Review of Classical Wave
Review of Classical Wave
Review of Classical Wave
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
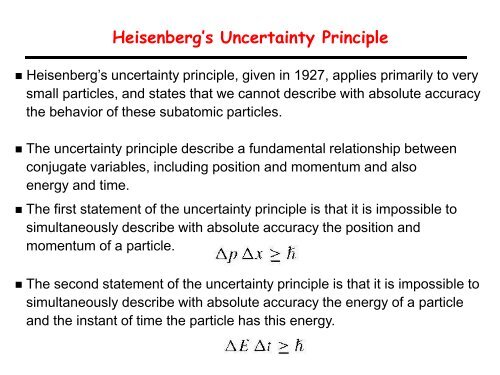
Heisenberg’s Uncertainty Principle<br />
• Heisenberg’s uncertainty principle, given in 1927, applies primarily to very<br />
small particles, and states that we cannot describe with absolute accuracy<br />
the behavior <strong>of</strong> these subatomic particles.<br />
• The uncertainty principle describe a fundamental relationship between<br />
conjugate variables, including position and momentum and also<br />
energy and time.<br />
• The first statement <strong>of</strong> the uncertainty principle is that it is impossible to<br />
simultaneously describe with absolute accuracy the position and<br />
momentum <strong>of</strong> a particle.<br />
• The second statement <strong>of</strong> the uncertainty principle is that it is impossible to<br />
simultaneously describe with absolute accuracy the energy <strong>of</strong> a particle<br />
and the instant <strong>of</strong> time the particle has this energy.