- Page 1 and 2:
Nuclear Science Pyrochemical Separa
- Page 3 and 4:
OECD PROCEEDINGS Proceedings of the
- Page 5:
FOREWORD In the future, nuclear fue
- Page 8 and 9:
J. Uhlir Pyrochemical Reprocessing
- Page 10 and 11:
Annex 1. LIST OF PARTICIPANTS......
- Page 12 and 13:
In the poster session, eight poster
- Page 14:
OPENING SESSION 11
- Page 18 and 19:
OPENING ADDRESS Philippe Savelli De
- Page 20:
SESSION I National and Internationa
- Page 23 and 24:
A sixth project might be funded by
- Page 25 and 26:
Selective extraction of minor actin
- Page 27 and 28:
Work programme The work programme i
- Page 29 and 30:
Introduction Commercial nuclear pow
- Page 31 and 32:
The ATW system is predicated on the
- Page 33 and 34:
In the electrorefiner, the transura
- Page 35 and 36:
these materials will be sent direct
- Page 37 and 38:
alternative were to be used instead
- Page 40 and 41:
OVERVIEW OF RIAR ACTIVITY ON PYROPR
- Page 42 and 43:
• Non-proliferation resistance (h
- Page 44 and 45:
nuclear fuel (burn-up of 7.7% h.a.,
- Page 46 and 47:
In the framework of the programme f
- Page 48 and 49:
Figure 1. Closed fuel cycles based
- Page 50 and 51:
BNFL’S MOLTEN SALTS PROGRAMME: IN
- Page 52 and 53:
technologies, demonstrated closure
- Page 54 and 55:
In summary, fast flux systems with
- Page 56 and 57:
ANL has utilised a design value of
- Page 58 and 59:
processes used for aqueous systems
- Page 60 and 61:
Investment in BNFL’s molten salts
- Page 62 and 63:
ASSESSMENT OF PYROCHEMICAL PROCESSE
- Page 64:
SESSION II Role and Requirements of
- Page 67 and 68:
Scope The present low price of uran
- Page 69 and 70:
At present, the transport of spent
- Page 71 and 72:
[11] V.K. Afonichkin, V.E. Komarov
- Page 73 and 74:
Figure 3. Electropotential relative
- Page 76 and 77:
PYROCHEMICAL REPROCESSING TECHNOLOG
- Page 78 and 79:
ability of the pyrochemical and pyr
- Page 80 and 81:
Figure 1. View of the experimental
- Page 82 and 83:
ACTINIDE RECYCLING BY PYROPROCESS F
- Page 84 and 85:
Characteristics of technology The p
- Page 86 and 87:
Experiments for separation of TRUs
- Page 88:
[12] M. Kurata, Y. Sakamura and T.
- Page 91 and 92:
Introduction Due to its simplicity,
- Page 93 and 94:
Atmosphere control For metal fuel e
- Page 95 and 96:
Recycling technology As for oxide f
- Page 97 and 98:
REFERENCES [1] “Metal Fuel Cycle
- Page 99 and 100:
Table 1. Oxide fuel electrowinning
- Page 102 and 103:
MOLTEN SALT DATA FOR PYROCHEMISTRY:
- Page 104 and 105:
Sample preparation Because of the h
- Page 106 and 107:
Computational procedure Two approac
- Page 108 and 109:
[10] M. Gaune-Escard, A. Bogacz, L.
- Page 110 and 111:
Figure 4. Molar heat capacity of Eu
- Page 112:
Figure 10. Experimental [32] and ca
- Page 115 and 116:
Introduction Pyrochemical technique
- Page 117 and 118:
Activity coefficient determination
- Page 119 and 120:
The free oxide concentration depend
- Page 121 and 122:
REFERENCES [1] CEA, “Assessment o
- Page 123 and 124:
Figure 2. Cyclic voltammograms of P
- Page 125 and 126:
Figure 6. Influence of the concentr
- Page 127 and 128:
Introduction The design of a metal
- Page 129 and 130:
i(t) is the experimental current at
- Page 131 and 132:
sweep rate. At high sweep rates the
- Page 133 and 134:
Conclusion The success of a pyropar
- Page 135 and 136:
Figure 2. Diagram of the gas circui
- Page 137 and 138:
Figure 8. Current reversal chronopo
- Page 139 and 140:
Introduction The reductive liquid-l
- Page 141 and 142:
0 [ MCl ex 3 in S] = f [ MCl 3, ] +
- Page 143 and 144:
There is no concrete theoretical ba
- Page 145 and 146:
Table 1. ∆G°f[M in Bi] summarise
- Page 147 and 148:
Figure 4. Estimated ∆G f0 [M in B
- Page 150 and 151:
RESULTS OF DEMONSTRATION PROGRAMME
- Page 152 and 153:
inventory are the SNF from commerci
- Page 154 and 155:
During the repeatability phase of t
- Page 156 and 157:
[4] “A Roadmap for Developing Acc
- Page 158:
Table 1. Free energies of formation
- Page 161 and 162:
Introduction The study of partition
- Page 163 and 164:
emained dissolved in Cd. Particular
- Page 165 and 166:
Figure 1. Double-strata fuel cycle
- Page 167 and 168:
Figure 5. Predicted E-p(O 2- ) diag
- Page 169 and 170:
Introduction Lithium has several ad
- Page 171 and 172:
time to allow them to equilibrate t
- Page 173 and 174:
The gas burette was calibrated with
- Page 175 and 176:
Table 1. Approximate Gibbs free ene
- Page 177 and 178:
Figure 2. Gas burette apparatus for
- Page 180 and 181:
SMALL-SCALE DEMONSTARATION OF PYROM
- Page 182 and 183:
Test plan The first phase of the jo
- Page 184 and 185:
Plutonium recovery into a liquid Cd
- Page 186 and 187:
Figure 1. Test schedule of joint st
- Page 188 and 189:
Photo 1. Stainless steel box with A
- Page 190 and 191:
STUDIES ON THE HEAD-END STEPS FOR P
- Page 192 and 193:
the cathode deposit to 1 300°C to
- Page 194 and 195:
REFERENCES [1] L. Burris, R.K. Steu
- Page 196:
Figure 3. Schematic diagram of salt
- Page 200 and 201:
DEVELOPMENT OF STRUCTURAL MATERIAL
- Page 202 and 203:
Due to the used contrast it was pos
- Page 204 and 205:
Molten salt storage tanks Molten sa
- Page 206:
REFERENCES [1] M. Hron, P. Hosnedl,
- Page 209 and 210:
Introduction Pyrochemical processin
- Page 211 and 212:
( µ ) K m s = 5× 10− 8 12 T ( )
- Page 213 and 214:
Conclusions For the development of
- Page 215 and 216:
Figure 1. Fuel salt flow rate requi
- Page 217 and 218:
Introduction The feasibility of a n
- Page 219 and 220:
conditional acidity scale, that is
- Page 221 and 222:
Cerium and plutonium chemical prope
- Page 223 and 224:
REFERENCES [1] L. Mouron, S. Grandj
- Page 225 and 226:
[27] M. Garcia, Y. Castrillejo, P.
- Page 227 and 228:
Figure 5. Possibilities for a selec
- Page 230:
SESSION IV Open Discussion and Reco
- Page 233 and 234:
Professor Madic concluded from the
- Page 236 and 237: TECHNETIUM METAL AND PYROMETALLURGI
- Page 238 and 239: Chemical preparations All reagents
- Page 240 and 241: REFERENCES [1] K. Ben Said, M. Fatt
- Page 242 and 243: Figure 1. Radiochemical hutch of RO
- Page 244: Figure 6. Tc K-edge k 3 -weighted E
- Page 247 and 248: Introduction The use of the molten
- Page 249 and 250: designs [1,2], in which a single mo
- Page 251 and 252: eactor inlet temperature. A few exp
- Page 253 and 254: In experiments [15] a successful at
- Page 255 and 256: REFERENCES [1] H.J. MacPherson, Rea
- Page 257 and 258: Figure 2. Separation factors betwee
- Page 259 and 260: Introduction The first axis of the
- Page 261 and 262: The metal was either cast into the
- Page 263 and 264: REFERENCES [1] J.A. Jensen, A.M. Pl
- Page 265 and 266: Figure 3. Lost of substances expres
- Page 268 and 269: STUDY OF OXIDISING URANIUM FOR PYRO
- Page 270 and 271: Thermodynamics simulation To examin
- Page 272 and 273: 400°C. It is thought that the rate
- Page 274 and 275: Figure 4. Outline of roasting oxida
- Page 276: Table 1. Roasting oxidation examina
- Page 279 and 280: Scope There is presently a surplus
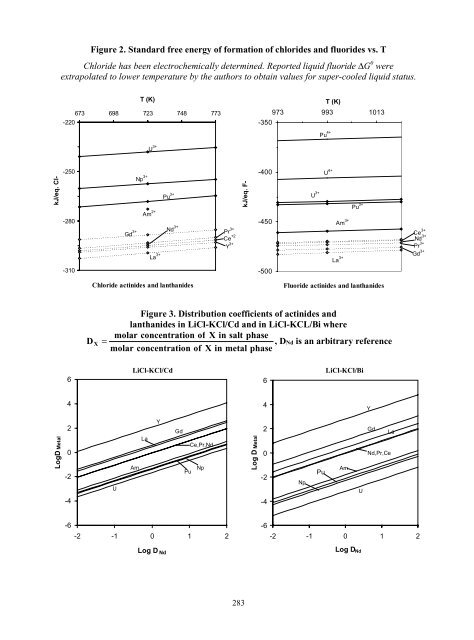
- Page 281 and 282: activity coefficient in liquid Bi t
- Page 283 and 284: [19] L. Martinot, J.C. Spirlet, G.
- Page 285: Element Table 2. Activity coefficie
- Page 290 and 291: PYRO-OXIDATION OF PLUTONIUM SPENT S
- Page 292 and 293: Input and output products are weigh
- Page 294 and 295: REFERENCES [1] J.L. McNeese, “Pyr
- Page 296 and 297: Figure 1. Equipment for pyro-oxidat
- Page 298 and 299: ELECTROCHEMICAL STUDIES OF EUCL3 AN
- Page 300 and 301: Results and discussion Cyclic volta
- Page 302 and 303: The values of the ψT function, obt
- Page 304 and 305: The formal standard potential E* Eu
- Page 306 and 307: Figure 1. Cyclic voltammetric curve
- Page 308: Figure 4. Cyclic volammetric curves
- Page 311 and 312: Introduction There is a remarkable
- Page 313 and 314: We shall not go into the details (w
- Page 315 and 316: It is a special feature of the molt
- Page 317 and 318: Appendix B CORRECTIONS OF PARAMETER
- Page 320 and 321: Annex 1 LIST OF PARTICIPANTS BELGIU
- Page 322 and 323: Mr. Olivier CONOCAR CEA VALRHO SPHA
- Page 324 and 325: Mme Sylvie PILLON CEA-DRN/DER/SIS C
- Page 326 and 327: Dr. Toru OGAWA Group Leader Researc
- Page 328 and 329: Dr. James J. LAIDLER Argonne Nation
- Page 330: Annex 2 WORKSHOP ORGANISATION Chair
- Page 333 and 334: The study would cover: • Review o
- Page 335 and 336: ORDER FORM OECD Nuclear Energy Agen