The effect of temperature on the flotation of pyrite - saimm
The effect of temperature on the flotation of pyrite - saimm
The effect of temperature on the flotation of pyrite - saimm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
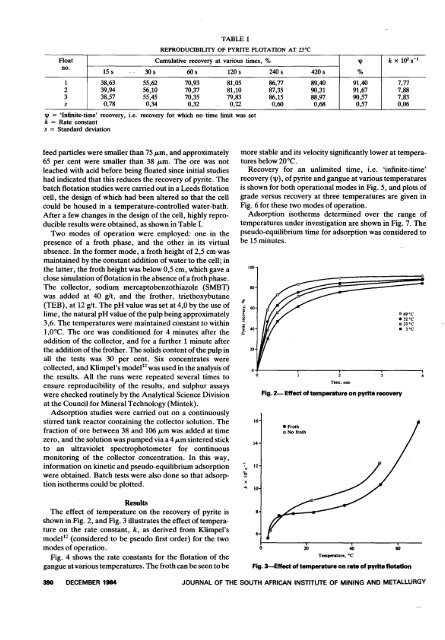
TABLE<br />
REPRODUCIBILITY OF PYRITE FLOTATION AT 23°C<br />
Float Cumulative recovery at various times, %<br />
k x 1OZS-I<br />
no.<br />
'"<br />
15 s - - 3Os 60s 120 s 240 s 420s %<br />
1 38,63 55,62 70,93 81,05 86,77 89,40<br />
I<br />
91,40 7,77<br />
2 39,94 56,10 70,37 81,10 87,35 90,31 91,67 7,88<br />
3 38,57 55,45 70,35 79,83 86,15 88,97 90,57 7,83<br />
s 0,78 0,34 0,32 0,12 0,60 0,68 0,57 0,06<br />
'Infinite-time' recovery, i.e. recovery for which no time limit was set<br />
'" k Rate c<strong>on</strong>stant<br />
s = Standard deviati<strong>on</strong><br />
I<br />
feed particles were smaller than 75/Lm, and approximately<br />
65 per cent were smaller than 38 /Lm. <str<strong>on</strong>g>The</str<strong>on</strong>g> ore was not<br />
leached with acid before being floated since initial studies<br />
had indicated that this reduces <strong>the</strong> recovery <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
batch flotati<strong>on</strong> studies were carried out in a Leeds flotati<strong>on</strong><br />
cell, <strong>the</strong> design <str<strong>on</strong>g>of</str<strong>on</strong>g> which had been altered so that <strong>the</strong> cell<br />
could be housed in a <str<strong>on</strong>g>temperature</str<strong>on</strong>g>-c<strong>on</strong>trolled water-bath.<br />
After a few changes in <strong>the</strong> design <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> cell, highly reproducible<br />
results were obtained, as shown in Table I.<br />
Two modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong> were employed: <strong>on</strong>e in <strong>the</strong><br />
presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase, and <strong>the</strong> o<strong>the</strong>r in its virtual<br />
absence. In <strong>the</strong> former mode, a froth height <str<strong>on</strong>g>of</str<strong>on</strong>g> 2,5 cmwas<br />
maintained by <strong>the</strong> c<strong>on</strong>stant additi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> water to <strong>the</strong> cell; in<br />
<strong>the</strong> latter, <strong>the</strong> froth height was below 0,5 cm, which gave a<br />
close simulati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> in <strong>the</strong> absence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> collector, sodium mercaptobenzothiazole (SMBT)<br />
was added at 40 glt, and <strong>the</strong> fro<strong>the</strong>r, triethoxybutane<br />
(TEB), at 12 glt. <str<strong>on</strong>g>The</str<strong>on</strong>g> pH value was set at 4,0 by <strong>the</strong> use <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
lime, <strong>the</strong> natural pH value <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp being approximately<br />
3,6. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s were maintained c<strong>on</strong>stant to within<br />
1,0°e. <str<strong>on</strong>g>The</str<strong>on</strong>g> ore was c<strong>on</strong>diti<strong>on</strong>ed for 4 minutes after <strong>the</strong><br />
additi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> collector, and for a fur<strong>the</strong>r 1 minute after<br />
<strong>the</strong> additi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> fro<strong>the</strong>r. <str<strong>on</strong>g>The</str<strong>on</strong>g> solids c<strong>on</strong>tent <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp in<br />
all <strong>the</strong> tests was 30 per cent. Six c<strong>on</strong>centrates were<br />
collected, and Klimpel's model12was used in <strong>the</strong> analysis <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>the</strong> results. All <strong>the</strong> runs were repeated several times to<br />
ensure reproducibility <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> results, and sulphur assays<br />
were checked routinely by <strong>the</strong> Analytical Science Divisi<strong>on</strong><br />
at <strong>the</strong> Council for Mineral Technology (Mintek).<br />
Adsorpti<strong>on</strong> studies were carried out <strong>on</strong> a c<strong>on</strong>tinuously<br />
stirred tank reactor c<strong>on</strong>taining <strong>the</strong> collector soluti<strong>on</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
fracti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> ore between 38 and 106 /Lm was added at time<br />
zero, and <strong>the</strong> soluti<strong>on</strong> was pumped via a 4/Lm sintered stick<br />
to an ultraviolet spectrophotometer for c<strong>on</strong>tinuous<br />
m<strong>on</strong>itoring <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> collector c<strong>on</strong>centrati<strong>on</strong>. In this way,<br />
informati<strong>on</strong> <strong>on</strong> kinetic and pseudo-equilibrium adsorpti<strong>on</strong><br />
were obtained. Batch tests were also d<strong>on</strong>e so that adsorpti<strong>on</strong><br />
iso<strong>the</strong>rms could be plotted.<br />
Results<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> recovery <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> is<br />
shown in Fig. 2, and Fig. 3 illustrates <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
<strong>on</strong> <strong>the</strong> rate c<strong>on</strong>stant, k, as derived from Klimpel's<br />
model12 (c<strong>on</strong>sidered to be pseudo first order) for <strong>the</strong> two<br />
modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong>.<br />
Fig. 4 shows <strong>the</strong> rate c<strong>on</strong>stants for <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong><br />
gangue at various <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s. <str<strong>on</strong>g>The</str<strong>on</strong>g> froth can be seen to be<br />
more stable and its velocity significantly lower at <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s<br />
below 20°e.<br />
Recovery for an unlimited time, i.e. 'infinite-time'<br />
recovery ('1\1),<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> and gangue at various <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s<br />
is shown for both operati<strong>on</strong>al modes in Fig. 5, and plots <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
grade versus recovery at three <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s are given in<br />
Fig. 6 for <strong>the</strong>se two modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong>.<br />
Adsorpti<strong>on</strong> iso<strong>the</strong>rms determined over <strong>the</strong> range <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<str<strong>on</strong>g>temperature</str<strong>on</strong>g>s under investigati<strong>on</strong> are shown in Fig. 7. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
pseudo-equilibrium time for adsorpti<strong>on</strong> was c<strong>on</strong>sidered to<br />
be 15 minutes.<br />
0<br />
~x<br />
...<br />
100<br />
80<br />
.r<br />
~ 60<br />
~<br />
u<br />
.~ 40<br />
...<br />
20<br />
0<br />
0<br />
Time. miD<br />
16<br />
14<br />
~12<br />
10<br />
6<br />
Fig. 2- Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>pyrite</strong> recovery<br />
. Froth<br />
.. N<str<strong>on</strong>g>of</str<strong>on</strong>g>roth<br />
0 20 40<br />
Temperature. .C<br />
Fig. 3-Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> flotati<strong>on</strong><br />
'"<br />
1i:<br />
069.C<br />
. S2.C<br />
m23"C<br />
. 3.C<br />
60<br />
390 DECEMBER 1984 JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY