The effect of temperature on the flotation of pyrite - saimm
The effect of temperature on the flotation of pyrite - saimm
The effect of temperature on the flotation of pyrite - saimm
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
J. S. A fr. Inst. Min. Metal/., vol. 84, no. 12.<br />
Dec. 1984. pp. 389-394.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong><br />
<strong>on</strong> <strong>the</strong> flotati<strong>on</strong><br />
by C.T. O'CONNOR*, R.C. DUNNEt, and A.M.A. BOTELHO DE SOUSA*<br />
SYNOPSIS<br />
In <strong>the</strong> investigati<strong>on</strong> described here, it was found that <strong>the</strong> main <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> is <strong>on</strong> <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong>,<br />
and that adsorpti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> reagent is not rate c<strong>on</strong>trolling. Above 10°C, <strong>the</strong> flotati<strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> and gangue<br />
increases almost exp<strong>on</strong>entially with a rise in <str<strong>on</strong>g>temperature</str<strong>on</strong>g>. Below that <str<strong>on</strong>g>temperature</str<strong>on</strong>g>, <strong>the</strong> flotati<strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> shows<br />
a sudden marked decrease, that <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue increases, and <strong>the</strong> sulphur grades are significantly poorer.<br />
In <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong>, <strong>the</strong> mass transfer <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> <strong>pyrite</strong> from <strong>the</strong> pulp to <strong>the</strong> troth is thought to be <strong>the</strong> ratec<strong>on</strong>trolling<br />
step, whereas <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue is c<strong>on</strong>trolled by <strong>the</strong> combined <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> stability and velocity<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth and <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> elutriating medium. <str<strong>on</strong>g>The</str<strong>on</strong>g> latter is probably <strong>the</strong> c<strong>on</strong>trolling <str<strong>on</strong>g>effect</str<strong>on</strong>g> at <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s<br />
below 10°C.<br />
Little difference was noted in <strong>the</strong> recoveries (provided no time limit was set) with changes in <str<strong>on</strong>g>temperature</str<strong>on</strong>g>; hence<br />
adequate recoveries can be obtained at Iow <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s by <strong>the</strong> introducti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> l<strong>on</strong>ger residence times for <strong>the</strong><br />
pulp. <str<strong>on</strong>g>The</str<strong>on</strong>g> adverse <str<strong>on</strong>g>effect</str<strong>on</strong>g>s <str<strong>on</strong>g>of</str<strong>on</strong>g> Iow <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s <strong>on</strong> grades can be overcome <strong>on</strong>ly by additi<strong>on</strong>al cleaning or by heating<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp.<br />
SAMEVATTING<br />
Daar is in die <strong>on</strong>dersoek wat hier beskryf word, gevind dat die vernaamste uitwerking van temperatuur is dat dit<br />
die flottasietempo raak en dat die reagentsabsorpsie nie tempobeherend<br />
van piriet en aarsteen byna eksp<strong>on</strong>ensiaal met 'n styging in temperatuur.<br />
is nie. Bo 10°C versnel die flottasietempo<br />
Onder hierdie temperatuur to<strong>on</strong> die flottasietempo<br />
van piriet 'n skielike duidelike afname, terwyl die van die aarsteen toeneem en die swawelgrade<br />
beduidend laer is.<br />
In die flottasie van piriet word daar gereken dat die massa-oordrag van die piriet van die pulp ha die skuim die<br />
tempobeherende stap is, terwyl die aarsteenflottasie beheer word deur die gekombineerde uitwerking van die<br />
stabiliteit en snelheid van die skuim en die viskositeit van die elutrieermiddel. Laasgenoemde is waarskynlik die<br />
beherende faktor by <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong>der 10°C.<br />
Daar is min verskil in die herwinnings waargeneem met 'n verandering in temperatuur (mits da.ar geen tydgrens<br />
gestel word nie); gevolglik kan toereikende herwinnings by laer <str<strong>on</strong>g>temperature</str<strong>on</strong>g> verkry word deur langer residensietye<br />
vir die pulp te'gebruik. Die nadelige uitwerking van lae <str<strong>on</strong>g>temperature</str<strong>on</strong>g> op grade kan net oorkom word deur verdere<br />
sko<strong>on</strong>maak, <str<strong>on</strong>g>of</str<strong>on</strong>g> deur die pulp te verhit.<br />
Introducti<strong>on</strong><br />
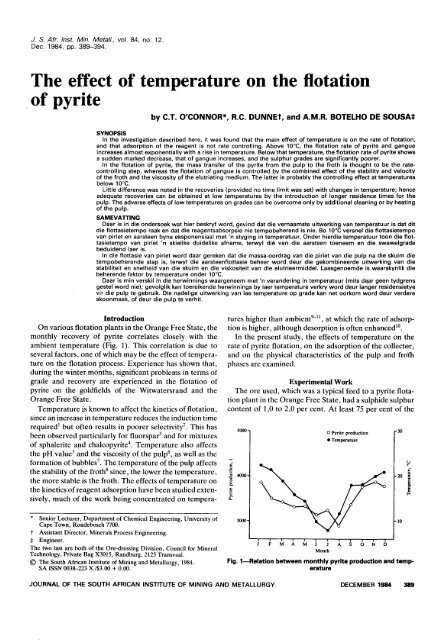
On various flotati<strong>on</strong> plants in <strong>the</strong> Orange Free State, <strong>the</strong><br />
m<strong>on</strong>thly recovery <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> correlates closely with <strong>the</strong><br />
ambient <str<strong>on</strong>g>temperature</str<strong>on</strong>g> (Fig. 1). This correlati<strong>on</strong> is due to<br />
several factors, <strong>on</strong>e <str<strong>on</strong>g>of</str<strong>on</strong>g> which may be <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
<strong>on</strong> <strong>the</strong> flotati<strong>on</strong> process. Experience has shown that,<br />
during <strong>the</strong> winter m<strong>on</strong>ths, significant problems in terms <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
grade and recovery are experienced in <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>pyrite</strong> <strong>on</strong> <strong>the</strong> goldfields <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> Witwatersrand and <strong>the</strong><br />
Orange Free State.<br />
Temperature is known to affect <strong>the</strong> kinetics <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong>,<br />
since an increase in <str<strong>on</strong>g>temperature</str<strong>on</strong>g> reduces <strong>the</strong> inducti<strong>on</strong> time<br />
requiredl but <str<strong>on</strong>g>of</str<strong>on</strong>g>ten results in poorer selectivity2. This has<br />
been observed particularly for fluorspar3 and for mixtures<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> sphalerite and chalco<strong>pyrite</strong>4. Temperature also affects<br />
<strong>the</strong> pH value5 and <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp6, as well as <strong>the</strong><br />
formati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> bubbles 7. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp affects<br />
<strong>the</strong> stability <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth8 since, <strong>the</strong> lower <strong>the</strong> <str<strong>on</strong>g>temperature</str<strong>on</strong>g>,<br />
<strong>the</strong> more stable is <strong>the</strong> froth. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g>s <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong><br />
<strong>the</strong> kinetics <str<strong>on</strong>g>of</str<strong>on</strong>g> reagent adsorpti<strong>on</strong> have been studied extensively,<br />
much <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> work being c<strong>on</strong>centrated <strong>on</strong> tempera-<br />
. Senior Lecturer, Department <str<strong>on</strong>g>of</str<strong>on</strong>g> Chemical Engineering, University <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
Cape Town, R<strong>on</strong>debosch 7700.<br />
t Assistant Director, Minerals Process Engineering.<br />
:j: Engineer.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> two last are both <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> Ore-dressing Divisi<strong>on</strong>, Council for Mineral<br />
Technology, Private Bag X3015, Randburg, 2125 Transvaal.<br />
@ <str<strong>on</strong>g>The</str<strong>on</strong>g> South African Institute <str<strong>on</strong>g>of</str<strong>on</strong>g> Mining and Metallurgy, 1984.<br />
SA ISSN 0038-223 X 1$3.00 + 0.00.<br />
tures higher than ambient9--11, at which <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> adsorpti<strong>on</strong><br />
is higher, although desorpti<strong>on</strong> is <str<strong>on</strong>g>of</str<strong>on</strong>g>ten enhancedlO.<br />
In <strong>the</strong> present study, <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g>s <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong><br />
rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> flotati<strong>on</strong>, <strong>on</strong> <strong>the</strong> adsorpti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> collect)[,<br />
and <strong>on</strong> <strong>the</strong> physical characteristics <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp and froth<br />
phases are examined.<br />
Experimental Work<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> ore used, which was a typical feed to a <strong>pyrite</strong> flotati<strong>on</strong><br />
plant in <strong>the</strong> Orange Free State, had a sulphide sulphur<br />
c<strong>on</strong>tent <str<strong>on</strong>g>of</str<strong>on</strong>g> 1,0 to 2,0 per cent. At least 75 per cent <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong><br />
c<br />
.g<br />
~4()()()<br />
0<br />
s.<br />
"~Po,<br />
5000 0 Pyrite producti<strong>on</strong><br />
. Temperature<br />
3000 10<br />
F M A M J J A s<br />
M<strong>on</strong>th<br />
0 N D<br />
30<br />
;;<br />
20 ~ "<br />
Fig. 1-Relati<strong>on</strong> between m<strong>on</strong>thly <strong>pyrite</strong> producti<strong>on</strong> and <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
8-<br />
!<br />
JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY DECEMBER 1984 389
TABLE<br />
REPRODUCIBILITY OF PYRITE FLOTATION AT 23°C<br />
Float Cumulative recovery at various times, %<br />
k x 1OZS-I<br />
no.<br />
'"<br />
15 s - - 3Os 60s 120 s 240 s 420s %<br />
1 38,63 55,62 70,93 81,05 86,77 89,40<br />
I<br />
91,40 7,77<br />
2 39,94 56,10 70,37 81,10 87,35 90,31 91,67 7,88<br />
3 38,57 55,45 70,35 79,83 86,15 88,97 90,57 7,83<br />
s 0,78 0,34 0,32 0,12 0,60 0,68 0,57 0,06<br />
'Infinite-time' recovery, i.e. recovery for which no time limit was set<br />
'" k Rate c<strong>on</strong>stant<br />
s = Standard deviati<strong>on</strong><br />
I<br />
feed particles were smaller than 75/Lm, and approximately<br />
65 per cent were smaller than 38 /Lm. <str<strong>on</strong>g>The</str<strong>on</strong>g> ore was not<br />
leached with acid before being floated since initial studies<br />
had indicated that this reduces <strong>the</strong> recovery <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
batch flotati<strong>on</strong> studies were carried out in a Leeds flotati<strong>on</strong><br />
cell, <strong>the</strong> design <str<strong>on</strong>g>of</str<strong>on</strong>g> which had been altered so that <strong>the</strong> cell<br />
could be housed in a <str<strong>on</strong>g>temperature</str<strong>on</strong>g>-c<strong>on</strong>trolled water-bath.<br />
After a few changes in <strong>the</strong> design <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> cell, highly reproducible<br />
results were obtained, as shown in Table I.<br />
Two modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong> were employed: <strong>on</strong>e in <strong>the</strong><br />
presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase, and <strong>the</strong> o<strong>the</strong>r in its virtual<br />
absence. In <strong>the</strong> former mode, a froth height <str<strong>on</strong>g>of</str<strong>on</strong>g> 2,5 cmwas<br />
maintained by <strong>the</strong> c<strong>on</strong>stant additi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> water to <strong>the</strong> cell; in<br />
<strong>the</strong> latter, <strong>the</strong> froth height was below 0,5 cm, which gave a<br />
close simulati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> in <strong>the</strong> absence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> collector, sodium mercaptobenzothiazole (SMBT)<br />
was added at 40 glt, and <strong>the</strong> fro<strong>the</strong>r, triethoxybutane<br />
(TEB), at 12 glt. <str<strong>on</strong>g>The</str<strong>on</strong>g> pH value was set at 4,0 by <strong>the</strong> use <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
lime, <strong>the</strong> natural pH value <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp being approximately<br />
3,6. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s were maintained c<strong>on</strong>stant to within<br />
1,0°e. <str<strong>on</strong>g>The</str<strong>on</strong>g> ore was c<strong>on</strong>diti<strong>on</strong>ed for 4 minutes after <strong>the</strong><br />
additi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> collector, and for a fur<strong>the</strong>r 1 minute after<br />
<strong>the</strong> additi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> fro<strong>the</strong>r. <str<strong>on</strong>g>The</str<strong>on</strong>g> solids c<strong>on</strong>tent <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp in<br />
all <strong>the</strong> tests was 30 per cent. Six c<strong>on</strong>centrates were<br />
collected, and Klimpel's model12was used in <strong>the</strong> analysis <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>the</strong> results. All <strong>the</strong> runs were repeated several times to<br />
ensure reproducibility <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> results, and sulphur assays<br />
were checked routinely by <strong>the</strong> Analytical Science Divisi<strong>on</strong><br />
at <strong>the</strong> Council for Mineral Technology (Mintek).<br />
Adsorpti<strong>on</strong> studies were carried out <strong>on</strong> a c<strong>on</strong>tinuously<br />
stirred tank reactor c<strong>on</strong>taining <strong>the</strong> collector soluti<strong>on</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
fracti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> ore between 38 and 106 /Lm was added at time<br />
zero, and <strong>the</strong> soluti<strong>on</strong> was pumped via a 4/Lm sintered stick<br />
to an ultraviolet spectrophotometer for c<strong>on</strong>tinuous<br />
m<strong>on</strong>itoring <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> collector c<strong>on</strong>centrati<strong>on</strong>. In this way,<br />
informati<strong>on</strong> <strong>on</strong> kinetic and pseudo-equilibrium adsorpti<strong>on</strong><br />
were obtained. Batch tests were also d<strong>on</strong>e so that adsorpti<strong>on</strong><br />
iso<strong>the</strong>rms could be plotted.<br />
Results<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> recovery <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> is<br />
shown in Fig. 2, and Fig. 3 illustrates <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
<strong>on</strong> <strong>the</strong> rate c<strong>on</strong>stant, k, as derived from Klimpel's<br />
model12 (c<strong>on</strong>sidered to be pseudo first order) for <strong>the</strong> two<br />
modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong>.<br />
Fig. 4 shows <strong>the</strong> rate c<strong>on</strong>stants for <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong><br />
gangue at various <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s. <str<strong>on</strong>g>The</str<strong>on</strong>g> froth can be seen to be<br />
more stable and its velocity significantly lower at <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s<br />
below 20°e.<br />
Recovery for an unlimited time, i.e. 'infinite-time'<br />
recovery ('1\1),<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> and gangue at various <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s<br />
is shown for both operati<strong>on</strong>al modes in Fig. 5, and plots <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
grade versus recovery at three <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s are given in<br />
Fig. 6 for <strong>the</strong>se two modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong>.<br />
Adsorpti<strong>on</strong> iso<strong>the</strong>rms determined over <strong>the</strong> range <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<str<strong>on</strong>g>temperature</str<strong>on</strong>g>s under investigati<strong>on</strong> are shown in Fig. 7. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
pseudo-equilibrium time for adsorpti<strong>on</strong> was c<strong>on</strong>sidered to<br />
be 15 minutes.<br />
0<br />
~x<br />
...<br />
100<br />
80<br />
.r<br />
~ 60<br />
~<br />
u<br />
.~ 40<br />
...<br />
20<br />
0<br />
0<br />
Time. miD<br />
16<br />
14<br />
~12<br />
10<br />
6<br />
Fig. 2- Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>pyrite</strong> recovery<br />
. Froth<br />
.. N<str<strong>on</strong>g>of</str<strong>on</strong>g>roth<br />
0 20 40<br />
Temperature. .C<br />
Fig. 3-Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> flotati<strong>on</strong><br />
'"<br />
1i:<br />
069.C<br />
. S2.C<br />
m23"C<br />
. 3.C<br />
60<br />
390 DECEMBER 1984 JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY
,<br />
~<br />
~<br />
x<br />
...<br />
0<br />
'100<br />
98<br />
11 96<br />
If<br />
.~<br />
'0 94<br />
..<br />
92<br />
90<br />
0<br />
. Froth<br />
0 N<str<strong>on</strong>g>of</str<strong>on</strong>g>roth<br />
0 20 40<br />
Temperature, .C<br />
Fig. 4-Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue flotati<strong>on</strong><br />
Pyrite<br />
.Froth<br />
0 No froth<br />
GanlUe<br />
. Froth<br />
" N<str<strong>on</strong>g>of</str<strong>on</strong>g>roth<br />
20 40 60<br />
Temperature, .C<br />
Fig. 5-Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> 'infinite-time' recovery ('1/')<str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>pyrite</strong> and gangue<br />
30 . Froth<br />
0 N<str<strong>on</strong>g>of</str<strong>on</strong>g>roth<br />
25<br />
23'C<br />
60<br />
21<br />
19<br />
17<br />
15<br />
11<br />
13 I<br />
'0<br />
..<br />
11<br />
Fig. 8 gives <strong>the</strong> results <str<strong>on</strong>g>of</str<strong>on</strong>g><strong>the</strong> tests <strong>on</strong> froth stability. For<br />
<strong>the</strong>se tests, <strong>the</strong> time taken for <strong>the</strong> froth to break down from<br />
its maximum height to a predetermined height (termed<br />
durati<strong>on</strong> and measured in sec<strong>on</strong>ds) was measured at<br />
different <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s. Also shown are similar results<br />
obtained <strong>on</strong> a two-phase froth by G6tte13 using sodium<br />
dodecyl sulphate as fro<strong>the</strong>r.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> results <str<strong>on</strong>g>of</str<strong>on</strong>g> varying reagent c<strong>on</strong>centrati<strong>on</strong>s, aerati<strong>on</strong><br />
rates, and impeller speeds are presented in Tabks 11to VII.<br />
As can be seen from Tables 11 to V, variati<strong>on</strong> in <strong>the</strong><br />
c<strong>on</strong>centrati<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> flotati<strong>on</strong> reagents had little significant<br />
<str<strong>on</strong>g>effect</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> or <strong>on</strong> <strong>the</strong> infinite-time<br />
recoveries <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong>.<br />
However, as Tables VI and VII indicate, variati<strong>on</strong>s in <strong>the</strong><br />
aerati<strong>on</strong> rate and <strong>the</strong> impeller speed had a significant <str<strong>on</strong>g>effect</str<strong>on</strong>g><br />
<strong>on</strong> <strong>the</strong> infinite-time recovery and flotati<strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong>.<br />
Fig. 9 shows <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
pulps <str<strong>on</strong>g>of</str<strong>on</strong>g> different densities. <str<strong>on</strong>g>The</str<strong>on</strong>g>se tests were carried out<br />
with a St6rmer viscometer. Also shown is a plot for which<br />
Mo<strong>on</strong>ey's correlati<strong>on</strong> for pUlpSl4 was used:<br />
In ILm = 2,5s<br />
ILl 1-cs'<br />
where ILmand ILl are <strong>the</strong> respective viscosities <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp<br />
and water in centipoises, sis <strong>the</strong> volume fracti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong><br />
solids, and <strong>the</strong> coefficient c has a value <str<strong>on</strong>g>of</str<strong>on</strong>g> from 1 to 1,5. For<br />
<strong>the</strong>se calculati<strong>on</strong>s, c was taken as 1 and swas taken as<br />
0,18. <str<strong>on</strong>g>The</str<strong>on</strong>g> results show <strong>the</strong> expected increase in viscosity<br />
with decrease in <str<strong>on</strong>g>temperature</str<strong>on</strong>g>.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> photographic studies showed that, at <strong>the</strong> higher<br />
viscosity, <strong>the</strong> rate at which <strong>the</strong> bubbles rose was lower and<br />
<strong>the</strong> number <str<strong>on</strong>g>of</str<strong>on</strong>g> bubbles per unit volume greater.<br />
Discussi<strong>on</strong><br />
As was expected from a c<strong>on</strong>siderati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> fundamentals<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> flotati<strong>on</strong> process, this study showed that<br />
<str<strong>on</strong>g>temperature</str<strong>on</strong>g> affects mainly <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> flotati<strong>on</strong> and<br />
has little <str<strong>on</strong>g>effect</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> final recoveries. It was also shown<br />
that, at <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s below 1O0C, a marked change occurs<br />
in <strong>the</strong> trend <str<strong>on</strong>g>of</str<strong>on</strong>g> decreasing rate c<strong>on</strong>stants for <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>pyrite</strong>. This change was highly reproducible, and occurred<br />
in <strong>the</strong> presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase and in its absence. In <strong>the</strong><br />
presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase, <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> was slightly<br />
lower, probably because <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> superimposed <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong><br />
3<br />
e } 2<br />
. sO'C<br />
.30.C<br />
0 IO'C<br />
[;J 2.C<br />
<strong>on</strong> 20<br />
If<br />
~<br />
0 15<br />
10<br />
3'C<br />
,.C~<br />
-0<br />
.t:><br />
CS "<br />
-g<br />
1<br />
'";<br />
~<br />
5<br />
20 40 60<br />
Pyrite<br />
recovery.<br />
Fig. 6--Grade versus recovery at different <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s and<br />
modes <str<strong>on</strong>g>of</str<strong>on</strong>g> operati<strong>on</strong><br />
'10<br />
80<br />
100<br />
0<br />
0 20 40<br />
60<br />
Equilibrium c<strong>on</strong>centrati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 5MBT <strong>on</strong>to <strong>pyrite</strong>. glt<br />
Fig. 7-Adsorpti<strong>on</strong> iso<strong>the</strong>rms for sodium mercaptobenzothiazole<br />
(SMBT) <strong>on</strong>to <strong>pyrite</strong><br />
JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY DECEMBER 1984 391
30<br />
'"<br />
ci' 20<br />
0<br />
';::<br />
C\S<br />
....<br />
::s<br />
"0<br />
..c:<br />
(5 10<br />
.....<br />
~<br />
0 This work<br />
. Gotte13<br />
300<br />
'"<br />
ci'<br />
0<br />
';::<br />
C\S<br />
....<br />
200 ::s<br />
"0<br />
..c:<br />
.....<br />
0 ....<br />
~100<br />
0<br />
0<br />
10<br />
20 30<br />
Temperature, °C<br />
40<br />
50<br />
0<br />
60<br />
Fig. 8- Tests <strong>on</strong> froth stability<br />
TABLE 11<br />
EFFECT OF COLLECTOR CONCENTRATION ON RECOVERY<br />
OF PYRITE AND GANGUE<br />
5MBT c<strong>on</strong>centrati<strong>on</strong><br />
Pyrite<br />
Gangue<br />
'1' k x 1O2s-1 '1' k X 1O2s-1<br />
g/t % %<br />
10 91,99 7,60 13,08 1,74<br />
20 92,65 7,59 12,47 1,40<br />
40 90,61 7,76 12,58 1,48<br />
100 95,04 8,68 14,44 1,50<br />
200 94,64 7,84 11,43 1,92<br />
TABLE V<br />
EFFECT OF ACTIVATOR CONCENTRAnON ON RECOVERY<br />
OF PYRITE AND GANGUE<br />
CUSO4<br />
Pyrite<br />
Gangue<br />
c<strong>on</strong>centrati<strong>on</strong><br />
g/t %'1' k x 1O2s-1<br />
%'1' k X IOZs-1<br />
'1' = 'Infinite-time'<br />
- 89,37 9,33 12,14 1,56<br />
20 88,61 9,19 12,67 1,51<br />
50 90,25 8,52 12,9 1,26<br />
100 89,32 8,39 11,30 1,43<br />
recovery<br />
'1' = 'Infinite-time' recovery TABLE III<br />
EFFECT OF FROTHER CONCENTRAnON ON RECOVERY<br />
OF PYRITE AND GANGUE<br />
Pyrite<br />
Gangue<br />
DOWFROTH 250<br />
c<strong>on</strong>centrati<strong>on</strong><br />
'1' kx1O2s-1<br />
g/t '1' kx 1O2s-1<br />
% %<br />
- 85,12 7,91 7,01 1,41<br />
10 87,37 7,96 10,26 1,65<br />
20 89,66 7,72 12,67 1,38<br />
40 88,55 8,02 15,72 1,72<br />
80 88,66 8,80 17,75 1,68<br />
160 91,20 8,33 21,64 1,50<br />
TABLE VI<br />
EFFECT OF AERATION RATE ON RECOVERY<br />
OF PYRITE AND GANGUE<br />
Pyrite<br />
Gangue<br />
Aerati<strong>on</strong> rate<br />
l/min '1' k X 1O2s-1 '1' k X 1O2s-1<br />
% %<br />
3 82,31 2,38 8,93 0,17<br />
5 86,38 5,89 8,96 0,70<br />
7 88,55 8,02 15,72 1,72<br />
9 91,06 10,75 19,69 1,92<br />
'1'<br />
= 'Infinite-time' recovery TABLE IV<br />
EFFECT OF DEPRESSANT CONCENTRAnON ON RECOVERY<br />
OF PYRITE AND GANGUE<br />
Pyrite<br />
Gangue<br />
ACROL (J2P350)<br />
c<strong>on</strong>centrati<strong>on</strong><br />
g/t '1' k x 1O2s-1 '1' kx IOZs-1<br />
% %<br />
- 89,37 9,33 12,14 1,56<br />
10 86,91 8,90 11,22 1,45<br />
20 88,00 8,11 11,55 1,17<br />
50 90,46 7,19 11,97 0,94<br />
lOO 89,15 6,68 9,73 0,87<br />
'1' = 'Infinite-time' recovery TABLE VII<br />
EFFECT OF IMPELLER SPEED ON RECOVERY<br />
OF PYRITE AND GANGUE<br />
Pyrite<br />
Gangue<br />
Impeller speed<br />
r/min '1' k x 1O2s-1 '1' k X 1O2s-]<br />
% %<br />
800 85,77 4,57 11,45 0,52<br />
950 83,97 6,89 10,24 1,02<br />
1100 88,11 5,97 11,04 0,68<br />
1300 89,66 7,72 12,67 1,38<br />
1450 89,11 8,94 11,90 1,49<br />
1600 89,63 8,45 12,12 1,57<br />
'1'<br />
= 'Infinite-time'<br />
recovery<br />
'1'<br />
= 'Infinite-time' recovery<br />
392 DECEMBER 1984 JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY
'"~<br />
0<br />
'h<br />
.~ ~~<br />
";: I<br />
IcP=I.OxlO-'P..s<br />
0<br />
0 10 20<br />
30<br />
Tem"",.!u,e. "C<br />
Fig. 9- Effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> pulp viscosity<br />
lower velocity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth. A similar trend in <strong>the</strong> rates <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
flotati<strong>on</strong> was observed by Klimpel12 in <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> silica<br />
from a tac<strong>on</strong>ite ore.<br />
Slightly less <strong>pyrite</strong> was recovered (92 per cent as<br />
compared with 98 per cent) when a froth phase was present,<br />
probably as a result <str<strong>on</strong>g>of</str<strong>on</strong>g> normal froth <str<strong>on</strong>g>effect</str<strong>on</strong>g>s such as crowding<br />
and bubble breakage. Lowering <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
decreased <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> since it decreased <strong>the</strong><br />
velocity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth, increased <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp, and<br />
reduced <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> mass transfer <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> <strong>pyrite</strong> from <strong>the</strong> pulp<br />
to <strong>the</strong> froth. <str<strong>on</strong>g>The</str<strong>on</strong>g> influence <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>pyrite</strong> flotati<strong>on</strong> was virtually <strong>the</strong> same in <strong>the</strong> presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a<br />
froth phase as it was in its absence (Figs. 3 and 5); so <strong>the</strong><br />
most important <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong>se factors is probably <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> mass<br />
transfer <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> <strong>pyrite</strong> from <strong>the</strong> pulp to <strong>the</strong> froth phase.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> reas<strong>on</strong> for <strong>the</strong> decrease in <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> mass transfer<br />
has not yet been identified, but this decrease may be due to<br />
<strong>on</strong>e <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> following factors:<br />
(a) poorer loading <str<strong>on</strong>g>of</str<strong>on</strong>g> bubbles as <strong>the</strong> result <str<strong>on</strong>g>of</str<strong>on</strong>g> decreased<br />
inducti<strong>on</strong> time,<br />
(b) lower rate <str<strong>on</strong>g>of</str<strong>on</strong>g> bubble rise, or<br />
(c) fewer bubbles per unit volume <str<strong>on</strong>g>of</str<strong>on</strong>g> pulp.<br />
Inducti<strong>on</strong> time, defined as <strong>the</strong> time necessary for <strong>the</strong><br />
thinning <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> bubble liquid layer to a critical thickness at<br />
which rupture occurs, is affected by several variables15 such<br />
as <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> bul~ liquid and <strong>the</strong> c<strong>on</strong>centrati<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>the</strong> flotati<strong>on</strong> reagents. Less collector is adsorbed at lower<br />
<str<strong>on</strong>g>temperature</str<strong>on</strong>g>s, but <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> flotati<strong>on</strong> is hardly<br />
affected by a change in collector c<strong>on</strong>centrati<strong>on</strong>. <str<strong>on</strong>g>The</str<strong>on</strong>g>refore,<br />
<strong>the</strong> extent <str<strong>on</strong>g>of</str<strong>on</strong>g> collector adsorpti<strong>on</strong> does not appear to be<br />
resp<strong>on</strong>sible for <strong>the</strong> marked change in <strong>the</strong> flotati<strong>on</strong> rate. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
o<strong>the</strong>r parameters that affect inducti<strong>on</strong> time have yet to be<br />
evaluated.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> bubble rise was observed to be slower for<br />
colder pulps <str<strong>on</strong>g>of</str<strong>on</strong>g> higher viscosity. Although this is c<strong>on</strong>sistent<br />
with <strong>the</strong> observed lower rate <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> at low <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s,<br />
o<strong>the</strong>r factors, e.g. inducti<strong>on</strong> time, may be as important,<br />
or more important.<br />
Photographic studies showed that, at low <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s,<br />
40<br />
o~.<br />
<strong>the</strong>re were more bubbles per unit volume than at high<br />
<str<strong>on</strong>g>temperature</str<strong>on</strong>g>s. This, and <strong>the</strong> fact that <strong>the</strong> flotati<strong>on</strong> rate did<br />
not vary c<strong>on</strong>siderably with varying quantities <str<strong>on</strong>g>of</str<strong>on</strong>g> fro<strong>the</strong>r, do<br />
not sustain <strong>the</strong> hypo<strong>the</strong>sis that <strong>the</strong> slower rate <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong><br />
at low <str<strong>on</strong>g>temperature</str<strong>on</strong>g> is caused by <strong>the</strong> presence <str<strong>on</strong>g>of</str<strong>on</strong>g> fewer<br />
bubbles per unit volume.<br />
At lower <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s, flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> gangue is<br />
affected as follows. <str<strong>on</strong>g>The</str<strong>on</strong>g> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> mass transfer from <strong>the</strong> pulp<br />
to <strong>the</strong> froth, <strong>the</strong> elutriati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue from <strong>the</strong> froth phase,<br />
and <strong>the</strong> velocity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth are lower, and <strong>the</strong> froth is more<br />
stable. At <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s below lOoC, <strong>the</strong> k and 'IjJ<str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
pr<str<strong>on</strong>g>of</str<strong>on</strong>g>iles for gangue (Figs. 4 and 5) are markedly<br />
different from those for <strong>pyrite</strong> in <strong>the</strong> presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth<br />
phase, in that <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue flotati<strong>on</strong> increases. This<br />
phenomen<strong>on</strong> is ascribed to <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> increased<br />
stability <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth and <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> elutriating<br />
medium, which cause less gangue to return to <strong>the</strong> pulp by<br />
bubble breakage or by elutriati<strong>on</strong>. In <strong>the</strong> flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
gangue at lower <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s, <strong>the</strong>se two <str<strong>on</strong>g>effect</str<strong>on</strong>g>s appear to<br />
overshadow <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> reduced froth velocity and <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
lower mass-transfer rates from <strong>the</strong> pulp to <strong>the</strong> froth. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
different pr<str<strong>on</strong>g>of</str<strong>on</strong>g>iles obtained for <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue flotati<strong>on</strong><br />
in <strong>the</strong> presence <str<strong>on</strong>g>of</str<strong>on</strong>g> a froth phase and in its absence indicate<br />
that, with respect to <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue flotati<strong>on</strong>, <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g><br />
<strong>on</strong> <strong>the</strong> froth phase at lower <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s is most significant,<br />
resulting in greater recoveries <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue and seriously<br />
reduced grades. This is shown clearly in Fig. 6.<br />
It has been shown 16 that <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> adsorpti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 5MBT<br />
<strong>on</strong>to <strong>pyrite</strong> decreases with <str<strong>on</strong>g>temperature</str<strong>on</strong>g>. Fig. 7 shows <strong>the</strong><br />
<str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> equilibrium adsorpti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
5MBT. However, in all instances, a pseudo-equilibrium<br />
adsorpti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> collector, approximately equivalent to a<br />
double layer <str<strong>on</strong>g>of</str<strong>on</strong>g> reagent molecules, is obtained after 4<br />
minutes. M<strong>on</strong>olayer coverage is obtained at all <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s<br />
after less than 1 minute. <str<strong>on</strong>g>The</str<strong>on</strong>g> adsorpti<strong>on</strong> iso<strong>the</strong>rms<br />
show that, at higher <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s, desorpti<strong>on</strong> may occur.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> equilibrium adsorpti<strong>on</strong> at 2 °C is significantly lower<br />
than at ambient <str<strong>on</strong>g>temperature</str<strong>on</strong>g>, but is never<strong>the</strong>less adequate<br />
for flotati<strong>on</strong> to occur. Hence, since adequate amounts <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
reagent are adsorbed within <strong>the</strong> normal c<strong>on</strong>diti<strong>on</strong>ing time,<br />
viz 4 minutes, <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> reagent adsorpti<strong>on</strong> is not c<strong>on</strong>sidered<br />
to be rate c<strong>on</strong>trolling.<br />
C<strong>on</strong>clusi<strong>on</strong>s<br />
This study showed that <strong>the</strong> flotati<strong>on</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong><br />
decreases with <str<strong>on</strong>g>temperature</str<strong>on</strong>g> owing to a reducti<strong>on</strong> in <strong>the</strong> rate<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> mass transfer <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> from <strong>the</strong> pulp to <strong>the</strong> froth. This,<br />
in turn, is probably partly because <strong>the</strong> bubbles rise more<br />
slowly at lower <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s, and hence higher viscosities.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> inducti<strong>on</strong> time was not investigated.<br />
It was also shown that <strong>the</strong> rate <str<strong>on</strong>g>of</str<strong>on</strong>g> gangue flotati<strong>on</strong><br />
is influenced mainly by <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong><br />
stability <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> froth and <strong>the</strong> viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> elutriating<br />
medium.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g>se <str<strong>on</strong>g>effect</str<strong>on</strong>g>s <str<strong>on</strong>g>of</str<strong>on</strong>g> a decrease in <str<strong>on</strong>g>temperature</str<strong>on</strong>g> cannot be<br />
overcome by changes in reagent c<strong>on</strong>centrati<strong>on</strong>s. As this<br />
study simulated <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>temperature</str<strong>on</strong>g> <strong>on</strong> <strong>the</strong> rougher<br />
banks <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong> plants, it is c<strong>on</strong>cluded that, for adequate<br />
grades to be maintained at lower <str<strong>on</strong>g>temperature</str<strong>on</strong>g>s, <strong>the</strong> load <strong>on</strong><br />
<strong>the</strong> cleaner will have to be increased. An increase in aera-<br />
JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY DECEMBER 1984 393
ti<strong>on</strong> rate would improve <strong>the</strong> flotati<strong>on</strong> rate and infinite-time<br />
recovery <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong>, This al<strong>on</strong>e does not <str<strong>on</strong>g>of</str<strong>on</strong>g>fer a soluti<strong>on</strong> to<br />
<strong>the</strong> problem encountered during <strong>the</strong> winter m<strong>on</strong>ths (Fig.<br />
1), since <strong>the</strong>re will be a similar increase in gangue flotati<strong>on</strong>.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g> use <str<strong>on</strong>g>of</str<strong>on</strong>g> higher impeller speeds results in improved<br />
flotati<strong>on</strong> rates and recoveries <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>pyrite</strong> without a reducti<strong>on</strong><br />
in grade, and this may overcome <strong>the</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g>s <str<strong>on</strong>g>of</str<strong>on</strong>g> decreased<br />
<str<strong>on</strong>g>temperature</str<strong>on</strong>g>s.<br />
Finally, it is c<strong>on</strong>cluded that, although heating <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> pulp<br />
may improve <strong>the</strong> recoveries, <strong>the</strong> ec<strong>on</strong>omics must be carefully<br />
evaluated for each flotati<strong>on</strong> plant. An example <str<strong>on</strong>g>of</str<strong>on</strong>g> such<br />
an evaluati<strong>on</strong> is given in <strong>the</strong> addendum.<br />
Acknowledgements<br />
This paper is published by permissi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> Council for<br />
Mineral Technology (Mintek) which, with <strong>the</strong> University<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> Cape Town, was resp<strong>on</strong>sible for funding this work. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
authors thank <strong>the</strong>m, as well as <strong>the</strong> Ore-dressing, Mineralogy,<br />
and Analytical Science Divisi<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> Mintek for <strong>the</strong>ir<br />
assistance in many aspects <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> work.<br />
References<br />
1. EIGELES,M.A., and VOLOVA,M.L. Kinetic investigati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>tact time, <str<strong>on</strong>g>temperature</str<strong>on</strong>g> and surface c<strong>on</strong>diti<strong>on</strong> <strong>on</strong> <strong>the</strong><br />
adhesi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> bubbles to mineral surfaces. Mineral Research Institute,<br />
Ministry <str<strong>on</strong>g>of</str<strong>on</strong>g> Geology and Protecti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> Mineral<br />
Resources <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> USSR.<br />
2. GLEMBOTSKII.,V.A., KLASSEN.V.L, and PLAKSIN,LN. Flotati<strong>on</strong>.<br />
New York, Primary Sources, 1972. pp. 355-356.<br />
3. KLASSEN.V.L, and MoKRoussov, V.A. An introducti<strong>on</strong> to <strong>the</strong><br />
<strong>the</strong>ory <str<strong>on</strong>g>of</str<strong>on</strong>g>flotati<strong>on</strong>. L<strong>on</strong>d<strong>on</strong>, Butterworth, 1963. p. 258.<br />
4. MARAIS, P. Some practical c<strong>on</strong>siderati<strong>on</strong>s in <strong>the</strong> design and<br />
operati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> a plant for <strong>the</strong> differential flotati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> mixed<br />
sulphides, especially copper and zinc. J. S. Afr. Inst. Min. Metal/.,<br />
vo!. 80, no. 11. 1980. pp. 385-394.<br />
5. SUTHERLAND,K.L., and WARK, LW. Principles <str<strong>on</strong>g>of</str<strong>on</strong>g> flotati<strong>on</strong>.<br />
Melbourne, Australasian Institute <str<strong>on</strong>g>of</str<strong>on</strong>g> Mining and Metallurgy,<br />
1955. pp. 178-188.<br />
6. MARSDEN, D.D. <str<strong>on</strong>g>The</str<strong>on</strong>g> <str<strong>on</strong>g>effect</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> pH value, <str<strong>on</strong>g>temperature</str<strong>on</strong>g> and density<br />
<strong>on</strong> <strong>the</strong> kinematic viscosity <str<strong>on</strong>g>of</str<strong>on</strong>g> some South African gold-mine slurries.<br />
J. S. Afr. Inst. Min. Metal/., vo!. 62, no. 6. 1962. pp. 391-398.<br />
7. DAVIDSON, J.F., and SHOLER, B.O.G. Bubble formati<strong>on</strong> at an<br />
orifice in a viscous liquid. Trans. Inst. Chem. Eng., vo!. 38. 1960.<br />
pp. 144-154.<br />
8. KLASSEN. V.L, and MoKRoussov, V.A., op. cit., p. 368.<br />
9. SUTHERLAND, K.L., and WARK, LW., op. cit., p. 350.<br />
10. KUBOTA, T., YOSHIDA, M., HASHIMOTO, S., and SHIMOIZAKA, J.<br />
A new method for copper-lead separati<strong>on</strong> by raising <strong>the</strong> <str<strong>on</strong>g>temperature</str<strong>on</strong>g><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> bulk float. Proceedings <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> I1 th Internati<strong>on</strong>al<br />
Mineral Processing C<strong>on</strong>gress, Cagliari, April 1975. Cagliari,<br />
Instituto di Arte Mineraria Universita di Cagliari, 1975.<br />
pp. 623-637.<br />
11. HUKKI, R. T. Hot flotati<strong>on</strong> improves selectivity and raises mineral<br />
recoveries. World Min., vo!. 26, no. 3.1973. pp. 74-76.<br />
12. KUMPEL, R.R. <str<strong>on</strong>g>The</str<strong>on</strong>g> engineering analysis <str<strong>on</strong>g>of</str<strong>on</strong>g> dispersi<strong>on</strong> <str<strong>on</strong>g>effect</str<strong>on</strong>g>s in<br />
selected mineral processing operati<strong>on</strong>s. Proceedings <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> Internati<strong>on</strong>al<br />
Symposium <strong>on</strong> Fine Particles Processing, Las Vegas,<br />
Nevada, February 1980. Somasundaran, P. (ed.). New York,<br />
AIME, 1980. pp. 1129-1151.<br />
13. KLASSEN, V.L, and MoKRoussov, V.A. op. cit., p. 368.<br />
14. MoONEY, J. J. Colloid Interface Sci., vo!. 6. 1951. p. 162.<br />
15. LEJA, J. Surface chemistry <str<strong>on</strong>g>of</str<strong>on</strong>g> froth flotati<strong>on</strong>. New York, Plenum<br />
Press, 1982.<br />
16. O'CONNOR, C.T., MccLEAN, D.M., and DELPoRT, L. University<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> Cape Town, private communicati<strong>on</strong>, 1982.<br />
Addendum<br />
If a flotati<strong>on</strong> plant with a feed rate <str<strong>on</strong>g>of</str<strong>on</strong>g> 19 000 t/d yields<br />
500 t <str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>centrate and 3800 g <str<strong>on</strong>g>of</str<strong>on</strong>g> gold per day during<br />
winter and a 10°C rise in <str<strong>on</strong>g>temperature</str<strong>on</strong>g> will result in<br />
an increase <str<strong>on</strong>g>of</str<strong>on</strong>g> 7,5 per cent in <strong>the</strong> amount <str<strong>on</strong>g>of</str<strong>on</strong>g> c<strong>on</strong>centrate<br />
produced,<br />
4085 gld.<br />
Thus,<br />
<strong>the</strong> feed rate <str<strong>on</strong>g>of</str<strong>on</strong>g> pulp<br />
<strong>the</strong> heat capacity <str<strong>on</strong>g>of</str<strong>on</strong>g> pulp<br />
<strong>the</strong> enthalpy <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong> steam<br />
(100 kPa, 250°C)<br />
<strong>the</strong> steam costs<br />
<strong>the</strong> gold recovery will be approximately<br />
= 19000 t/d<br />
= 3,15 kJ/(kg.K), calculated<br />
<strong>on</strong> a weighted<br />
average at a pulp density<br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> 30 per cent and<br />
a heat capacity <str<strong>on</strong>g>of</str<strong>on</strong>g> <strong>the</strong><br />
solids <str<strong>on</strong>g>of</str<strong>on</strong>g> 0,7 kJ/(kg.K)<br />
= 2944 kJ/kg<br />
= R3,;>O(including capital<br />
costs)<br />
<strong>the</strong> gold price<br />
= R12000per kilogram.<br />
<str<strong>on</strong>g>The</str<strong>on</strong>g>refore, if <strong>the</strong> heat losses are 40 per cent, <strong>the</strong> cost <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
heating <strong>the</strong> pulp will be RU85 per day, and <strong>the</strong> increased<br />
revenue from gold will be R3420 per day.<br />
394 DECEMBER 1984 JOURNAL OF THE SOUTH AFRICAN INSTITUTE OF MINING AND METALLURGY