Novel Organic Synthesis in Supercritical Carbon Dioxide - ISASF
Novel Organic Synthesis in Supercritical Carbon Dioxide - ISASF
Novel Organic Synthesis in Supercritical Carbon Dioxide - ISASF
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Novel</strong> <strong>Organic</strong> <strong>Synthesis</strong> <strong>in</strong> <strong>Supercritical</strong> <strong>Carbon</strong> <strong>Dioxide</strong><br />
Sarah A. Brough, Christopher M. Rayner* and Anthony A. Clifford, Department of Chemistry, University of<br />
Leeds, Leeds, LS2 9JT, UK, C.M.Rayner@leeds.ac.uk, Fax +44 113 343 6565<br />
INTRODUCTION<br />
We have pursued a programme of <strong>in</strong>vestigat<strong>in</strong>g the substitution of environmentally benign<br />
supercritical carbon dioxide (scCO 2 ) for organic solvents <strong>in</strong> synthetic chemistry [1]. The<br />
focus of the present study is to synthesise novel bis-oxazol<strong>in</strong>e (BOX) ligands for use as<br />
catalysts <strong>in</strong> asymmetric cyclopropanation reactions utilis<strong>in</strong>g scCO 2 as a solvent [2-3]. BOX<br />
ligands have become one of the most successful, versatile and commonly used class of<br />
ligands for asymmetric catalysis [4], and can be applied <strong>in</strong> a wide range of metal catalysed<br />
organic transformations [5].<br />
FORMATION OF BOX LIGANDS<br />
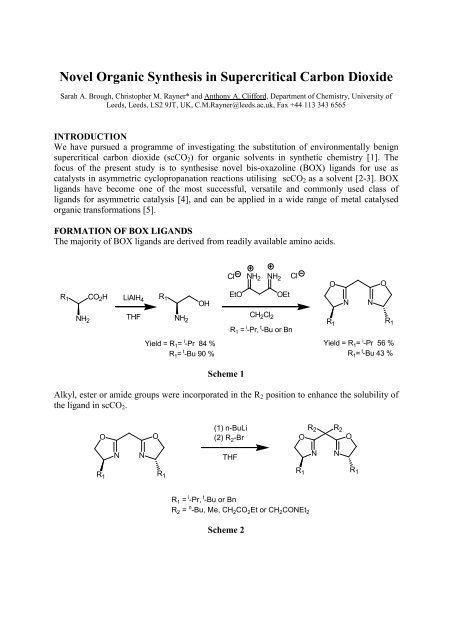
The majority of BOX ligands are derived from readily available am<strong>in</strong>o acids.<br />
R 1 CO 2 H LiAlH 4<br />
R 1<br />
OH<br />
NH 2<br />
THF NH 2<br />
Yield = R 1 = i -Pr 84 %<br />
R 1 = t -Bu 90 %<br />
Cl NH 2 NH 2 Cl<br />
EtO OEt<br />
CH 2 Cl 2<br />
R 1 = i -Pr, t -Bu or Bn<br />
O<br />
R 1<br />
N<br />
N<br />
O<br />
R 1<br />
Yield = R 1 = i -Pr 56 %<br />
R 1 = t -Bu 43 %<br />
Scheme 1<br />
Alkyl, ester or amide groups were <strong>in</strong>corporated <strong>in</strong> the R 2 position to enhance the solubility of<br />
the ligand <strong>in</strong> scCO 2 .<br />
O<br />
R 1<br />
N<br />
N<br />
O<br />
R 1<br />
(1) n-BuLi<br />
(2) R 2 -Br<br />
THF<br />
R 2 R 2<br />
O<br />
N N<br />
R 1<br />
O<br />
R 1<br />
R 1 = i -Pr, t -Bu or Bn<br />
R 2 = n -Bu, Me, CH 2 CO 2 Et or CH 2 CONEt 2<br />
Scheme 2
BOX CATALYSED ASYMMETRIC CYCLOPROPANATION REACTIONS<br />
The cyclopropyl group is found as a basic structural element <strong>in</strong> a wide range of naturally<br />
occurr<strong>in</strong>g plants and micro-organisms [2,6]. Asymmetric cyclopropanation reactions have<br />
been extensively studied, and are usually carried out <strong>in</strong> conventional solvents such as CHCl 3<br />
and CH 2 Cl 2 . Studies have also been carried out <strong>in</strong> scCHF 3 demonstrat<strong>in</strong>g <strong>in</strong>terest<strong>in</strong>g solvent<br />
effects [7]. In conventional solvents, the gem-dimethyl derivative typically gave the best<br />
enantioselectivity, hence is the most widely used chiral BOX ligand [8].<br />
O O<br />
N N<br />
Cu<br />
t-Bu t-Bu<br />
R 2 TfO OTf<br />
+<br />
R R R 1 R 2<br />
1 1 R<br />
+<br />
2<br />
N2 CHCl 3 or CH 2 Cl 2<br />
+<br />
R 2<br />
R 2<br />
R 1 or R 2 = aliphatic or aromatic<br />
Scheme 3<br />
BOX CATALYSED CYCLOPROPANATION REACTIONS IN scCO 2 COMPARED<br />
WITH CONVENTIONAL SOLVENTS<br />
Cyclopropanation reactions of styrene and ethyl diazoacetate were performed <strong>in</strong> scCO 2 us<strong>in</strong>g<br />
1 mol% BOX catalyst at different pressures to identify conditions for optimum selectivity.<br />
The same reactions were also carried out <strong>in</strong> chloroform and toluene as conventional solvents<br />
comparison. Results are presented for the four ligands shown <strong>in</strong> Scheme 4.<br />
Ph<br />
+<br />
N 2<br />
CO 2 Et<br />
R 2 R 2<br />
O O<br />
N N<br />
Cu<br />
R 1 R 1<br />
TfO OTf<br />
scCO 2<br />
Ph<br />
CO 2 Et<br />
+<br />
Ph<br />
CO 2 Et<br />
+<br />
EtO 2 C<br />
CO 2 Et<br />
R 1 = i Pr, t Bu or Bn<br />
n-Bu<br />
O<br />
n-Bu<br />
O<br />
EtO 2 CH 2 C<br />
O<br />
CH 2 CO 2 Et<br />
O<br />
N<br />
N<br />
N<br />
N<br />
R 2 = n Bu, CH 2 CO 2 Et, CH 2 CONEt 2<br />
N N<br />
1 2<br />
Et 2 NOCH 2 C<br />
O<br />
CH 2 CONEt 2<br />
O<br />
O<br />
O<br />
N<br />
N<br />
3 4<br />
Scheme 4
Table 1: Enantiometric excess (%) for the<br />
reaction <strong>in</strong> conventional solvents for the four<br />
ligands<br />
Enantiomeric exess (%)<br />
100<br />
90<br />
75<br />
50<br />
Ligand Chloroform Toluene<br />
1 >95 80<br />
2 45 23<br />
3 59 58<br />
4 87 88<br />
80<br />
100<br />
Pressure (bar)<br />
Ligand 1: Ligand 2: Ligand 3: Ligand 4:<br />
120<br />
Figure 1: ee values for the cyclopropanation<br />
reaction studies <strong>in</strong> scCO 2 .<br />
Enantiomeric excess values were<br />
determ<strong>in</strong>ed us<strong>in</strong>g HPLC (chiracel OJ<br />
column and elution with hexane/2-<br />
propanol (98:2) and a flow rate of 0.5<br />
ml/ m<strong>in</strong>ute, after reduction to the<br />
primary alcohol). Table 1 shows<br />
results for the enantiomeric excess<br />
(ee) obta<strong>in</strong>ed <strong>in</strong> conventional<br />
solvents. Ligands 1 and 4 perform<br />
relatively well, with >95% ee<br />
obta<strong>in</strong>ed for Ligand 1 <strong>in</strong> chloroform,<br />
but the others perform badly.<br />
Results for experiments carried<br />
out <strong>in</strong> scCO 2 are summarised <strong>in</strong><br />
Figure 1. As can be seen, the pressure<br />
can be altered to optimise<br />
enantioselectivity, with the optimum<br />
pressure around 115 bar. Ligand 2<br />
gave most <strong>in</strong>terest<strong>in</strong>g results as low ee<br />
was observed <strong>in</strong> chloroform (45%)<br />
and toluene (23%), but was much<br />
higher <strong>in</strong> scCO 2 at 115 bar (91%).<br />
Such high selectivities are rare with<br />
val<strong>in</strong>e-derived BOX ligands. Thus,<br />
cheaper L-val<strong>in</strong>e (€4.00 per g) derived<br />
BOX can be used <strong>in</strong> place of L-tertleuc<strong>in</strong>e<br />
(€33.50 per g) derived BOX<br />
ligands for cyclopropanation reactions<br />
<strong>in</strong> scCO 2 at 115 bar.<br />
YIELD OPTIMISATION<br />
Literature procedures for the reaction of styrene and ethyl diazoacetate <strong>in</strong> conventional<br />
solvents gave optimum yields (~50-95 %) if the diazoacetate was added over 24 hours via a<br />
syr<strong>in</strong>ge pump [9]. This reduced unwanted dimerisation of ethyl diazoacetate.<br />
EtO 2 C<br />
+<br />
CuBOX<br />
N 2<br />
CO 2 Et<br />
BOXCu<br />
EtO 2 C<br />
CO 2 Et<br />
N 2<br />
EtO 2 C<br />
CO 2 Et<br />
Scheme 5<br />
As this cannot be achieved us<strong>in</strong>g scCO 2 on current small scales, we have constructed a<br />
system, shown <strong>in</strong> Figure 2, which allows the ethyl diazoacetate to be added slowly to a 250<br />
ml reactor over a fixed period of time. Initial results show that slow addition of diazoacetate<br />
can decrease the extent of dimerisation, and this is currently be<strong>in</strong>g optimised.
enantiomeric excess of 91%.<br />
Figure 2: 250 mL reactor<br />
CONCLUSIONS<br />
1. It is possible to optimise the<br />
enantioselectivity of the<br />
asymmetric cyclopropanation<br />
reaction us<strong>in</strong>g BOX ligands <strong>in</strong><br />
scCO 2 us<strong>in</strong>g pressure.<br />
2. In some cases higher<br />
selectivities are obta<strong>in</strong>ed, than<br />
those available <strong>in</strong> conventional<br />
solvents with comparable<br />
ligands.<br />
3. In particular, cheaper L-val<strong>in</strong>e<br />
derived BOX ligands can be<br />
used <strong>in</strong> place of L-tert-leuc<strong>in</strong>e<br />
derived BOX ligands <strong>in</strong><br />
asymmetric cyclopropanation<br />
reactions, with an optimum<br />
REFERENCES:<br />
[1] OAKES, R.S.; CLIFFORD, A.A.; RAYNER, C.M. J. Chem. Soc., Perk<strong>in</strong> Trans. 1,<br />
2001, p. 918<br />
[2] GHOSH, A.K.; MATHIVANAN, P.; CAPPIELLO, J. Tetrahedron asymmetry, Vol. 9,<br />
1998, p. 1<br />
[3] DOYLE, M.P.; PROTOPOPOVA, M.N. Tetrahedron, 1998, p. 7919<br />
[4] MCMANUS, H.A.; GUIRY, P.T. Chem. Rev., 2004, p. 4151<br />
[5] CHRISTENSEN, C.; JULH, K.; JORGENSEN, K.A. Chem. Comm., 2001, p. 2222<br />
[6] LEBEL, H.; MARCOUX, J-F.; MOLINARO, C.; CHARETTE, A.B. Chem. Rev.,<br />
2003, p. 997<br />
[7] WYNNE, D.C., JESSOP, P.G. Angew. Chem. Int. Ed., Vol. 38, 1999, p. 1143<br />
[8] SALAUN, J. Chem. Rev., 1989, p. 147<br />
[9] LOWENTHAL, R.E.; ABIKO, A.; MASUME, S. Tetrahedron Lett., Vol. 31, 1990, p.<br />
6005