EuroMEEting - Genetic Alliance UK
EuroMEEting - Genetic Alliance UK
EuroMEEting - Genetic Alliance UK
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Lunch<br />
Lunch<br />
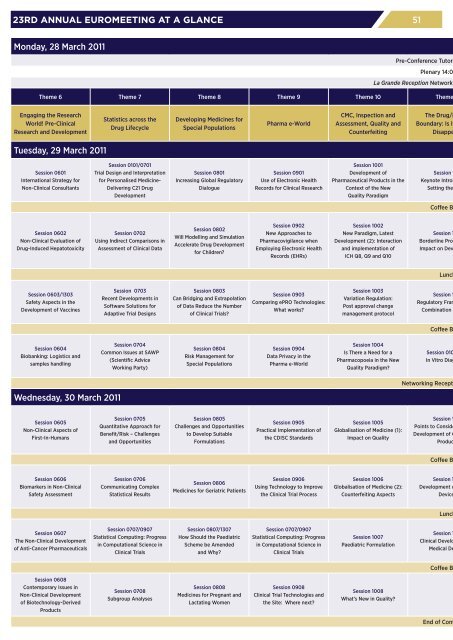
23RD ANNUAL EUROMEETING AT A GLANCE<br />
51<br />
Monday, 28 March 2011<br />
Pre-Conference Tutori<br />
Plenary 14:0<br />
La Grande Reception Networki<br />
Theme 6 Theme 7 Theme 8 Theme 9 Theme 10 Theme<br />
Engaging the Research<br />
World! Pre-Clinical<br />
Research and Development<br />
Statistics across the<br />
Drug Lifecycle<br />
Developing Medicines for<br />
Special Populations<br />
Pharma e-World<br />
CMC, Inspection and<br />
Assessment, Quality and<br />
Counterfeiting<br />
The Drug/D<br />
Boundary: Is It<br />
Disappe<br />
Tuesday, 29 March 2011<br />
Session 0601<br />
International Strategy for<br />
Non-Clinical Consultants<br />
Session 0101/0701<br />
Trial Design and Interpretation<br />
for Personalised Medicine-<br />
Delivering C21 Drug<br />
Development<br />
Session 0801<br />
Increasing Global Regulatory<br />
Dialogue<br />
Session 0901<br />
Use of Electronic Health<br />
Records for Clinical Research<br />
Session 1001<br />
Development of<br />
Pharmaceutical Products in the<br />
Context of the New<br />
Quality Paradigm<br />
Session 1<br />
Keynote Introd<br />
Setting the<br />
Coffee B<br />
Session 0602<br />
Non-Clinical Evaluation of<br />
Drug-Induced Hepatotoxicity<br />
Session 0702<br />
Using Indirect Comparisons in<br />
Assessment of Clinical Data<br />
Session 0802<br />
Will Modelling and Simulation<br />
Accelerate Drug Development<br />
for Children?<br />
Session 0902<br />
New Approaches to<br />
Pharmacovigilance when<br />
Employing Electronic Health<br />
Records (EHRs)<br />
Session 1002<br />
New Paradigm, Latest<br />
Development (2): Interaction<br />
and implementation of<br />
ICH Q8, Q9 and Q10<br />
Session 1<br />
Borderline Prod<br />
Impact on Dev<br />
Session 0603/1303<br />
Safety Aspects in the<br />
Development of Vaccines<br />
Session 0703<br />
Recent Developments in<br />
Software Solutions for<br />
Adaptive Trial Designs<br />
Session 0803<br />
Can Bridging and Extrapolation<br />
of Data Reduce the Number<br />
of Clinical Trials?<br />
Session 0903<br />
Comparing ePRO Technologies:<br />
What works?<br />
Session 1003<br />
Variation Regulation:<br />
Post approval change<br />
management protocol<br />
Session 1<br />
Regulatory Fram<br />
Combination P<br />
Coffee B<br />
Session 0604<br />
Biobanking: Logistics and<br />
samples handling<br />
Session 0704<br />
Common Issues at SAWP<br />
(Scientific Advice<br />
Working Party)<br />
Session 0804<br />
Risk Management for<br />
Special Populations<br />
Session 0904<br />
Data Privacy in the<br />
Pharma e-World<br />
Session 1004<br />
Is There a Need for a<br />
Pharmacopoeia in the New<br />
Quality Paradigm?<br />
Session 010<br />
In Vitro Diag<br />
Wednesday, 30 March 2011<br />
Networking Recept<br />
Session 0605<br />
Non-Clinical Aspects of<br />
First-In-Humans<br />
Session 0705<br />
Quantitative Approach for<br />
Benefit/Risk – Challenges<br />
and Opportunities<br />
Session 0805<br />
Challenges and Opportunities<br />
to Develop Suitable<br />
Formulations<br />
Session 0905<br />
Practical Implementation of<br />
the CDISC Standards<br />
Session 1005<br />
Globalisation of Medicine (1):<br />
Impact on Quality<br />
Session 1<br />
Points to Conside<br />
Development of C<br />
Produc<br />
Coffee B<br />
Session 0606<br />
Biomarkers in Non-Clinical<br />
Safety Assessment<br />
Session 0706<br />
Communicating Complex<br />
Statistical Results<br />
Session 0806<br />
Medicines for Geriatric Patients<br />
Session 0906<br />
Using Technology to Improve<br />
the Clinical Trial Process<br />
Session 1006<br />
Globalisation of Medicine (2):<br />
Counterfeiting Aspects<br />
Session 1<br />
Development o<br />
Device<br />
Session 0607<br />
The Non-Clinical Development<br />
of Anti-Cancer Pharmaceuticals<br />
Session 0707/0907<br />
Statistical Computing: Progress<br />
in Computational Science in<br />
Clinical Trials<br />
Session 0807/1307<br />
How Should the Paediatric<br />
Scheme be Amended<br />
and Why?<br />
Session 0707/0907<br />
Statistical Computing: Progress<br />
in Computational Science in<br />
Clinical Trials<br />
Session 1007<br />
Paediatric Formulation<br />
Session 1<br />
Clinical Develo<br />
Medical De<br />
Session 0608<br />
Contemporary Issues in<br />
Non-Clinical Development<br />
of Biotechnology-Derived<br />
Products<br />
Session 0708<br />
Subgroup Analyses<br />
Session 0808<br />
Medicines for Pregnant and<br />
Lactating Women<br />
Session 0908<br />
Clinical Trial Technologies and<br />
the Site: Where next?<br />
Session 1008<br />
What’s New in Quality?<br />
Coffee B<br />
End of Conf