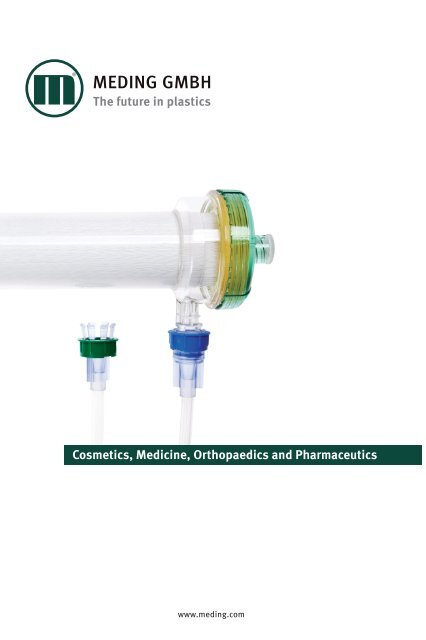
Cosmetics, Medicine, Orthopaedics and Pharmaceutics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
The future in plastics<br />
<strong>Cosmetics</strong>, <strong>Medicine</strong>, <strong>Orthopaedics</strong> <strong>and</strong> <strong>Pharmaceutics</strong><br />
www.meding.com
Integrated productivity – solutions become plannable<br />
Integrated productivity st<strong>and</strong>s for total project implementation, which has been a firm part of<br />
Meding GmbH's philosophy for generations. From the manufacturing of a prototype to tool<br />
development to series production <strong>and</strong> assembly – everything comes from a single source, <strong>and</strong><br />
takes place ideally within seven days.<br />
In addition, we have committed employees who plan, construct <strong>and</strong> manufacture your<br />
products using modern, advanced manufacturing equipment. In a qualified <strong>and</strong> controlled<br />
environment, up to clean room class 7, we produce customer-specific <strong>and</strong> technically sophisticated<br />
plastic parts with a piece weight from 0.01 to 1,500 grams.<br />
develops designs manufactures your product<br />
For our partners in the cosmetics, medical technology, orthopaedics <strong>and</strong> pharmacy sectors,<br />
this principle of "overall solutions" offers major advantages. In the shortest time, you receive<br />
products which, both in terms of their use <strong>and</strong> design, precisely meet the users' needs. And<br />
almost before you know it, we put into practice the innovative ideas which you want to use to<br />
open up new markets.<br />
Our decades of experience with plastics, our extensive knowledge of the industry, <strong>and</strong> on-going<br />
quality awareness form the basis for the tried-<strong>and</strong>-tested Meding principle. But there is<br />
much more! For finishing, we provide you with additional <strong>and</strong> comprehensive services:<br />
printing, module assembly, improvement, commissioning <strong>and</strong> just-in-time delivery complete<br />
the project flow in the best possible way.
From specialisation to the specialists<br />
With Meding, you can count on decades of tradition <strong>and</strong> experience. Since 1949, when our<br />
firm was set up as a metal-processing company, we have consistently been a trendsetter. At<br />
the same time, the triumph of plastic moulded parts started with the development of thermoplastics<br />
in the 1950s, <strong>and</strong> this was a process which we followed with great interest. The material<br />
fascinated us more <strong>and</strong> more, <strong>and</strong> that is why we started manufacturing plastic parts in<br />
1976.<br />
Since then, <strong>and</strong> with the same amount of enthusiasm as in 1976, we have concentrated on the<br />
many processing <strong>and</strong> application opportunities provided by this material. In the years which<br />
followed, we invested in new manufacturing technologies <strong>and</strong> our own know-how <strong>and</strong> skills.<br />
With the development <strong>and</strong> production start of an exact-dosing applicator in 1986, we entered<br />
the sophisticated <strong>and</strong> extremely interesting market of medical technology, <strong>and</strong> this was a step<br />
which still characterises us today. This is shown not only by the product range for the entire<br />
health sector, which we continue to develop, but also by the numerous design registrations<br />
<strong>and</strong> patents which document the passion with which we develop innovative new products.<br />
Excerpt from the range of our products<br />
Ampoule openers<br />
Applicators with cap<br />
Blade protection caps<br />
Buckles<br />
Clamp-on profiles<br />
Corner connectors<br />
Cosmetic spatulas<br />
Cream spatulas<br />
Dialysis connectors<br />
Display frames<br />
Dosing systems<br />
Double measuring spoons<br />
Eye-cups<br />
Faeces sample sets<br />
Finger sleeve toothbrush<br />
Flange end covering caps<br />
Frames<br />
H<strong>and</strong> guards<br />
H<strong>and</strong>-held transmitters<br />
Headrest caps<br />
Housings<br />
Insulator sleeves<br />
Knee stabilisers<br />
Measuring caps<br />
Measuring cups<br />
Measuring spoons<br />
Mouth spatulas<br />
Nail polish colour scheme cards<br />
Perforated strips<br />
Pill dispensers<br />
Pipettes<br />
Plasma container<br />
Plastic back frame<br />
Protective caps<br />
Protective rings<br />
Pump housings<br />
Pushbuttons<br />
Release levers<br />
Salve spatulas<br />
Screw-on caps<br />
Shakers<br />
Sliders<br />
Spring b<strong>and</strong> rods<br />
Tablet tubes<br />
Tweezers<br />
2-component caps
The MEDING principle<br />
Idea/Order<br />
Receipt of order<br />
within 24 hours<br />
Manufacture of<br />
prototype<br />
within 24 hours<br />
Article released<br />
by customer<br />
within 4 days<br />
Tool<br />
manufacture<br />
within 5 days<br />
Manufacture of<br />
the pilot series<br />
Series<br />
manufacture<br />
Delivery<br />
Milestones<br />
1949<br />
Meding GmbH is established in<br />
Iserlohn as a metal-processing<br />
company.<br />
1974<br />
Klaus <strong>and</strong> Ursula Pietzner take<br />
over Meding GmbH <strong>and</strong> move the<br />
company headquarters to<br />
Lüdenscheid.<br />
1976<br />
The company starts manufacturing<br />
plastic products.<br />
1986<br />
The company starts manufacturing<br />
products for the cosmetic <strong>and</strong><br />
pharmaceutical industries.<br />
1992<br />
Construction <strong>and</strong> transfer to a<br />
production <strong>and</strong> administrative<br />
building in Halver, where we still<br />
operate.<br />
1995<br />
Certification of the quality<br />
management system according to<br />
DIN EN ISO 9002. The warehouse<br />
areas are exp<strong>and</strong>ed.<br />
1998<br />
Granting of the CE mark for<br />
medical products.<br />
1999<br />
Expansion of the production area<br />
for compliance with pharmaceutical<br />
requirements.<br />
2001<br />
A new warehouse is built.<br />
2003<br />
Adaptation of the QM system to<br />
DIN EN ISO 9001:2000 <strong>and</strong> DIN EN<br />
ISO 13488.<br />
2006<br />
Expansion of the production area<br />
by 30%. Adaptation of the QM<br />
system to EN ISO 13485.<br />
2007<br />
Setting-up of a processing centre<br />
for servicing <strong>and</strong> maintaining<br />
tools.<br />
2008<br />
Expansion of the product range to<br />
include plastic-metal compounds<br />
(hybrid technology).<br />
2009<br />
Certification acc. to DIN EN ISO<br />
9001:2008 <strong>and</strong> DIN EN ISO<br />
13485:2007.<br />
2010<br />
Extended development of<br />
two-component <strong>and</strong> three component<br />
parts. Manufacture starts of<br />
prototypes using 3D printing<br />
processes.<br />
2011<br />
Addition of a high-bay warehouse.<br />
2012<br />
Adaptation of the QM systems to<br />
EN ISO 13485:2003+AC:2009.<br />
2013<br />
Energy-saving injection-moulding<br />
machines are added. An energy<br />
audit takes place.
Quality at the heart of all activities<br />
Technology <strong>and</strong> automation are the basic requirements for a good product, but it is the interaction<br />
between qualified employees <strong>and</strong> highly-developed, precise production engineering<br />
which makes it perfect. Our high quality st<strong>and</strong>ards form the basis for manufacturing <strong>and</strong> using<br />
our delicate products, <strong>and</strong> this is a task which we are happy to accept.<br />
All the medical products manufactured in our company meet, as a matter of course, the requirements<br />
of EU directive 93/42/EEC <strong>and</strong> the Medical Devices Act. They have carried the CE mark<br />
since 1998. These high quality requirements form part of our living company culture. Regular<br />
certification according to DIN EN ISO 9001 <strong>and</strong> EN ISO 13485 is not just documented – our<br />
employees <strong>and</strong> management carry out the process of continuous improvement on a daily<br />
basis. Only physiologically harmless plastics are processed, <strong>and</strong> these plastics correspond at<br />
a minimum to the recommendations of the German Federal Institute for Risk Assessment<br />
<strong>and</strong>/or European Pharmacopeia. To ensure that this happens, our own measuring <strong>and</strong> test<br />
equipment is manufactured in-house.<br />
The many characteristics of plastic materials, together with the versatility which processing<br />
offers, constantly give us the momentum to deal intensively with the on-going development of<br />
our products, <strong>and</strong> to carry out research into improvements for the materials <strong>and</strong> innovative<br />
processing methods. To achieve all this, Meding GmbH works closely with the "Fachhochschule<br />
Südwestfalen" university of applied sciences <strong>and</strong> the Kunststoffinstitut (Plastics Institute),<br />
with which, for example, materials suitability tests or migration tests are carried out.<br />
You can find more information<br />
on our website:<br />
www.meding.com
Kruppstraße 8<br />
D-58553 Halver<br />
Telefon: +49 23 53 - 91 58-0<br />
Telefax: +49 23 53 - 91 58-28<br />
E-Mail: info@meding.com<br />
Internet: www.meding.com<br />
To visit our Facebook fan page,<br />
just scan the QR code:<br />
05/2013