
0.0100 0.0100<br />
0.0000 0.0000<br />
0.0300 0.0300<br />
0.0200 0.0200<br />
AU AU<br />
0.0100 0.0100<br />
0.0000 0.0000<br />
0.00 20.00 40.00<br />
Minutes<br />
0.00 0.00 20.00 20.00 40.00 40.00<br />
Minutes Minutes<br />
60.00 60.00<br />
78 Peaks<br />
15 Minutes<br />
98 Peaks<br />
25 Minutes<br />
114 Peaks<br />
50 Minutes<br />
137 Peaks<br />
75 Minutes<br />
162 Peaks<br />
150 Minutes<br />
0.00 0.00 50.00 Minutes Minutes<br />
100.00 100.00 150.00 150.00<br />
Conditions<br />
-Column: Symmetry300, C 18, 5<br />
µm,<br />
4.6 x 150 mm<br />
- Sample: Tryptic digests of<br />
bovine serum albumin<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in<br />
acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Detection: 214 nm<br />
- Flow rate: 0.75 mL/min.<br />
- Temperature: 35 ºC<br />
-Longer RT;<br />
Shallower the slope;<br />
Increases Rs<br />
-Rs~ 1/c where c =<br />
gradient slope. All<br />
other variables are<br />
kept constant<br />
Alden<br />
Summary of Part I - <strong>Gradient</strong> Slope<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Slope is one of the most powerful<br />
operational parameter you have at your<br />
disposal<br />
Resolution increases as gradient slope<br />
decreases.<br />
Change in the initial percent organic can<br />
decrease the run time, maintain the resolution<br />
of your separation and preserve your elution<br />
pattern.<br />
Alden © 1998 Waters Corporation<br />
© 1998 Waters Corporation<br />
Dr. Shulamit Levin 17-20
Volumes in an HPLC System<br />
A B C<br />
D<br />
Proportioning<br />
Valve<br />
System Volume<br />
Waters 2690
Column Volume to to <strong>Gradient</strong> Volume Relationship (Approach 1) 1)<br />
-- <strong>Gradient</strong> volume scaled to to column volume<br />
50 mm column<br />
Column volume = 0.5 mL Column volume = 2.5 mL<br />
5 minute gradient @ 1 mL/min<br />
gradient volume = tg x f.r. = 5<br />
Total volume = g.v./c.v. = 10 column vols.<br />
250 mm column<br />
25 minute gradient @ 1 mL/min<br />
gradient volume = tg x f.r. = 25<br />
Total volume = g.v./c.v. = 10 column vols.<br />
Variation in Column Lengths at Equal Ratio of<br />
<strong>Gradient</strong> Volumes to Column Volumes<br />
0.0600<br />
AU AU0.0400<br />
0.0400<br />
0.0200<br />
0.1500<br />
0.1000<br />
AU<br />
0.0500<br />
0.1500<br />
0.1000<br />
AU<br />
0.0500<br />
4.00 5.00 6.00<br />
Minutes<br />
10.00 12.00 14.00 16.00<br />
Minutes<br />
Alden<br />
Column Volume = 0.83 mL<br />
Total Volume = 4.5 c.v.<br />
Column Volume = 2.5 mL<br />
Total Volume = 4.5 c.v.<br />
Column Volume = 4.2 mL<br />
Total Volume = 4.5 c.v.<br />
20.00 25.00<br />
Minutes<br />
5 minutes<br />
4.6 X 50 mm<br />
© 1998 Waters Corporation<br />
Conditions<br />
- Column: Symmetry300, C 18 , 5 µm<br />
- Sample: Tryptic digests of bovine<br />
serum albumin<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- 0 - 30 %B in the time shown.<br />
- Flow rate: 0.75 mL/min.<br />
15 minutes<br />
4.6 X 150 mm, - Detection: 214 nm<br />
- Temperature: 35 ºC<br />
25 minutes<br />
4.6 X 250 mm,<br />
- Elution pattern<br />
stays the same.<br />
- Resolution inc. as<br />
the # of plates inc.<br />
- Run time inc. as<br />
column length inc.<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Rs =<br />
t<br />
w<br />
N<br />
∼<br />
4<br />
}<br />
× ln α ×<br />
}<br />
. % . εt . πr .L/F + 1<br />
tg<br />
2<br />
Efficiency Selectivity<br />
Retention<br />
L (column length) is is varied. <strong>Gradient</strong><br />
volume is is scaled in in proportion to to the<br />
column volume.<br />
B<br />
1<br />
}<br />
Column Volume to <strong>Gradient</strong> Volume<br />
Relationship (Approach 2)<br />
-- <strong>Gradient</strong> volume not scaled to column volume<br />
50 mm column<br />
Column volume = 0.5 mL Column volume = 2.5 mL<br />
5 minute gradient @ 1 mL/min<br />
gradient volume = tg x f.r. = 5<br />
Total volume = g.v./c.v. = 10 column vols.<br />
250 mm column<br />
5 minute gradient @ 1 mL/min<br />
Terms are<br />
constant<br />
© 1998 Waters Corporation<br />
gradient volume = t g x f.r. = 5<br />
Total volume = g.v./c.v. = 2 column vols.<br />
Dr. Shulamit Levin 25-28<br />
© 1998 Waters Corporation
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I - Factors influencing efficiency and<br />
retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Rs =<br />
t<br />
w<br />
N<br />
∼ × ln α ×<br />
4<br />
B<br />
}<br />
}<br />
Efficiency Selectivity<br />
1<br />
. % . . εt πr .L/F + 1<br />
tg<br />
2<br />
}<br />
Retention<br />
© 1998 Waters Corporation<br />
Column Length Effects on Resolution at a Constant<br />
<strong>Gradient</strong> Duration (cont'd)<br />
0.02000<br />
0.01500<br />
0.01000<br />
AU<br />
0.00500<br />
0.00000<br />
-0.00500<br />
0.00<br />
20.00<br />
Minutes<br />
130 Peaks<br />
Symmetry300 4.6 X 50 mm,<br />
C 18, 5 µm<br />
40.00<br />
0.1200<br />
0.1000<br />
0.0800<br />
AU 0.0600<br />
0.0400<br />
0.0200<br />
131 Peaks<br />
Symmetry300 4.6 X 150<br />
mm,<br />
C 5 µm<br />
18,<br />
0.0000<br />
0.00 20.00<br />
Minutes<br />
40.00<br />
Conditions<br />
- Sample: Tryptic digests of bovine serum<br />
albumin<br />
- Injection: 20 µL (7 µL for 4.6 X 50 mm)<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Temperature: 35 ºC<br />
- Flow rate: 0.75 mL/min.<br />
- Detection: 214 nm<br />
-50 mm column has a<br />
similar resolving power<br />
as 250 mm column if the<br />
gradient duration<br />
remains the same.<br />
<strong>Gradient</strong> Operation<br />
0.2500 0.2500<br />
0.2000 0.2000<br />
0.1500 0.1500<br />
AU AU0.1000 0.1000<br />
0.0500 0.0500<br />
1<br />
0.0000 0.0000<br />
-0.0500 -0.0500<br />
0.00 0.00<br />
0.500 0.500<br />
0.400 0.400<br />
0.300 0.300<br />
AU AU<br />
0.200 0.200<br />
0.100 0.100<br />
0.000 0.000<br />
0.800<br />
0.600<br />
AU 0.400<br />
0.200<br />
0.00 0.00<br />
Column Length Effects on Resolution at a<br />
Constant <strong>Gradient</strong> Duration<br />
Column Volume = 0.83 mL<br />
Total Volume =40.7 c.v..<br />
1<br />
2<br />
1<br />
3<br />
4<br />
5,6<br />
20.00 20.00<br />
Column Volume = 2.5 mL<br />
Total Volume =13.5 c.v.<br />
5,6<br />
2 4<br />
3<br />
20.00 20.00<br />
Column Volume = 4.2 mL<br />
Total Volume = 8.0 c.v.<br />
0.000<br />
0.00 20.00<br />
Minutes<br />
40.00<br />
Alden<br />
2<br />
3<br />
4<br />
7<br />
5 6<br />
9<br />
8<br />
Minutes Minutes<br />
Minutes Minutes<br />
7<br />
10<br />
11<br />
7<br />
9<br />
8<br />
Symmetry300 4.6 X 50 mm,<br />
C18, 5 µm<br />
Conditions<br />
13<br />
- Sample: Tryptic digests of bovine<br />
12<br />
cytochrome c<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
Symmetry300 4.6 X 150<br />
mm,<br />
- Flow rate: 0.75 mL/min.<br />
- Detection: 214 nm<br />
10<br />
C 5 µm<br />
18, 13<br />
- Temperature: 35 ºC<br />
40.00 40.00<br />
8<br />
11<br />
40.00 40.00<br />
9 10<br />
11<br />
12<br />
Symmetry300 4.6 X 250<br />
mm,<br />
C 5 µm<br />
18,<br />
13<br />
12<br />
-Will observe elution<br />
pattern changes.<br />
-Resolution changes<br />
-Run time remains<br />
the same.<br />
Summary Part I - Column Length<br />
If the gradient volume is scaled proportionally to the<br />
column volume<br />
elution pattern does not change<br />
resolution increases with column length.<br />
© 1998 Waters Corporation<br />
If the gradient volume is not scaled in proportion to the<br />
column volume<br />
0.1200<br />
0.1000<br />
0.0800<br />
AU 0.0600<br />
0.0400<br />
0.0200<br />
134 Peaks<br />
Symmetry300 4.6 X 250<br />
mm,<br />
C 5 µm<br />
18,<br />
elution pattern and resolution changes<br />
50 mm column exhibits similar resolving power to a 250<br />
mm column.<br />
0.0000<br />
0.00 20.00<br />
Minutes<br />
40.00<br />
Alden © 1998 Waters Corporation<br />
© 1998 Waters Corporation<br />
Dr. Shulamit Levin 29-32
Rs (Resolution)<br />
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Neue<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Flow Rate; FF<br />
Part II II - Factors influencing selectivity<br />
Concentration and Type of Modifier<br />
Temperature<br />
Chemistry and Pore Size of the Packing Material<br />
Part III - Factors influencing reproducibility<br />
Column<br />
HPLC system<br />
© 1998 Waters Corporation<br />
Resolution as a Function of Flow Rate at a Constant<br />
<strong>Gradient</strong> Duration<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
(50 mm Column)<br />
0.33<br />
1 mL/min<br />
Flow Rate (mL/min)<br />
3.3<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Flow Rate; F<br />
Rs =<br />
t<br />
w<br />
N<br />
∼ × lnα<br />
×<br />
4<br />
B<br />
}<br />
}<br />
Efficiency Selectivity<br />
Flow Rate Effects on Resolution<br />
at a Constant <strong>Gradient</strong> Duration<br />
0.0500<br />
0.0400<br />
AU<br />
0.0300<br />
0.0200<br />
0.0100<br />
0.0000<br />
0.00<br />
0.0400<br />
0.0300<br />
20.00<br />
Minutes<br />
40.00<br />
75 Peaks<br />
0.3 mL/minute<br />
100 Peaks<br />
AU 0.0200<br />
0.0100<br />
0.0000<br />
0.00 20.00 Minutes 40.00<br />
0.5 mL/minute<br />
135 Peaks<br />
0.02500<br />
0.02000<br />
0.01500<br />
AU 0.01000<br />
0.00500<br />
0.00000<br />
-0.00500<br />
0.00 20.00 Minutes<br />
1.0 mL/minute<br />
40.00<br />
0.02000<br />
0.01500<br />
AU AU<br />
0.01000<br />
0.00500<br />
0.00000<br />
-0.00500 -0.00500<br />
0.00 0.00 20.00 20.00 Minutes Minutes 40.00<br />
Alden<br />
1<br />
. % . εt . πr .L/F + 1<br />
tg<br />
2<br />
}<br />
Retention<br />
F (flow rate) is is varied. All other<br />
variables are kept constant<br />
130 Peaks<br />
2.0 mL/minute<br />
Conditions<br />
© 1998 Waters Corporation<br />
- Column: Symmetry300, C 18,<br />
5 µm, 4.6x50mm<br />
- Sample: Tryptic digests of bovine<br />
serum albumin<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Temperature: 35 ºC<br />
- Detection: 214 nm<br />
-Best resolution<br />
occurred at a flow rate<br />
of 1.0 mL/min. for this<br />
peptide under these<br />
<strong>conditions</strong>.<br />
-Elution pattern<br />
changes.<br />
Dr. Shulamit Levin 33-36<br />
© 1998 Waters Corporation
Summary of Part I - Flow Rate<br />
Maximum resolution is achieved at an<br />
optimal flow rate:<br />
As flow rate changes N changes<br />
As flow rate changes the elution pattern<br />
changes.<br />
Type of Modifiers<br />
Solvation<br />
Ionization<br />
Ion-pairing<br />
Volatility (Collection)<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
What Factors Influence <strong>Gradient</strong> RP-HPLC<br />
Separations...<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Flow Rate; F<br />
Part II II - Factors influencing selectivity<br />
Concentration and Type of of Modifier<br />
Temperature<br />
Chemistry and Pore Size of the Packing Material<br />
Part III - Factors influencing reproducibility<br />
Column<br />
HPLC system<br />
Effects of TFA Concentration on Resolution<br />
- Typical gradient <strong>conditions</strong><br />
0.300<br />
0.200 0.200<br />
AU AU<br />
0.100 0.100<br />
0.000 0.000<br />
Alden<br />
0.1% TFA in<br />
solvents A and B<br />
0.00 0.00<br />
1<br />
5,6<br />
2 4<br />
3<br />
9<br />
7 8<br />
10<br />
11<br />
20.00 Minutes Minutes 40.00<br />
13<br />
12<br />
Conditions<br />
- Column: Symmetry300, C 18,<br />
5 µm, 3.9x150mm,<br />
© 1998 Waters Corporation<br />
- Sample: Tryptic digests of bovine<br />
cytochrome c<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: water<br />
Solvent B: acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Flow rate: 0.75 mL/min.<br />
- Temperature: 35 ºC<br />
- Detection: 214 nm<br />
Dr. Shulamit Levin 37-40<br />
© 1998 Waters Corporation
The Power of Different TFA Concentrations in<br />
Your Mobile Phase<br />
- 0.05% TFA in<br />
solvents A and B<br />
2<br />
1 3<br />
- 0.1% TFA in solvents<br />
A and B<br />
- 0.2% TFA in<br />
solvents A and B<br />
2<br />
1 3<br />
1<br />
4<br />
5 6<br />
2 4<br />
3<br />
5,6<br />
5,6<br />
4<br />
0.00 20.00 40.00<br />
Minutes<br />
Alden<br />
Absorbance (214 nm)<br />
Alternate Ion Pairing Reagents<br />
TFA and HFBA (Heptafluorobutyric Acid)<br />
0.40<br />
0.02<br />
0.00<br />
0.40<br />
0.20<br />
0.00<br />
TFA<br />
HFBA<br />
20 40 60 80<br />
Time (min)<br />
7<br />
7<br />
7<br />
8<br />
9<br />
8<br />
10<br />
9<br />
11<br />
10<br />
9<br />
8<br />
11<br />
10<br />
11<br />
12<br />
13<br />
12 13<br />
13<br />
12<br />
© 1998 Waters Corporation<br />
Sample: Rabbit cytochrome c<br />
tryptic digest, 500 pmol<br />
Column: Delta-Pak C 18, 5µm,<br />
300Å, 2.0 x 150 mm<br />
Eluents: A=water/ 0.1% TFA or<br />
HFBA<br />
B=acetonitrile/ 0.1%<br />
TFA or HFBA<br />
<strong>Gradient</strong>: O-60 % B 120 min<br />
Flow: 0.18 mL/min<br />
Temp: 35 ºC<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
0.200<br />
0.100 0.100<br />
0.000 0.000<br />
The Power of Different TFA<br />
Concentrations in Your Mobile Phase<br />
0.300 0.300<br />
AU AU<br />
0.3000<br />
0.2500<br />
0.2000<br />
AU 0.1500<br />
0.1000<br />
0.0500<br />
0.0000<br />
Alden<br />
0.00 0.00<br />
0.1% TFA in Solvent A<br />
0.1% TFA in Solvent B<br />
1<br />
1<br />
2<br />
3<br />
20.00 20.00<br />
2<br />
3 4<br />
5,6<br />
4<br />
Minutes Minutes<br />
0.05% TFA in Solvent A<br />
0.1% TFA in Solvent B<br />
7<br />
8<br />
9<br />
10<br />
11<br />
40.00 40.00<br />
0.00 20.00 40.00<br />
Minutes<br />
5 6<br />
7<br />
8<br />
9<br />
10<br />
11<br />
12<br />
13<br />
12 13<br />
Conditions<br />
- Column: Symmetry300, C 18, 5 µm,<br />
3.9x150mm<br />
- Sample: Tryptic Digests of Bovine<br />
Cytochrome c<br />
- Injection: 20 µL<br />
What Factors Influence <strong>Gradient</strong><br />
RP-HPLC Separations...<br />
- Mobile Phase:<br />
Solvent A: water<br />
Solvent B: acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Flow rate: 0.75 mL/min.<br />
- Temperature: 35 ºC<br />
- Detection: 214 nm<br />
Part I I - - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Flow Rate; FF<br />
Part II II - - Factors influencing selectivity<br />
Concentration and Type of Modifier<br />
Temperature<br />
Chemistry and Pore Size of the Packing Material<br />
Part III III - - Factors influencing reproducibility<br />
Column<br />
HPLC system<br />
Dr. Shulamit Levin 41-44<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation
Temperature Effects on Resolution<br />
Resolution is temperature dependent<br />
Temperature is a critical parameter to control<br />
in order to achieve reproducible separations.<br />
What Factors Influence <strong>Gradient</strong><br />
RP-HPLC Separations...<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and and N<br />
Flow Rate; F<br />
Part II - Factors influencing selectivity<br />
Concentration and Type of Modifier<br />
Temperature<br />
Pore Size and Chemistry of of the Packing Material<br />
Part III - Factors influencing reproducibility<br />
Column<br />
HPLC system<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
0.300<br />
0.200<br />
AU<br />
0.100<br />
0.000<br />
0.300<br />
0.200<br />
AU<br />
0.100<br />
0.000<br />
0.300<br />
0.200<br />
AU<br />
0.100<br />
0.000<br />
Temperature Effects on Resolution<br />
Alden<br />
0.300 0.300<br />
AU AU<br />
0.200 0.200<br />
5, 6<br />
7<br />
25.00 30.00 35.00 40.00<br />
Minutes<br />
5, 6<br />
7<br />
25.00 30.00 35.00 40.00<br />
Minutes<br />
5 6<br />
Rs (5,6) = 0<br />
Rs (8,9) = 2.39<br />
Rs (5,6)= 0<br />
Rs (8,9)= 2.07<br />
Rs (5,6)= 0.84<br />
Rs (8,9)= 1.63<br />
7<br />
25.00 30.00 35.00 40.00<br />
Minutes<br />
8<br />
8<br />
9<br />
9<br />
8<br />
9<br />
10<br />
10<br />
10<br />
11<br />
11<br />
11<br />
30 °C<br />
35 °C<br />
40 °C<br />
Conditions<br />
- Column: Symmetry300, C 18 ,<br />
5 µm, 3.9x150mm<br />
- Sample: Tryptic digests of bovine<br />
cytochrome c<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-30%B<br />
- Flow rate: 0.75 mL/min.<br />
- Detection: 214 nm<br />
Pore Size Effects on Resolution<br />
0.30<br />
0.20<br />
AU<br />
0.10<br />
0.00<br />
0.500 0.500<br />
0.400 0.400<br />
0.100 0.100<br />
0.000 0.000<br />
0.00 0.00<br />
1<br />
1<br />
5 6<br />
2 4<br />
3<br />
-0.10<br />
0.00 20.00<br />
Minutes<br />
40.00<br />
Carmody<br />
2 4<br />
3<br />
20.00 20.00<br />
5,6<br />
7<br />
Minutes Minutes<br />
8<br />
7<br />
Symmetry300, C 18, 5 µm,<br />
4.6 X 150 mm, 300Å<br />
10<br />
13<br />
9<br />
Symmetry®, C18, 5µm,<br />
4.6 X 150 mm, 100Å<br />
9<br />
8<br />
11<br />
10<br />
11<br />
40.00 40.00<br />
12<br />
13<br />
12<br />
Conditions<br />
© 1998 Waters Corporation<br />
- Sample: Tryptic digests of<br />
cytochrome c (bovine)<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.1% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- <strong>Gradient</strong>: 0-50 min., 0-30%B<br />
- Temperature: 35 ºC<br />
- Flow Rate: 0.75 mL/min.<br />
- Detection: 214 nm<br />
-Different pore<br />
sizes change<br />
selectivity.<br />
Dr. Shulamit Levin 45-48<br />
© 1998 Waters Corporation
Selectivity Differences Between Packings<br />
Alberta Peptides on Symmetry® Reversed-Phase Columns<br />
0.10<br />
AUFS<br />
AUFS<br />
0.05<br />
0.00<br />
0.10<br />
0.05<br />
0.00<br />
SymmetryShield RP 8, 100Å<br />
1<br />
2<br />
3<br />
5.00 10.00 15.00<br />
Symmetry® C 8, 100Å<br />
1 2<br />
5.00 10.00 15.00<br />
Minutes<br />
3<br />
4<br />
4<br />
5<br />
5<br />
Ac-Arg-Gly-X-X-Gly-Gly-Leu-Gly-LysAmide<br />
-X-X-:<br />
1: Ala-Gly with free alpha amino group<br />
2: Gly-Gly 3: Ala-Gly<br />
4: Val-Gly 5: Val-Val<br />
Peptide Mapping Validation<br />
-Robustness Testing<br />
Choice and quality of enzyme<br />
Digestion <strong>conditions</strong><br />
HPLC <strong>conditions</strong><br />
Equipment<br />
System<br />
Column<br />
Conditions:<br />
Columns: 3.9 mm x 150 mm<br />
Flow Rates: 0.8 mL/min<br />
Mobile Phase: A. 0.1% TFA aqueous;<br />
B. acetonitrile with 0.1% TFA<br />
<strong>Gradient</strong>: 10% to 40% B in 30 minutes,<br />
step to 60% B for 5 min<br />
Sample: 9 µL Alberta Peptides, mix<br />
with 5 decapeptides<br />
Detector: 214 nm<br />
Temperature: 35°C<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
What Factors Influence <strong>Gradient</strong><br />
RP-HPLC Separations...<br />
Part I - Factors influencing efficiency and retention<br />
<strong>Gradient</strong> Slope; c<br />
Column Length; L and N<br />
Flow Rate; FF<br />
Part II II - Factors influencing selectivity<br />
Concentration and Type of Modifier<br />
Temperature<br />
Chemistry and Pore Size of the Packing Material<br />
Part III - Factors influencing reproducibility<br />
Column<br />
HPLC HPLC system system<br />
Effects of Irreproducible <strong>Gradient</strong> Delivery<br />
-- Traditional HPLC System<br />
Experience resolution<br />
differences and<br />
retention time shifts<br />
0.00 20.00 40.00<br />
Minutes<br />
Carmody<br />
Dr. Shulamit Levin 49-52<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation
Waters 2690 Separations Module<br />
<strong>Gradient</strong> Accuracy & Precision<br />
0.08<br />
0.07<br />
0.06<br />
0.05<br />
AU 0.04<br />
0.03<br />
0.02<br />
0.01<br />
0.00<br />
10%B<br />
10.0 20.0 30.0 40.0 50.0 60.0<br />
Minutes<br />
Reproducible <strong>Gradient</strong> Delivery<br />
-Waters Alliance HPLC System<br />
-Overlay of 15 Consecutive Injections<br />
Conditions:<br />
Column: Symmetry300 C18, 5 µm, 3.9 x 150 nm<br />
Sample: 20 µL of bovine, cytochrome c (tryptic digest)<br />
Flow rate: 1 mL/min.<br />
Temperature: 35 ºC<br />
Linear gradient: 0 - 28%B in 45 min.<br />
Mobile phase: A: 0.05% TFA in water<br />
B: 0.1% TFA in Methanol<br />
Alden<br />
1% Step <strong>Gradient</strong> at 1.0 mL/min<br />
Accuracy
Complex <strong>Gradient</strong> Conditions:<br />
Column:<br />
Column:<br />
AccQ<br />
AccQ<br />
Tag<br />
Tag<br />
Column,<br />
Column,<br />
3.9<br />
3.9<br />
x<br />
x<br />
150<br />
150<br />
mm<br />
mm<br />
Eluent<br />
Eluent<br />
A:<br />
A:<br />
AccQ<br />
AccQ<br />
Tag<br />
Tag<br />
Eluent<br />
Eluent<br />
A<br />
Eluent<br />
Eluent<br />
B:<br />
B:<br />
Acetonitrile<br />
Acetonitrile<br />
Eluent C: C: Water<br />
Flow<br />
Flow<br />
Rate:<br />
Rate:<br />
1.0<br />
1.0<br />
mL/min.<br />
mL/min.<br />
Column<br />
Column<br />
Temp.:<br />
Temp.:<br />
37<br />
37<br />
°C<br />
°C<br />
Detection:<br />
Detection:<br />
Fluorescence,<br />
Fluorescence, kk ex =<br />
ex =<br />
250<br />
250<br />
nm,<br />
nm,<br />
kem k<br />
=<br />
em=<br />
395 nm nm<br />
Sample: 50 50 pmol Hydrolysate Standard<br />
Sample<br />
Sample<br />
Temp.:<br />
Temp.:<br />
5<br />
5<br />
°C<br />
°C<br />
<strong>Gradient</strong>:<br />
<strong>Gradient</strong>:<br />
Time Flow %A %B %C %D Curve<br />
0.00 1.00 100.0 0.0 0.0 0.0 *<br />
0.50 1.00 99.0 1.0 0.0 0.0 11<br />
18.0 1.00 95.0 5.0 0.0 0.0 6<br />
19.0 1.00 91.0 9.0 0.0 0.0 6<br />
29.5 1.00 83.0 17.0 0.0 0.0 6<br />
33.0 1.00 0.0 60.0 40.0 0.0 11<br />
36.0 1.00 100.0 0.0 0.0 0.0 11<br />
65.0 1.00 0.0 60.0 40.0 0.0 11<br />
100.0 0.00 100.0 0.0 0.0 0.0 6<br />
"Consideration must be given to<br />
column-to-column variation attributed to<br />
production differences from a recommended<br />
manufacturer. Column-to-column variability<br />
was recently bemoaned as the 'Achilles heel' in<br />
the HPLC of protein pharmaceuticals (20).<br />
Although this problem has been both ignored by<br />
many and overstated by others, peptide<br />
mapping does place very high demands on<br />
column performance. Thus, column-to-column<br />
variability is of special concern (21,22)."<br />
© 1998 Waters Corporation<br />
Garnick et al. Biologicals (1996) 24 , 255-275<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
Why is Column Reproducibility<br />
Important?<br />
Validated RP-HPLC assays require HPLC columns to<br />
be reproducible from column-to-column and<br />
batch-to-batch.<br />
Columns which perform reproducibly in terms of<br />
selectivity and separation characteristics from<br />
batch-to-batch ensures reliable, reproducible and<br />
robust assays over the life of of the product.<br />
© 1998 Waters Corporation<br />
Column-to-Column Reproducibility<br />
Employed 8 different Symmetry300, C 18, , 5 µm<br />
18<br />
columns from the same batch (#107)<br />
Conducted evaluation over a 3-week time period<br />
Different mobile phase preparations for each run<br />
Dr. Shulamit Levin 57-60<br />
© 1998 Waters Corporation
Symmetry300<br />
Batch-to-Batch Reproducibility<br />
Batch 107<br />
Batch 106<br />
Batch 104<br />
Batch 103<br />
10 30 50<br />
Minutes<br />
Costello<br />
Conclusions (continued)<br />
Conditions<br />
- Column: Symmetry300, C 18,<br />
5 µm, 3.9x150mm<br />
- Sample: Tryptic digests of<br />
cytochrome c (bovine)<br />
- Injection: 20 µL<br />
- Mobile Phase:<br />
Solvent A: 0.05% TFA in water<br />
Solvent B: 0.1% TFA in acetonitrile<br />
- <strong>Gradient</strong>: 0-45 min., 0-28%B<br />
- Flow rate: 1.0 mL/min.<br />
- Temperature: 35 °C<br />
- Detection: 220 nm<br />
For Rugged/Robust gradient method<br />
development consider...<br />
System performance<br />
reproducibility of gradient delivery<br />
system delay volume<br />
Column performance<br />
column-to-column reproducibility<br />
batch-to-batch reproducibility<br />
- Instrument:<br />
Waters Alliance<br />
HPLC system<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation<br />
<strong>Gradient</strong> Operation<br />
Conclusion<br />
There are several factors to assess when<br />
developing/optimizing a gradient RP-HPLC<br />
method.<br />
Primary tools for gradient optimization are<br />
gradient slope, column length and modifier<br />
type.<br />
Secondary tools to be used for fine tuning<br />
your gradient are temperature, modifier<br />
concentration and flow rate.<br />
Acknowledgments:<br />
Bonnie Alden<br />
Ray Crowley<br />
Uwe Neue<br />
Tom Walter<br />
Ray Fisk<br />
Dave Costello<br />
Edouard Bouvier<br />
...and Waters' Symmetry® Team<br />
Dr. Shulamit Levin 61-64<br />
© 1998 Waters Corporation<br />
© 1998 Waters Corporation