7. Superconductivity - University of Liverpool
7. Superconductivity - University of Liverpool
7. Superconductivity - University of Liverpool
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
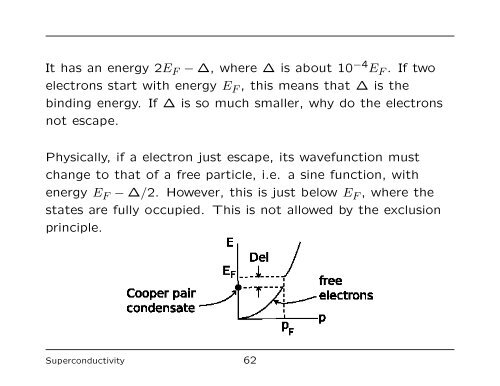
It has an energy 2E F − ∆, where ∆ is about 10 −4 E F . If two<br />
electrons start with energy E F , this means that ∆ is the<br />
binding energy. If ∆ is so much smaller, why do the electrons<br />
not escape.<br />
Physically, if a electron just escape, its wavefunction must<br />
change to that <strong>of</strong> a free particle, i.e. a sine function, with<br />
energy E F − ∆/2. However, this is just below E F , where the<br />
states are fully occupied. This is not allowed by the exclusion<br />
principle.<br />
<strong>Superconductivity</strong> 62