DOE-NE-0088
DOE-NE-0088
DOE-NE-0088
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The History<br />
Of Nuclear Energy<br />
Energy From The Atom<br />
Although they are tiny, atoms have a large<br />
amount of energy holding their nuclei together.<br />
Certain isotopes of some elements can be split<br />
and will release part of their energy as heat.<br />
This splitting is called fission. The heat released<br />
in fission can be used to help generate electricity<br />
in powerplants.<br />
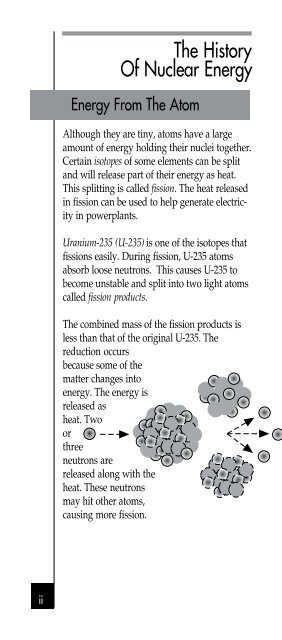
Uranium-235 (U-235) is one of the isotopes that<br />
fissions easily. During fission, U-235 atoms<br />
absorb loose neutrons. This causes U-235 to<br />
become unstable and split into two light atoms<br />
called fission products.<br />
The combined mass of the fission products is<br />
less than that of the original U-235. The<br />
reduction occurs<br />
because some of the<br />
matter changes into<br />
energy. The energy is<br />
released as<br />
heat. Two<br />
or<br />
three<br />
neutrons are<br />
released along with the<br />
heat. These neutrons<br />
may hit other atoms,<br />
causing more fission.<br />
ii