Growth Hormone Improves Body Composition, Fat ... - GGH Journal
Growth Hormone Improves Body Composition, Fat ... - GGH Journal
Growth Hormone Improves Body Composition, Fat ... - GGH Journal
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
were no noted side effects from GH therapy. Specifically, frequent<br />
complete blood cell counts were performed to monitor for the risk<br />
of acute lymphoblastic leukemia, which is known to occur more<br />
frequently in children with Down syndrome. Prior to treatment,<br />
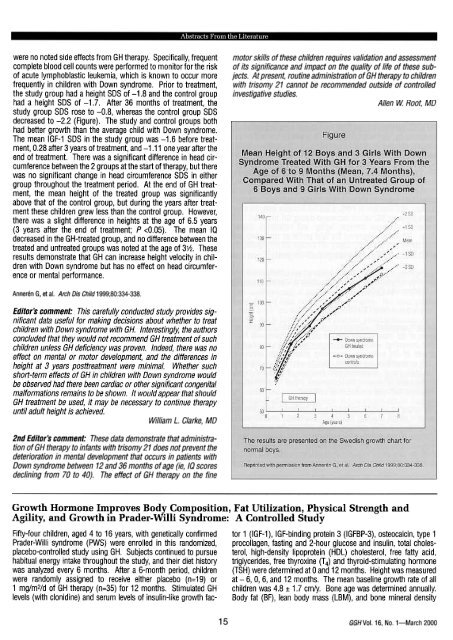
the study group had a height SDS of -1.8 and the control group<br />
had a height SDS of -1.7. After 36 months of treatment, the<br />
study group SDS rose to -0.8, whereas the control group SDS<br />
decreased to -2.2 (Figure). The study and control groups both<br />
had better growth than the average child with Down syndrome.<br />
The mean IGF-1 SDS in the study group was -1.6 before treatment,<br />
0.28 after 3 years of treatment, and -1.11 one year after the<br />
end of treatment. There was a significant difference in head circumference<br />
between the 2 groups at the start of therapy, but there<br />
was no significant change in head circumference SDS in either<br />
group throughouthe treatment period. At the end of GH treatment,<br />
the mean height of the treated group was significantly<br />
above that of the control group, but during the years after treatment<br />
these children grew less than the control group. However,<br />
there was a slight difference in heights at the age of 6.5 years<br />
(3 years after the end of treatment; P
(BMD) were determined by dual energy X-ray absorptiometry<br />
(DXA). Resting energy expenditures (REE) and respiratory quotients<br />
(RQ) were determined by indirect calorimetry. Physical<br />
strength and agility were determined by using a modified Bruininks-<br />
Oseretsky test.<br />
After 12 months, GH-treated children had a mean growth rate of<br />
10.1 cm/y, compared with 5 cm/y among controls. Mean IGF-1,<br />
IGFBP-3, osteocalcin, and type 1 procollagen levels significantly<br />
increased in GH-treated children. Baseline body composition<br />
analysis revealed increased BF (45.2%:I: 8.3% vs 18% :I: 3.6%)<br />
and lower LBM (50% vs 80%) compared with healthy children<br />
without PWS. GH-treated patients experienced an 8% decrease in<br />
BF. Their LBM increased compared with controls (Table). Ninetyfive<br />
percent of PWS children had normal baseline BMD. Femoral<br />
BMD increased by 0.09 :I: 0.02 g/cm2, while lumbar spine and total<br />
body BMD remained unchangeduring GH treatment. Although<br />
baseline REE was low in PWS, it did not increase after GH treatment;<br />
however, RQ values decreaseduring GH treatment. GH<br />
treatment increased strength, agility, and respiratory muscle<br />
force. GH therapy improved the lipid profile as cholesterol and<br />
HDL cholesterol levels fell. Lastly, GH therapy did not significantly<br />
affect fasting and 2-hour postprandial insulin levels.<br />
which could be aggravated by GH treatment. Finally, the chromosomal<br />
abnormalities characteristic of PWS may add a risk factor<br />
for potential GH-associated malignancies. Therefore, the estimated<br />
potential benefit of this kind of treatment should be evaluated<br />
on a long-term basis.<br />
Fima Lifshitz, MD<br />
2nd Editor's comment: This topic is of much current interest. To<br />
supplement your knowledge about the recent literature, you can<br />
refer to 2 articles abstracted in <strong>GGH</strong> previously (1999;15[1 J).<br />
1. Thacker MJ, et al. <strong>Growth</strong> failure in Prader-Willi syndrome is<br />
secondary to GH deficiency. Harm Res 1998;49:216-220.<br />
2. Lindgren AC, et al. <strong>Growth</strong> hormone treatment of children<br />
with Prader-Willi syndrome affects linear growth and body<br />
composition favorably. Acta Paediatr 1998;87:28-31.<br />
The data and conclusions in these articles are consistent.<br />
Robert M. Blizzard, MD<br />
The authors concluded that GH treatment promoted growth and<br />
positively affected body composition, physical strength, and agility<br />
in children with PWS.<br />
Carrell A, et al. J Pediatr 1999;134:215-221<br />
Editors comment: Obesity, short stature, and hypotonia are<br />
some of the most striking physical signs of children with PWS.<br />
This interesting, well-designed, prospective study describes the<br />
effects of GH treatment-promoting growth while improving<br />
body composition and physical capacity-in children with PWS.<br />
The data are in agreement with previous studies and clearly<br />
showed that GH therapy could represent one of the most important<br />
therapeutic approaches to this disorder.<br />
The response of PWS patients to GH treatment resembles the<br />
positive effects of GH in hypopituitary patients. In addition, the<br />
possibility that PWS patients are GH deficient because of hypothalamic<br />
abnormalities needs to be considered, although the GH<br />
axis has been difficult to assess because of the obesity of PWS<br />
patients. The deleterious effects of GH treatment should be considered<br />
as PWS children have an increased incidence of scoliosis,<br />
<strong>Growth</strong> <strong>Hormone</strong> Treatment Increases CO2 Response, Ventilation and Central Inspiratory<br />
Drive in Children With Prader- Willi Syndrome<br />
Hypoventilation commonly occurs in subjects with Prader-Willi syn- rhGH (0.23 mg/kg/wk). This therapeutic program did not alter<br />
drome (PWS). This has been attributed in large part to obesity and body mass index in this interval, although changes in body comthe<br />
imposition of a large mechanical load, thereby impairing move- position were not reported. They found that in response to rhGH,<br />
ment of the thoracic cage. However, there also is a problem with minute ventilation (mUkg/min) increased 126%; the ventilatory<br />
ventilatory control in PWS patients, as evidenced by abnormal response (ie, sensitivity) to 4% CO2 increased 8-fold; and the cenresponses<br />
to hyperoxia, hypoxia, and hypercarbia, as well as tral inspiratory drive, a measurement of airway occlusion pressure<br />
decreased peripheral chemoreceptor activity (Menendez AA. fur J that is little affected by pulmonary mechanics (determined by the<br />
Pediatr 1999;158:941-942). change in airway pressure during the first 0.1 second after beginning<br />
an inspiration), increased 170%. In contrast, the response to<br />
Lindgren et al studied resting ventilatory regulation in 9 prepubertal<br />
children with PWS prior to and after 6 to 9 months of therapy with<br />
hyperoxia, measured as an increase of the inspired O2 concentration<br />
from room air to 100%, did not change during rhGH administration.<br />
<strong>GGH</strong>Vol. 16. No.1-March 2000<br />
16