Acrylamide from Maillard reaction products
Acrylamide from Maillard reaction products
Acrylamide from Maillard reaction products
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Acrylamide</strong> formed<br />
(µmol per mol amino acid)<br />
e-mail: d.s.mottram@reading.ac.uk<br />
†Procter Department of Food Science, University of<br />
Leeds, Leeds LS2 9JT, UK<br />
1. Rosen, J. & Hellenas, K.-E. Analyst 127, 880–882 (2002).<br />
2. Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S. & Törnqvist, M.<br />
J. Agric. Food Chem. 50, 4998–5006 (2002).<br />
3. IARC IARC Monographs on the Evaluation of Carcinogenic Risks<br />
to Humans 60, 389 (1994).<br />
4. Belitz, H.-D. & Grosch, W. Food Chemistry (Springer,<br />
New York, 1999).<br />
5. Martin, F. L. & Ames, J. M. J. Agric. Food Chem. 49,<br />
3885–3892 (2001).<br />
6. Dembinski, E. & Bany, S. J. Plant Physiol. 138, 494–496 (1991).<br />
7. Castle, L. J. Agric. Food Chem. 41, 1261–1263 (1993).<br />
Competing financial interests: declared none.<br />
Food chemistry<br />
<strong>Acrylamide</strong> <strong>from</strong> <strong>Maillard</strong><br />
<strong>reaction</strong> <strong>products</strong><br />
The discovery of the adventitious formation<br />
of the potential cancer-causing<br />
agent acrylamide in a variety of foods<br />
during cooking 1,2 has raised much concern 3,4 ,<br />
but the chemical mechanism(s) governing<br />
its production are unclear. Here we show<br />
that acrylamide can be released by the<br />
thermal treatment of certain amino acids<br />
(asparagine, for example), particularly in<br />
combination with reducing sugars, and of<br />
early <strong>Maillard</strong> <strong>reaction</strong> <strong>products</strong> (N-glycosides)<br />
5 . Our findings indicate that the<br />
<strong>Maillard</strong>-driven generation of flavour and<br />
colour in thermally processed foods can —<br />
under particular conditions — be linked to<br />
the formation of acrylamide.<br />
We heated 20 amino acids individually<br />
at 180 °C for 30 min and found that acrylamide<br />
is formed under these conditions<br />
<strong>from</strong> methionine and <strong>from</strong> asparagine<br />
(3.651.4 and 0.5650.05 µmol acrylamide<br />
per mol amino acid, respectively; all results<br />
are averages of n46 independent determinations<br />
unless stated otherwise).<br />
When pyrolysed at 180 °C with an<br />
equimolar amount of glucose, asparagine in<br />
particular generates significant amounts of<br />
acrylamide, reaching an average of<br />
368 m mol mol 11 after an incubation time (t i )<br />
of 30 min. If asparagine monohydrate was<br />
used in the incubation or water was added to<br />
the <strong>reaction</strong> (0.05 ml) before thermolysis, the<br />
release of acrylamide was enhanced nearly<br />
threefold (9605210 m mol mol 11 ), or over<br />
1,700 times the amount formed <strong>from</strong><br />
asparagine alone under the same conditions.<br />
Reaction of methionine and glutamine<br />
with equimolar amounts of glucose at<br />
180 °C also increased the formation of<br />
acrylamide, which occurred rapidly in each<br />
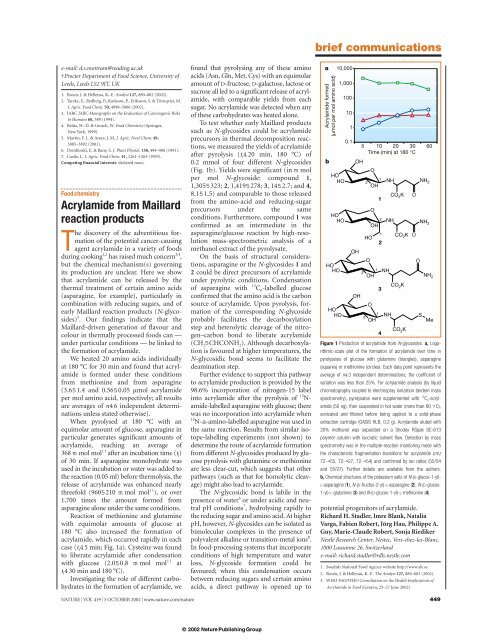
case (t i 45 min; Fig. 1a). Cysteine was found<br />
to liberate acrylamide after condensation<br />
with glucose (2.050.8 m mol mol 11 at<br />
t i 430 min and 180 °C).<br />
Investigating the role of different carbohydrates<br />
in the formation of acrylamide, we<br />
found that pyrolysing any of these amino<br />
acids (Asn, Gln, Met, Cys) with an equimolar<br />
amount of D-fructose, D-galactose, lactose or<br />
sucrose all led to a significant release of acrylamide,<br />
with comparable yields <strong>from</strong> each<br />
sugar. No acrylamide was detected when any<br />
of these carbohydrates was heated alone.<br />
To test whether early <strong>Maillard</strong> <strong>products</strong><br />
such as N-glycosides could be acrylamide<br />
precursors in thermal decomposition <strong>reaction</strong>s,<br />
we measured the yields of acrylamide<br />
after pyrolysis (t i 420 min, 180 °C) of<br />
0.2 mmol of four different N-glycosides<br />
(Fig. 1b). Yields were significant (in m mol<br />
per mol N-glycoside: compound 1,<br />
1,3055323; 2, 1,4195278; 3,1452.7; and 4,<br />
8.151.5) and comparable to those released<br />
<strong>from</strong> the amino-acid and reducing-sugar<br />
precursors under the same<br />
conditions. Furthermore, compound 1 was<br />
confirmed as an intermediate in the<br />
asparagine/glucose <strong>reaction</strong> by high-resolution<br />
mass-spectrometric analysis of a<br />
methanol extract of the pyrolysate.<br />
On the basis of structural considerations,<br />
asparagine or the N-glycosides 1 and<br />
2 could be direct precursors of acrylamide<br />
under pyrolytic conditions. Condensation<br />
of asparagine with 13 C 6 -labelled glucose<br />
confirmed that the amino acid is the carbon<br />
source of acrylamide. Upon pyrolysis, formation<br />
of the corresponding N-glycoside<br />
probably facilitates the decarboxylation<br />
step and heterolytic cleavage of the nitrogen–carbon<br />
bond to liberate acrylamide<br />
(CH 2 5CHCONH 2 ). Although decarboxylation<br />
is favoured at higher temperatures, the<br />
N-glycosidic bond seems to facilitate the<br />
deamination step.<br />
Further evidence to support this pathway<br />
to acrylamide production is provided by the<br />
98.6% incorporation of nitrogen-15 label<br />
into acrylamide after the pyrolysis of 15 N-<br />
amide-labelled asparagine with glucose; there<br />
was no incorporation into acrylamide when<br />
15 N-a-amino-labelled asparagine was used in<br />
the same <strong>reaction</strong>. Results <strong>from</strong> similar isotope-labelling<br />
experiments (not shown) to<br />
determine the route of acrylamide formation<br />
<strong>from</strong> different N-glycosides produced by glucose<br />
pyrolysis with glutamine or methionine<br />
are less clear-cut, which suggests that other<br />
pathways (such as that for homolytic cleavage)<br />
might also lead to acrylamide.<br />
The N-glycosidic bond is labile in the<br />
presence of water 6 or under acidic and neutral<br />
pH conditions 7 , hydrolysing rapidly to<br />
the reducing sugar and amino acid. At higher<br />
pH, however, N-glycosides can be isolated as<br />
bimolecular complexes in the presence of<br />
polyvalent alkaline or transition-metal ions 8 .<br />
In food-processing systems that incorporate<br />
conditions of high temperature and water<br />
loss, N-glycoside formation could be<br />
favoured; when this condensation occurs<br />
between reducing sugars and certain amino<br />
acids, a direct pathway is opened up to<br />
brief communications<br />
a<br />
b<br />
HO<br />
HO<br />
HO<br />
HO<br />
10,000<br />
HO<br />
HO<br />
HO<br />
HO<br />
1,000<br />
100<br />
10<br />
1<br />
0.1<br />
5 10 20 30 60<br />
Time (min) at 180 °C<br />
OH<br />
O<br />
1<br />
2<br />
OH<br />
NH NH 2<br />
Figure 1 Production of acrylamide <strong>from</strong> N-glycosides. a, Logarithmic-scale<br />
plot of the formation of acrylamide over time in<br />
pyrolysates of glucose with glutamine (triangles), asparagine<br />
(squares) or methionine (circles). Each data point represents the<br />
average of n43 independent determinations; the coefficient of<br />
variation was less than 25%. For acrylamide analysis (by liquid<br />
chromatography coupled to electrospray ionization tandem mass<br />
spectrometry), pyrolysates were supplemented with 13 C 3 -acrylamide<br />
(50 ng), then suspended in hot water (more than 90 7C),<br />
sonicated and filtered before being applied to a solid-phase<br />
extraction cartridge (OASIS HLB, 0.2 g). <strong>Acrylamide</strong> eluted with<br />
20% methanol was separated on a Shodex RSpak DE-613<br />
polymer column with isocratic solvent flow. Detection by mass<br />
spectrometry was in the multiple-<strong>reaction</strong> monitoring mode with<br />
the characteristic fragmentation transitions for acrylamide (m/z<br />
72➝55, 72➝27, 72➝54) and confirmed by ion ratios (55/54<br />
and 55/27). Further details are available <strong>from</strong> the authors.<br />
b, Chemical structures of the potassium salts of N-(D-glucos-1-yl)-<br />
L-asparagine (1), N-(D-fructos-2-yl)-L-asparagine (2), N-(D-glucos-<br />
1-yl)-L-glutamine (3) and N-(D-glucos-1-yl)-L-methionine (4).<br />
potential progenitors of acrylamide.<br />
Richard H. Stadler, Imre Blank, Natalia<br />
Varga, Fabien Robert, Jörg Hau, Philippe A.<br />
Guy, Marie-Claude Robert, Sonja Riediker<br />
Nestlé Research Center, Nestec, Vers-chez-les-Blanc,<br />
1000 Lausanne 26, Switzerland<br />
e-mail: richard.stadler@rdls.nestle.com<br />
1. Swedish National Food Agency website http://www.slv.se<br />
2. Rosén, J. & Hellenäs, K.-E. The Analyst 127, 880–882 (2002).<br />
3. WHO FAO/WHO Consultation on the Health Implications of<br />
<strong>Acrylamide</strong> in Food (Geneva, 25–27 June 2002)<br />
1<br />
4<br />
CO 2 K<br />
O<br />
2 NH NH<br />
OH<br />
2<br />
HO 1 CO 2 K O<br />
2<br />
OH<br />
O<br />
O<br />
1<br />
2<br />
OH<br />
NH<br />
NH 2<br />
3<br />
CO 2 K<br />
OH<br />
O<br />
1<br />
2<br />
OH<br />
NH<br />
S<br />
Me<br />
CO 2 K<br />
O<br />
NATURE | VOL 419 | 3 OCTOBER 2002 | www.nature.com/nature 449<br />
© 2002 Nature Publishing Group
ief communications<br />
http://www.who.int/fsf/<br />
4. European Commission Scientific Committee on Food (SCF)<br />
Opinion of the Scientific Committee on Food on New Findings<br />
Regarding the Presence of <strong>Acrylamide</strong> in Food<br />
(SCF/CS/CNTM/CONT/4 Final, 3 July 2002)<br />
http://europa.eu.int/comm/food/fs/scf/index_en.html<br />
5. Ledl, F. & Schleicher, E. Angew. Chem. Int. Ed. Engl. 29,<br />
565–594 (1990).<br />
6. Paulsen, H. & Pflughaupt, H. in The Carbohydrates —<br />
Chemistry and Biochemistry (eds Pigman, W. & Hortin, D.)<br />
Vol. 1B, 881–927 (Academic, New York, 1980).<br />
7. Von Euler, H. & Brunius, E. Chem. Ber. 59, 1581–1585 (1926).<br />
8. Chen, J., Pill, T. & Beck, W. Z. Naturforsch. B 44,<br />
459–464 (1989).<br />
Competing financial interests: declared none.<br />
Quantum cryptography<br />
A step towards global<br />
key distribution<br />
Large random bit-strings known as<br />
‘keys’ are used to encode and decode<br />
sensitive data, and the secure distribution<br />
of these keys is essential to secure communications<br />
across the globe 1 . Absolutely<br />
secure key exchange 2 between two sites has<br />
now been demonstrated over fibre 3 and<br />
free-space 4–6 optical links. Here we describe<br />
the secure exchange of keys over a freespace<br />
path of 23.4 kilometres between two<br />
mountains. This marks a step towards<br />
accomplishing key exchange with a near-<br />
Earth orbiting satellite and hence a global<br />
key-distribution system.<br />
The security of our key-exchange system<br />
is guaranteed by encoding single photons<br />
using two sets of orthogonal polarizations.<br />
Our transmitter module (Alice; Fig. 1)<br />
incorporates a miniature source of polarization-coded<br />
faint pulses (approximating<br />
single photons; C.K., P.Z., M.H. and H.W.,<br />
unpublished results), where 0° or 45° polarization<br />
encode binary zero, and 90° or 135°<br />
code binary one. These light pulses are<br />
expanded and collimated in a simple<br />
telescope to a beam of about 50 mm and<br />
then accurately aligned on the receiver<br />
(Bob; Fig. 1), a 25-cm-diameter commercial<br />
telescope. Light is collected and focused<br />
onto a compact four-detector photoncounting<br />
module (Fig. 1). A detection in<br />
any one detector then has an associated bit<br />
value, measurement basis (0° or 45°) and<br />
detection time. The bit values then form a<br />
raw key string. Valid bits are measured in<br />
the same basis as that in which they were<br />
encoded.<br />
Alice and Bob use a standard communications<br />
channel, such as a mobile telephone,<br />
to ascertain which bits arrived (many are<br />
lost) and which measurement basis was used,<br />
then they both discard the invalid bits —<br />
which leaves them with nearly identical<br />
random bit-strings, the sifted key. Eavesdropping<br />
measurements on the single<br />
photons disturb the encoding and introduce<br />
errors of up to 25%, so Alice and Bob test for<br />
errors in a short section of sifted key to<br />
Alice<br />
Zugspitze<br />
(2,950 m)<br />
Computer<br />
Fast pulse<br />
generator<br />
Alice<br />
Figure 1 Overview of the experiment against a relief map of the trial site. In the Alice module, four separate lasers (LDs) encode the four<br />
polarizations based on a random bit-string fed <strong>from</strong> the Alice computer. They are combined in a spatial filter (A,A) using a conical mirror<br />
(M) and a lens (L). The beam expands to 50 mm and is collimated in an output lens (L8). In the Bob module, a telescope (T) collects the<br />
light, which is filtered (F) and then spilt in a polarization-insensitive beam-splitter (BS), passing on to polarizing beam-splitters (PBS) and<br />
four photon-counting detectors (D). One polarizing beam-splitter is preceded by a 45° polarization rotator (R). A click in one of the photoncounting<br />
detectors D(u, B) sets the bit value B and the measurement basis u.<br />
verify the security of the channel. Low error<br />
rates due to background light detection and<br />
polarization settings are securely eliminated<br />
by using classical error-correcting codes sent<br />
over the mobile-telephone link.<br />
In the long-range experiment, Alice was<br />
located at a small experimental facility on<br />
the summit of Zugspitze in southern<br />
Germany, and Bob was on the neighbouring<br />
mountain of Karwendelspitze, 23.4 km<br />
away. At this distance, the transmitted<br />
beam was 1–2 m in diameter and was<br />
only weakly broadened by air-turbulence<br />
effects at this altitude. Lumped optical losses<br />
of about 18–20 decibels were measured<br />
and, using faint pulses containing 0.1<br />
photons per bit, the detected bit rate at<br />
Bob was 1.5–2 kilobits per second (receiver<br />
efficiency of 15%).<br />
Operating at night with filters of 10-nm<br />
bandwidth reduced the background<br />
counts, and errors appeared in less than<br />
5% of key bits. After sifting and error<br />
correction, net key exchange rates were<br />
hundreds of bits per second. In a series of<br />
experiments, several hundreds of kilobits<br />
of identical key string were generated at<br />
Alice and Bob.<br />
In associated experiments in poorer visibility,<br />
we showed that key exchange could<br />
be carried out when transmission losses<br />
were up to 27 decibels, but improvements<br />
in receiver efficiency and background<br />
counts should take us beyond 33 decibels.<br />
With this performance, key exchange to<br />
near-Earth orbit (500–1,000 km range)<br />
should become possible.<br />
Until now, the principal method of<br />
high-security key exchange has been the<br />
M<br />
4 LDs<br />
S<br />
A<br />
A<br />
L<br />
L'<br />
Bob<br />
Westliche<br />
karwendespitze<br />
(2,244 m)<br />
23.4 km<br />
Mobile phone<br />
link<br />
T F BS R<br />
Bob<br />
PBS<br />
D(0°,1)<br />
D(0°,0)<br />
PBS<br />
D(45°,0)<br />
D(45°,1)<br />
Computer<br />
‘trusted courier’ carrying a long random<br />
bit-string, the key, <strong>from</strong> one location to the<br />
other. Our experiment paves the way for<br />
the development of a secure global keydistribution<br />
network based on optical links<br />
to low-Earth-orbit satellites. We note that a<br />
10-kilometre key-exchange experiment has<br />
recently been announced 7 .<br />
C. Kurtsiefer*, P. Zarda*, M. Halder*,<br />
H. Weinfurter*, P. M. Gorman†,<br />
P. R. Tapster †, J. G. Rarity†<br />
*Ludwig-Maximilian University, 80799 Munich,<br />
Germany<br />
†Photonics Department, QinetiQ, Malvern ,<br />
Worcestershire WR14 3PS, UK<br />
e-mail: jgrarity@qinetiq.com<br />
1. Singh, S. The Code Book (Anchor, New York, 1999).<br />
2. Bennett, C. H. et al. J. Cryptol. 5, 3–28 (1992).<br />
3. Gisin, N., Ribordy, G., Tittel, W. & Zbinden, H. Rev. Mod. Phys.<br />
74, 145–196 (2002).<br />
4. Buttler, W. T. et al. Phys. Rev. Lett. 84, 5652–5655 (2000).<br />
5. Rarity, J. G., Gorman, P. M. & Tapster, P. R. Electron. Lett.<br />
37, 512–514 (2001).<br />
6. Rarity, J. G., Gorman, P. M. & Tapster, P. R. J. Mod. Opt.<br />
48, 1887–1901 (2001).<br />
7. Hughes, R. J., Nordholt, J. E., Derkacs, D. & Peterson, C. G.<br />
New J. Phys. 4, 43.1–43.14 (2002).<br />
Competing financial interests: declared none.<br />
erratum<br />
Cognitive change and the APOE ;4 allele<br />
I. J. Deary, M. C. Whiteman, A. Pattie, J. M. Starr,<br />
C. Hayward, A. F. Wright, A. Carothers, L. J. Whalley<br />
Nature 418, 932 (2002)<br />
In the second sentence of the seventh paragraph of this<br />
communication, the MMSE scores are incorrectly specified<br />
as less than or equal to 28; these should read as<br />
greater than or equal to 28.<br />
450 NATURE | VOL 419 | 3 OCTOBER 2002 | www.nature.com/nature<br />
© 2002 Nature Publishing Group