Racemization Studies - CEM Gmbh
Racemization Studies - CEM Gmbh
Racemization Studies - CEM Gmbh
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Page 1 of 2<br />
Application Note BIO-0003<br />
Microwave Synthesis: <strong>Racemization</strong> <strong>Studies</strong><br />
INTRODUCTION<br />
One potential issue in peptide synthesis is<br />
racemization of cysteine and histidine. This<br />
is a concern for microwave peptide syntheis<br />
because of the elevated temperatures<br />
during deprotection and coupling. <strong>Studies</strong><br />
have shown that lowering the temperature<br />
to 50 °C during the coupling of histidine and<br />
cysteine will decrease racemization.<br />
MATERIALS AND METHODS<br />
Reagents<br />
All Fmoc amino acids were obtained from<br />
Novabiochem and contained the following<br />
side chain protecting groups: Asn(Trt),<br />
Asp(OtBu), Arg(Pbf), Cys(Trt), Gln(Trt),<br />
Glu(OtBu), His(Trt), Lys(Boc), Ser(tBu),<br />
Thr(tBu), Trp(Boc), and Tyr(tBu). O-<br />
(Benzotriazol-1-yl)-N,N,N’,N’tetramethyluronium<br />
hexafluorophosphate<br />
(HBTU), N-hydroxybenzotriazole (HOBt),<br />
benzotriazol-1-yl-N-oxytris(pyrrolidino)phosphonium<br />
hexaflourophosphate (PyBOP), and Rink<br />
amide MBHA resin were also obtained from<br />
Novabiochem. Diisopropylethyamine<br />
(DIEA), N-methylmorpholine (NMM),<br />
collidine (TMP), piperidine, piperazine,<br />
trifluoroacetic acid (TFA), thioanisole, 1,2ethanedithiol<br />
(EDT), and phenol were<br />
obtained from Sigma Aldrich.<br />
Dichloromethane (DCM), N,N-<br />
Dimethylformamide (DMF), Nmethylpyrrolidone<br />
(NMP), anhydrous ethyl<br />
ether, acetic acid, HPLC grade water and<br />
acetonitrile were obtained from VWR.<br />
Peptide Synthesis:<br />
VYWTSPFMKLIHEQCNRADG-NH2<br />
A peptide containing all 20 amino acids was<br />
synthesized under a variety of conditions<br />
using the <strong>CEM</strong> Liberty automated<br />
microwave peptide synthesizer on 0.152 g<br />
Rink amide MBHA resin (0.66 meq/g<br />
substitution). Deprotection was performed<br />
in two stages with an initial deprotection of<br />
30 s followed by 3 min at 50 W with a<br />
maximum temperature of 80 °C (5 min<br />
followed by 15 min at 0 W for conventional<br />
synthesis). Coupling reactions were<br />
performed in the presence of a 5-fold molar<br />
excess of 0.2 M Fmoc-protected amino<br />
acids dissolved in DMF with various types of<br />
activation. Coupling reactions were 5 min at<br />
40 W (30 min at 0 W for conventional<br />
synthesis) with a maximum temperature of<br />
80 °C. In latter experiments, coupling<br />
conditions of cysteine and histidine were<br />
altered to 2 min at 0 W followed by 4 min at<br />
40 W with a maximum temperature of 50<br />
°C. Cleavage was performed using 10 mL of<br />
Reagent K (TFA/phenol/water/thioanisole/<br />
EDT; 82.5:5:5:5:2.5) for 180 minutes.<br />
Following cleavage, peptides were<br />
precipitated out and washed using ice cold<br />
anhydrous ethyl ether.<br />
Peptide Analysis<br />
All peptides were dissolved in 10% acetic<br />
acid solution and lyophilized to dryness. The<br />
peptide was analyzed using a Waters<br />
Atlantis dC18 column (3µm, 2.1 × 100mm)<br />
at 214nm. Separation was achieved by<br />
gradient elution of 5-60% solvent B (solvent
A=0.05% TFA in water; solvent B=0.025%<br />
TFA in acetonitrile) over 60 min at a flow<br />
rate of 0.5 ml/min. <strong>Racemization</strong> analysis of<br />
amino acids was performed by C.A.T. GmbH<br />
& Co. using a published GC-MS method that<br />
involves hydrolysis of the peptide in 6 N<br />
DCl/D2O.<br />
RESULTS<br />
In order to optimize the peptide purity by<br />
lowering the racemization of histidine and<br />
cysteine during microwave enhanced<br />
synthesis, multiple deprotection and<br />
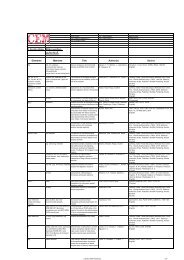
cleavage solutions were tested (Table 1).<br />
For experiments where the coupling<br />
temperature of Cys and His were changed,<br />
the remaining amino acids in the peptide<br />
were coupled at 80 °C.<br />
Conventional synthesis yields less than 1.5%<br />
d-enantiomer for all amino acids, yet crude<br />
purity is only 68.4% because the<br />
conventional synthesis results in numerous<br />
amino acid deletions. A microwave<br />
enhanced synthesis with piperidine<br />
deprotection and HBTU/DIEA activation<br />
indicates the sequence is susceptible to<br />
racemization at histidine (9.4% d-His) and<br />
Page 2 of 2<br />
Application Note BIO-0003<br />
cysteine (4.48% d-Cys) residues.<br />
<strong>Racemization</strong> levels of other amino acids<br />
during microwave synthesis were<br />
comparable to those of conventional<br />
synthesis (data not shown). The addition of<br />
HOBt to the activator or substituting PyBOP<br />
produced similar degrees of racemization as<br />
HBTU activation. Lowering the temperature<br />
from 80 °C to 50 °C during the coupling of<br />
cysteine and histidine reduces the<br />
racemization of His from >8.00% to 1.59%<br />
and Cys from >3.96% to 3.16%.<br />
CONCLUSION<br />
The increased racemization associated with<br />
microwave synthesis can be decreased by<br />
lowering the temperature to 50 °C during<br />
the coupling of histidine and cysteine. After<br />
the histidine or cysteine has been coupled<br />
there is no further increase in racemization<br />
throughout the peptide chain synthesis.<br />
Microwave synthesis, along with lower<br />
coupling temperatures for histidine and<br />
cysteine, leads to increased crude purity<br />
and reduced synthesis time.<br />
REFERENCES<br />
Palasek S, Cox Z, Collins J. J. Pept. Sci. 2007, 13, 143-148.<br />
Table 1. <strong>Racemization</strong> of amino acids measured by GC-MS after hydrolysis with 6 N DCl/D2O<br />
Conventional Microwave<br />
Deprotection A, 25 °C A, 80 °C B, 80 °C B, 80 °C C, 80 °C C, 80 °C C, 80 °C C, 80 °C<br />
Coupling D, 25 °C D, 80 °C E, 80 °C F, 80 °C D, 80 °C G, 80 °C H, 50 °C H, 25 °C (0 W)<br />
D-Cys 1.09