ILIF⢠Fact Sheet
ILIF⢠Fact Sheet
ILIF⢠Fact Sheet
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ILIF <strong>Fact</strong> <strong>Sheet</strong><br />
Treating Stenosis with Decompression and<br />
Instrumented Fusion<br />
A laminectomy is a type of decompression procedure used to<br />
treat lumbar spinal stenosis. It involves removing the spinous<br />
processes and the lamina to create a “window” above the<br />
spinal canal. This process requires the removal and/ or<br />
disruption of the supporting ligaments, muscles, and bones<br />
that stabilize the spine.<br />
To mitigate the instability of the spine caused by this<br />
procedure, patients often require fusion. This involves inserting<br />
screws and rods into the spine for stabilization followed by<br />
placing bone graft along both sides of the spine.<br />
Some of the disadvantages associated with traditional surgical<br />
treatments for stenosis include: resulting instability which can<br />
create pain, scar tissue that causes pressure on the spinal cord<br />
and/or nerves, or the complexity of a fusion procedure with<br />
screws and rods.<br />
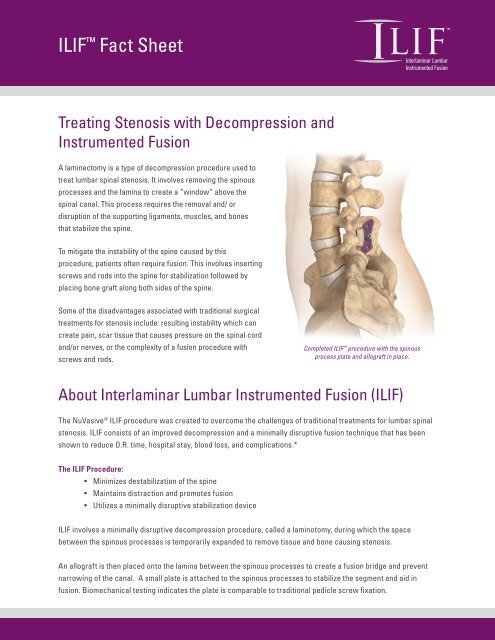
Completed ILIF procedure with the spinous<br />
process plate and allograft in place.<br />
About Interlaminar Lumbar Instrumented Fusion (ILIF)<br />
The NuVasive ® ILIF procedure was created to overcome the challenges of traditional treatments for lumbar spinal<br />
stenosis. ILIF consists of an improved decompression and a minimally disruptive fusion technique that has been<br />
shown to reduce O.R. time, hospital stay, blood loss, and complications.*<br />
The ILIF Procedure:<br />
• Minimizes destabilization of the spine<br />
• Maintains distraction and promotes fusion<br />
• Utilizes a minimally disruptive stabilization device<br />
ILIF involves a minimally disruptive decompression procedure, called a laminotomy, during which the space<br />
between the spinous processes is temporarily expanded to remove tissue and bone causing stenosis.<br />
An allograft is then placed onto the lamina between the spinous processes to create a fusion bridge and prevent<br />
narrowing of the canal. A small plate is attached to the spinous processes to stabilize the segment and aid in<br />
fusion. Biomechanical testing indicates the plate is comparable to traditional pedicle screw fixation.
ILIF <strong>Fact</strong> <strong>Sheet</strong><br />
ILIF Patient Benefits<br />
Reduced Blood Loss and Minimal Scarring<br />
The ILIF procedure does not significantly disrupt the<br />
supporting ligaments, muscles, and bones of the<br />
back, so patients are usually walking the same day.*<br />
Reduced Hospital Stay<br />
ILIF may require only an overnight stay in the<br />
hospital, compared to several days of immobility and<br />
hospitalization typical of traditional instrumented<br />
fusion approaches.*<br />
Reduced Operative Time<br />
Traditional procedures can take 1-2 hours to perform,<br />
the ILIF procedure can be successfully completed in<br />
as little as one hour or less, reducing the amount of<br />
anesthesia time.*<br />
Faster Return to Normal Activity<br />
Patients usually walk the same day after surgery<br />
and recovery is typically around 4 weeks,<br />
compared to 3 months or more.*<br />
About NuVasive ®<br />
NuVasive is an innovative medical device company focused on developing minimally disruptive surgical products<br />
and procedures for the spine. Our mission is to improve the lives of patients who suffer from debilitating back,<br />
neck, or leg pain by creating cutting-edge products and procedures that revolutionize spine surgery through<br />
focusing on Speed of Innovation ® , Absolute Responsiveness ® , and Superior Clinical Results.<br />
Only your physician can determine if ILIF is right for you. Individual results will vary. It is important to discuss the possible risks and potential benefits of ILIF with<br />
a physician prior to receiving treatment. The ILIF procedure may be an option if you require a posterior decompression and interspinous fusion between L2 and S1.<br />
As with any major surgical procedure, there are risks involved in spinal surgery. Operative and postoperative complications known to occur may include: early or<br />
late infection which may result in the need for additional surgeries; damage to blood vessels, spinal cord or peripheral nerves; pulmonary emboli; loss of sensory<br />
and/or motor function; impotence; permanent pain and/or deformity. Rarely, some complications may be fatal.<br />
The NuVasive ® ILIF System includes Affix ® which is a posterior, non-pedicle supplemental fixation device, intended for use in the non-cervical spine (T1 – S1). It is<br />
intended for plate fixation/attachment to spinous process for the purpose of achieving supplemental fusion in the following conditions: degenerative disc<br />
disease - defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies; spondylolisthesis; trauma (i.e.,<br />
fracture or dislocation); and/or tumor. It is not intended for stand-alone use.<br />
*Data on file<br />
NuVasive, Inc., 7475 Lusk Boulevard, San Diego, CA 92121 • Tel 800.475.9131 Fax 800.475.9134<br />
www.nuvasive.com<br />
© 2013. NuVasive, Inc. All rights reserved. , NuVasive, Speed of Innovation,<br />
Absolute Responsiveness, and Affix are registered trademarks of NuVasive, Inc.<br />
ILIF is a trademark of NuVasive, Inc.<br />
12-NUVA-1442