THE ATOMIC SPECTRUM OF HYDROGEN
THE ATOMIC SPECTRUM OF HYDROGEN
THE ATOMIC SPECTRUM OF HYDROGEN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Name:_______________________________ Date:_________________ Hr:____<br />
AP CHEMISTRY<br />
Ch 7 – Atomic Structure and Periodicity LAB 5<br />
<strong>THE</strong> <strong>ATOMIC</strong> <strong>SPECTRUM</strong> <strong>OF</strong> <strong>HYDROGEN</strong><br />
Introduction:<br />
In your chemical studies, you learned that after electrons are excited from the ground<br />
state (by an external source of energy), they naturally fall back to lower energy levels emitting<br />
energy in the process. It has been determined that electrons that fall to the first energy level<br />
emit energy in the range of ultraviolet light [Lyman Series], visible light is emitted if they fall to<br />
the second energy level [Balmer Series], and infrared light emitted if they fall to the third<br />
energy level [Paschen Series]. If one uses appropriate equipment, it is possible to view the<br />
visible emission lines of substances and make appropriate measurements. Using these<br />
measurements, one can determine the principal quantum number of the orbital from which the<br />
electron fell.<br />
A diffraction grating acts similar to a prism. It spreads a light into its component parts.<br />
When one looks through a diffraction grating off to the side, visible emission lines from the light<br />
source can be seen. Multiple sets of lines, like double rainbows, are visible as you look further<br />
out.<br />
Safety:<br />
Use caution when operating high voltage electrical equipment. DO NOT TOUCH the gas<br />
spectrum tube if it is plugged in and/or turned on.<br />
Equipment and Supplies:<br />
meter sticks, optical bench, hydrogen spectrum tube, diffraction grating, power supply<br />
Procedure:<br />
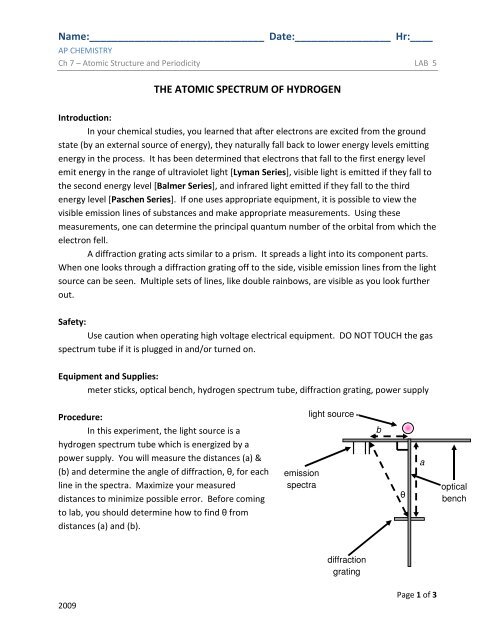
In this experiment, the light source is a<br />
hydrogen spectrum tube which is energized by a<br />
power supply. You will measure the distances (a) &<br />
(b) and determine the angle of diffraction, θ, for each<br />
line in the spectra. Maximize your measured<br />
distances to minimize possible error. Before coming<br />
to lab, you should determine how to find θ from<br />
distances (a) and (b).<br />
emission<br />
spectra<br />
light source<br />
| ||<br />
b<br />
θ<br />
a<br />
optical<br />
bench<br />
diffraction<br />
grating<br />
2009<br />
Page 1 of 3
Name:_______________________________ Date:_________________ Hr:____<br />
AP CHEMISTRY<br />
Ch 7 – Atomic Structure and Periodicity LAB 5<br />
Calculations:<br />
Calculate the wavelength of the bright first order violet, blue and red lines in the<br />
hydrogen spectrum, and one of the 2 nd order lines, using the formula: nλ = A sin θ, where λ is<br />
the wavelength in meters; A = 1/N with N being the number of lines/unit length of the<br />
diffraction grating; and n is the order of the spectrum. From this, one can proceed to calculate<br />
the number of the principal energy level from which the electrons fell to produce each line<br />
from the equations: c = λν, E = hν, ∆ . <br />
<br />
, where n 2 = 2 for visible<br />
light (see introduction). When considering the sign for ∆E, remember that the photons you<br />
observe have the energy that is released electrons.<br />
In the calculations section of your write-up, a sample calculation should be shown for<br />
the determination of the wavelength of one emitted line, the energy emitted, and the energy<br />
level from which the electrons fell. Present the results of calculations for all emitted lines<br />
(including the decimal equivalent before rounding to the integer energy level) in tabular form.<br />
Include the theoretical values for each of the calculations in your table as well (see your<br />
textbook or notes for the expected visible emission spectrum of hydrogen).<br />
Questions:<br />
1. Discuss the relationships among the wavelength of light emitted, the energy emitted,<br />
and the energy level from which the electrons fell.<br />
2. What limitations of this experimental procedure prevented you from calculating the<br />
energies emitted for electrons that fell to the 1 st principal energy level? the 3 rd principal<br />
energy level?<br />
3. Explain your experimental results as they pertain to Bohr’s model of the atom?<br />
Error Analysis:<br />
Be sure to evaluate the error for the decimal equivalent before rounding to the integer<br />
energy level compared to the theoretical values. Discuss possible sources for that error AND<br />
explain how those sources of error affected the calculations. Please do not discuss “calculation<br />
errors,” “human errors,” or “measurement errors” because these are not specific enough for<br />
error analysis.<br />
2009<br />
Page 2 of 3
Name:_______________________________ Date:_________________ Hr:____<br />
AP CHEMISTRY<br />
Ch 7 – Atomic Structure and Periodicity LAB 5<br />
<strong>THE</strong> <strong>ATOMIC</strong> <strong>SPECTRUM</strong> <strong>OF</strong> <strong>HYDROGEN</strong><br />
Grading Rubric<br />
HEADINGS ON EACH PAGE<br />
Title of Experiment<br />
Date Experiment Started<br />
Successive Page Numbers<br />
Name & Partner<br />
Item<br />
Points<br />
DATA SHEET<br />
Appropriate Purpose /2<br />
Clear & Complete Procedure /1<br />
Procedure Initialed by Instructor /1<br />
Clear & Complete Data and Observations /3<br />
Data and Observations Initialed by Instructor /1<br />
WRITE UP<br />
Clear & Complete Pertinent Data /1<br />
Clear & Complete Sample Calculations Present /11<br />
Clear & Complete Answers to Questions /9<br />
Clear & Complete Error Analysis<br />
Sources of Error<br />
/4<br />
Effects of Errors<br />
Clear & Complete Conclusion /2<br />
TOTAL /35<br />
2009<br />
Page 3 of 3