As a PDF file - E-thesis - Helsinki.fi
As a PDF file - E-thesis - Helsinki.fi
As a PDF file - E-thesis - Helsinki.fi
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
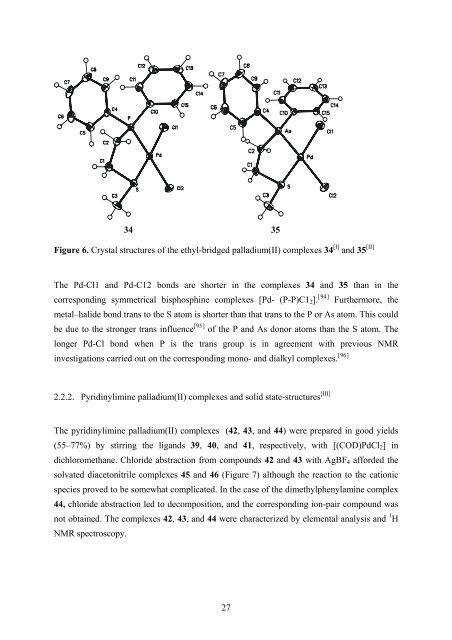
34 35<br />
Figure 6. Crystal structures of the ethyl-bridged palladium(II) complexes 34 [I] and 35 [II]<br />
The Pd-Cl1 and Pd-C12 bonds are shorter in the complexes 34 and 35 than in the<br />
corresponding symmetrical bisphosphine complexes [Pd- (P-P)C1 2 ]. [ 94]<br />
Furthermore, the<br />
metal–halide bond trans to the S atom is shorter than that trans to the P or <strong>As</strong> atom. This could<br />
be due to the stronger trans influence [ 95]<br />
of the P and <strong>As</strong> donor atoms than the S atom. The<br />
longer Pd-Cl bond when P is the trans group is in agreement with previous NMR<br />
investigations carried out on the corresponding mono- and dialkyl complexes. [ 96]<br />
2.2.2. Pyridinylimine palladium(II) complexes and solid state-structures [III]<br />
The pyridinylimine palladium(II) complexes (42, 43, and 44) were prepared in good yields<br />
(55–77%) by stirring the ligands 39, 40, and 41, respectively, with [(COD)PdCl 2 ] in<br />
dichloromethane. Chloride abstraction from compounds 42 and 43 with AgBF 4 afforded the<br />
solvated diacetonitrile complexes 45 and 46 (Figure 7) although the reaction to the cationic<br />
species proved to be somewhat complicated. In the case of the dimethylphenylamine complex<br />
44, chloride abstraction led to decomposition, and the corresponding ion-pair compound was<br />
not obtained. The complexes 42, 43, and 44 were characterized by elemental analysis and 1 H<br />
NMR spectroscopy.<br />
27