January/February 2004 - Ontario College of Pharmacists
January/February 2004 - Ontario College of Pharmacists
January/February 2004 - Ontario College of Pharmacists
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The Regulations are not intended to regulate the practice<br />
<strong>of</strong> complementary and alternative health care practitioners<br />
nor the practice <strong>of</strong> traditional Aboriginal medicine.<br />
The NHP Directorate intends to adopt a guidance document<br />
regarding the distinction between manufacture and<br />
sale <strong>of</strong> NHPs and compounding and distribution <strong>of</strong><br />
compounded products by both complementary and alternative<br />
health care practitioners and Aboriginal Healers.<br />
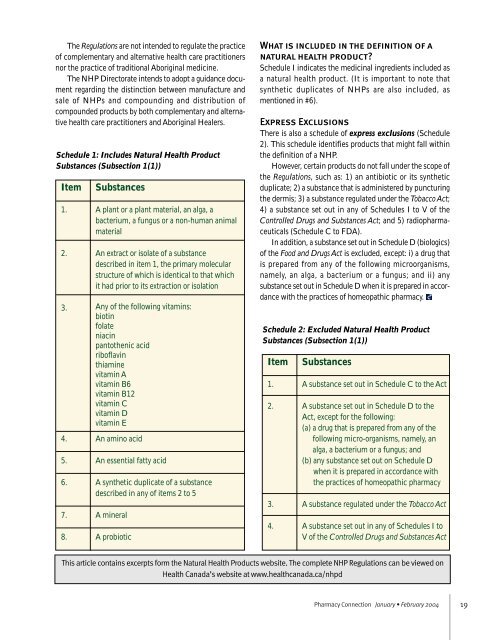
Schedule 1: Includes Natural Health Product<br />
Substances (Subsection 1(1))<br />
Item<br />
1.<br />
2.<br />
3.<br />
4.<br />
5.<br />
6.<br />
7.<br />
8.<br />
Substances<br />
A plant or a plant material, an alga, a<br />
bacterium, a fungus or a non-human animal<br />
material<br />
An extract or isolate <strong>of</strong> a substance<br />
described in item 1, the primary molecular<br />
structure <strong>of</strong> which is identical to that which<br />
it had prior to its extraction or isolation<br />
Any <strong>of</strong> the following vitamins:<br />
biotin<br />
folate<br />
niacin<br />
pantothenic acid<br />
rib<strong>of</strong>lavin<br />
thiamine<br />
vitamin A<br />
vitamin B6<br />
vitamin B12<br />
vitamin C<br />
vitamin D<br />
vitamin E<br />
An amino acid<br />
An essential fatty acid<br />
A synthetic duplicate <strong>of</strong> a substance<br />
described in any <strong>of</strong> items 2 to 5<br />
A mineral<br />
A probiotic<br />
What is included in the definition <strong>of</strong> a<br />
natural health product?<br />
Schedule I indicates the medicinal ingredients included as<br />
a natural health product. (It is important to note that<br />
synthetic duplicates <strong>of</strong> NHPs are also included, as<br />
mentioned in #6).<br />
Express Exclusions<br />
There is also a schedule <strong>of</strong> express exclusions (Schedule<br />
2). This schedule identifies products that might fall within<br />
the definition <strong>of</strong> a NHP.<br />
However, certain products do not fall under the scope <strong>of</strong><br />
the Regulations, such as: 1) an antibiotic or its synthetic<br />
duplicate; 2) a substance that is administered by puncturing<br />
the dermis; 3) a substance regulated under the Tobacco Act;<br />
4) a substance set out in any <strong>of</strong> Schedules I to V <strong>of</strong> the<br />
Controlled Drugs and Substances Act; and 5) radiopharmaceuticals<br />
(Schedule C to FDA).<br />
In addition, a substance set out in Schedule D (biologics)<br />
<strong>of</strong> the Food and Drugs Act is excluded, except: i) a drug that<br />
is prepared from any <strong>of</strong> the following microorganisms,<br />
namely, an alga, a bacterium or a fungus; and ii) any<br />
substance set out in Schedule D when it is prepared in accordance<br />
with the practices <strong>of</strong> homeopathic pharmacy.<br />
Schedule 2: Excluded Natural Health Product<br />
Substances (Subsection 1(1))<br />
Item<br />
1.<br />
2.<br />
3.<br />
4.<br />
Substances<br />
A substance set out in Schedule C to the Act<br />
A substance set out in Schedule D to the<br />
Act, except for the following:<br />
(a) a drug that is prepared from any <strong>of</strong> the<br />
following micro-organisms, namely, an<br />
alga, a bacterium or a fungus; and<br />
(b) any substance set out on Schedule D<br />
when it is prepared in accordance with<br />
the practices <strong>of</strong> homeopathic pharmacy<br />
A substance regulated under the Tobacco Act<br />
A substance set out in any <strong>of</strong> Schedules I to<br />
V <strong>of</strong> the Controlled Drugs and Substances Act<br />
This article contains excerpts form the Natural Health Products website. The complete NHP Regulations can be viewed on<br />
Health Canada’s website at www.healthcanada.ca/nhpd<br />
Pharmacy Connection <strong>January</strong> • <strong>February</strong> <strong>2004</strong> 19