As a PDF file - E-thesis - Helsinki.fi
As a PDF file - E-thesis - Helsinki.fi
As a PDF file - E-thesis - Helsinki.fi
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
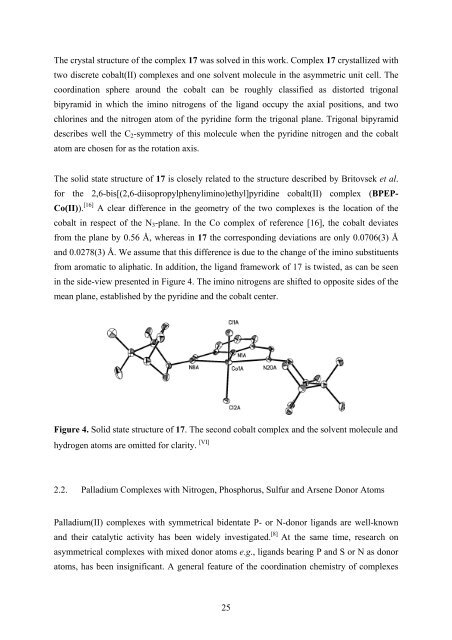
The crystal structure of the complex 17 was solved in this work. Complex 17 crystallized with<br />
two discrete cobalt(II) complexes and one solvent molecule in the asymmetric unit cell. The<br />
coordination sphere around the cobalt can be roughly classi<strong>fi</strong>ed as distorted trigonal<br />
bipyramid in which the imino nitrogens of the ligand occupy the axial positions, and two<br />
chlorines and the nitrogen atom of the pyridine form the trigonal plane. Trigonal bipyramid<br />
describes well the C 2 -symmetry of this molecule when the pyridine nitrogen and the cobalt<br />
atom are chosen for as the rotation axis.<br />
The solid state structure of 17 is closely related to the structure described by Britovsek et al.<br />
for the 2,6-bis[(2,6-diisopropylphenylimino)ethyl]pyridine cobalt(II) complex (BPEP-<br />
Co(II)). [ 16]<br />
A clear difference in the geometry of the two complexes is the location of the<br />
cobalt in respect of the N 3 -plane. In the Co complex of reference [16], the cobalt deviates<br />
from the plane by 0.56 Å, whereas in 17 the corresponding deviations are only 0.0706(3) Å<br />
and 0.0278(3) Å. We assume that this difference is due to the change of the imino substituents<br />
from aromatic to aliphatic. In addition, the ligand framework of 17 is twisted, as can be seen<br />
in the side-view presented in Figure 4. The imino nitrogens are shifted to opposite sides of the<br />
mean plane, established by the pyridine and the cobalt center.<br />
Figure 4. Solid state structure of 17. The second cobalt complex and the solvent molecule and<br />
hydrogen atoms are omitted for clarity. [VI]<br />
2.2. Palladium Complexes with Nitrogen, Phosphorus, Sulfur and Arsene Donor Atoms<br />
Palladium(II) complexes with symmetrical bidentate P- or N-donor ligands are well-known<br />
and their catalytic activity has been widely investigated. [ 8]<br />
At the same time, research on<br />
asymmetrical complexes with mixed donor atoms e.g., ligands bearing P and S or N as donor<br />
atoms, has been insigni<strong>fi</strong>cant. A general feature of the coordination chemistry of complexes<br />
25