Chemistry of High Energy Materials - The Scripps Research Institute
Chemistry of High Energy Materials - The Scripps Research Institute
Chemistry of High Energy Materials - The Scripps Research Institute
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
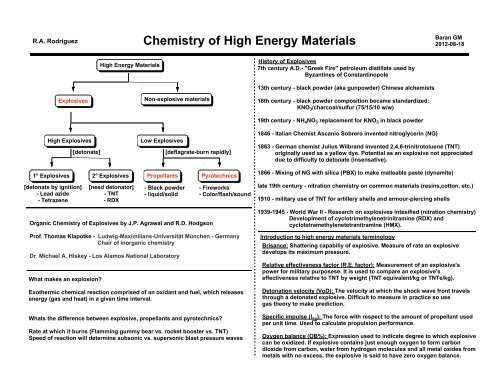
<strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
History <strong>of</strong> Explosives<br />
7th century A.D.- "Greek Fire" petroleum distillate used by<br />
Byzantines <strong>of</strong> Constantinopole<br />
Explosives<br />
<strong>High</strong> Explosives<br />
[detonate]<br />
Non-explosive materials<br />
Low Explosives<br />
[deflagrate-burn rapidly]<br />
13th century - black powder (aka gunpowder) Chinese alchemists<br />
18th century - black powder composition became standardized:<br />
KNO 3 /charcoal/sulfur (75/15/10 w/w)<br />
19th century - NH 4 NO 3 replacement for KNO 3 in black powder<br />
1846 - Italian Chemist Ascanio Sobrero invented nitroglycerin (NG)<br />
1863 - German chemist Julius Wilbrand invented 2,4,6-trinitrotoluene (TNT)<br />
originally used as a yellow dye. Potential as an explosive not appreciated<br />
due to difficulty to detonate (insensative).<br />
1° Explosives 2° Explosives Propellants Pyrotechnics<br />
[detonate by ignition]<br />
- Lead azide<br />
- Tetrazene<br />
[need detonator]<br />
- TNT<br />
- RDX<br />
- Black powder<br />
- liquid/solid<br />
Organic <strong>Chemistry</strong> <strong>of</strong> Explosives by J.P. Agrawal and R.D. Hodgson<br />
- Fireworks<br />
- Color/flash/sound<br />
Pr<strong>of</strong>. Thomas Klapotke - Ludwig-Maximilians-Universität München - Germany<br />
Chair <strong>of</strong> inorganic chemistry<br />
Dr. Michael A. Hiskey - Los Alamos National Laboratory<br />
What makes an explosion?<br />
Exothermic chemical reaction comprised <strong>of</strong> an oxidant and fuel, which releases<br />
energy (gas and heat) in a given time interval.<br />
Whats the difference between explosive, propellants and pyrotechnics?<br />
Rate at which it burns (Flamming gummy bear vs. rocket booster vs. TNT)<br />
Speed <strong>of</strong> reaction will determine subsonic vs. supersonic blast pressure waves<br />
1866 - Mixing <strong>of</strong> NG with silica (PBX) to make malleable paste (dynamite)<br />
late 19th century - nitration chemistry on common materials (resins,cotton, etc.)<br />
1910 - military use <strong>of</strong> TNT for artillery shells and armour-piercing shells<br />
1939-1945 - World War II - <strong>Research</strong> on explosives intesified (nitration chemistry)<br />
Developlment <strong>of</strong> cyclotrimethylenetrinitramine (RDX) and<br />
cyclotetramethylenetetranitramine (HMX).<br />
Introduction to high energy materials terminology<br />
Brisance: Shattering capabiliy <strong>of</strong> explosive. Measure <strong>of</strong> rate an explosive<br />
develops its maximum pressure.<br />
Relative effectiveness factor (R.E. factor): Measurement <strong>of</strong> an explosive's<br />
power for military purposese. It is used to compare an explosive's<br />
effectiveness relative to TNT by weight (TNT equivalent/kg or TNTe/kg).<br />
Detonation velocity (VoD): <strong>The</strong> velocity at which the shock wave front travels<br />
through a detonated explosive. Difficult to measure in practice so use<br />
gas theory to make prediction.<br />
Specific impulse (I sp ): <strong>The</strong> force with respect to the amount <strong>of</strong> propellant used<br />
per unit time. Used to calculate propulsion performance.<br />
Oxygen balance (OB%): Expression used to indicate degree to which explosive<br />
can be oxidized. If explosive contains just enough oxygen to form carbon<br />
dioxide from carbon, water from hydrogen molecules and all metal oxides from<br />
metals with no excess, the explosive is said to have zero oxygen balance.
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
Chemical types/class <strong>of</strong> organic explosives<br />
Aromatic C-nitro compounds<br />
Aliphatic C-nitro compounds<br />
NO<br />
NO 2 NO 2<br />
2<br />
R 2<br />
NO 2<br />
Me<br />
HO<br />
(NO<br />
acidic protons<br />
2 )x<br />
R NO 2 R 1 NO 2<br />
R NO 2 (condensation<br />
1° nitroalkane 2° nitroalkane Terminal<br />
chemistry) O 2 N NO<br />
O 2 N NO 2 O 2 N NO 2<br />
x ≥ 4<br />
2<br />
poor chemical<br />
gem-dinitroalkane<br />
TNT 1,3,5-trinitrobenzene 2,4,6-trinitrophenol<br />
stability<br />
(TNB)<br />
(picric acid)<br />
R 2 NO 2<br />
R 3<br />
R 2<br />
R 2<br />
NO 2<br />
high explosive with Aliphatic O-nitro compounds Aliphatic N-nitro compounds<br />
R 1 R 1 NO 2<br />
R 1 NO high thermal and<br />
2<br />
NO 2<br />
NO 2 Internal chemical stability<br />
O 2 N<br />
NO<br />
3° nitroalkane Trinitromethyl O 2 NO<br />
ONO 2<br />
NO 2<br />
2<br />
gem-dinitroalkane<br />
N N<br />
ONO 2<br />
N<br />
NO 2 Nitroglycerin (VOD=7750 m/s)<br />
heterocycles<br />
O 2 NN NNO 2<br />
N N<br />
N N<br />
NO 2<br />
ONO 2<br />
O 2 N NO 2 O 2 N<br />
NO<br />
O 2<br />
2 NO<br />
RDX (VOD=8440 m/s) HMX (VOD=9110 m/s)<br />
O 2 N NO O 2 2 NN NNO O<br />
2<br />
ONO 2<br />
NO 2<br />
N<br />
O 2 NO<br />
O 2 N<br />
NO 2<br />
N<br />
H<br />
S O<br />
N N<br />
Pentaerythritol tetranitrate<br />
H H<br />
H<br />
(PETN) (VOD=8310 m/s)<br />
2 N NH<br />
Nitro derivatives <strong>of</strong> pyrroles, thiopenes, and furans are not practical explosives:<br />
N,N'-dinitrourea<br />
2<br />
1. heat <strong>of</strong> formation <strong>of</strong>fers no benefits over standard arylene hydrocarbons<br />
(DNU)<br />
nitroguanadine<br />
2. during nitration, these heterocycles are much more prone to oxidation and<br />
NO<br />
O 2 NO 2<br />
ONO 2<br />
acid cat. ring opening compared to arenes<br />
O 2 N NO 2<br />
O 2 N<br />
NO<br />
O ONO N N 2<br />
H-bonding<br />
O 2 NO<br />
H NO 2<br />
2 N NO 2 N<br />
O<br />
2<br />
2<br />
O 2 NN<br />
O NO<br />
NNO 2 N<br />
reduction in<br />
2<br />
N N 2<br />
N NH<br />
N<br />
novel energetic nitrate eseter<br />
sensativity<br />
N<br />
O ONO<br />
N N<br />
2 N<br />
N<br />
O<br />
2<br />
F 2 N NF 2<br />
O 2 N<br />
H<br />
O NO 2 NO<br />
2<br />
NO 2<br />
N<br />
4-amino-3,5-dinitropyrazole 2,4-dinitroimidazole furazans/benz<strong>of</strong>urazans<br />
Si<br />
3,3-bis(difluoroamino)<br />
Hexanitrohexaazaisowurtzitane<br />
(LLM-116)<br />
(2,4-DNI) furoxans/benz<strong>of</strong>uroxans<br />
ONO<br />
octahydro-1,5,7,7-<br />
2<br />
O 2 NO<br />
tetranitro-1,5-diazocine<br />
(HNIW or CL-20)<br />
NH O<br />
2 NH 2<br />
(TNFX)<br />
O 2 N<br />
N<br />
Si-PETN<br />
(VOD=9380 m/s)<br />
O 2 N<br />
NO 2 O 2 N N NO 2<br />
O<br />
N N<br />
N<br />
H H 2 N<br />
H<br />
2 N<br />
N N<br />
N<br />
N<br />
N<br />
N<br />
O 2 N<br />
O<br />
N N<br />
H 2 N N NH 2 H 2 N N NH 2 O<br />
NO<br />
Ar<br />
N<br />
Ar<br />
Ar<br />
2<br />
N N<br />
N<br />
NO<br />
O<br />
N NH<br />
NH N<br />
2<br />
O<br />
2<br />
O<br />
benzotriazoles H<br />
H<br />
1,3,4-oxadiazoles 3-nitro-1,2,4-triazol-5-one<br />
pyridine N-oxide pyrazine N-oxide tetrazine N-oxide<br />
tetrazoles 1,2,4 triazoles<br />
(NTO)
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
Nitration chemistry<br />
<strong>The</strong> nitro gorup whether attached to aromatic or aliphatic carbon, is probably<br />
the most widely studied <strong>of</strong> the functional groups and this is in part attributed<br />
to its use as an 'explosophore' in many energetic materials.<br />
Borgardt et al. Chem Rev 1964. 64, 19 (polynitro functionality)<br />
Routes to C-Nitro functionality<br />
Direct nitration <strong>of</strong> aliphatic and alicyclic hydrocarbons possible in the vapor<br />
phase using HNO 3 or NO 2 (toxic redish-brown gas) at elevated temperatures.<br />
[2+2]<br />
TMS AcONO2 NO 2<br />
TMS<br />
O<br />
N<br />
O<br />
_<br />
OAc<br />
NO 2<br />
+<br />
NO 2<br />
O<br />
N<br />
O<br />
_<br />
OAc<br />
NO 2<br />
NO 2<br />
ONO 2<br />
AcONO 2<br />
Nef<br />
NO 2<br />
O<br />
N<br />
O OAc<br />
O<br />
ONO 2<br />
H<br />
Me<br />
Me<br />
Me<br />
NO 2<br />
Me<br />
Me<br />
Me<br />
O<br />
O<br />
Radical methods<br />
Ph<br />
HNO 3<br />
25%<br />
N 2 O 4<br />
N<br />
N<br />
O<br />
O<br />
- colorless liquid<br />
1 atm NO<br />
DCE/ rt<br />
O 2 N<br />
H<br />
Me<br />
O 2 N<br />
Me<br />
Me<br />
dinitro<br />
Ph<br />
NO 2<br />
NO 2<br />
Me<br />
NO 2<br />
Me<br />
Me<br />
Mukaiyama et al. Chem Lett 1995. 505<br />
H<br />
R<br />
H<br />
R<br />
H<br />
R<br />
NO 2<br />
H<br />
R<br />
NaNO 3 xs,<br />
CAN 2eq<br />
AcOH/CHCl 3<br />
80 - 90%<br />
H<br />
R<br />
H<br />
R<br />
+<br />
Nitration with HNO 3 is difficult<br />
O 2 N<br />
Me<br />
Me<br />
ONO<br />
Me<br />
Me<br />
nitro-nitrite<br />
NO 2<br />
+<br />
Ph<br />
+<br />
O 2 N<br />
Me<br />
Me<br />
ONO 2<br />
Me<br />
Me<br />
nitro-nitrate<br />
OH<br />
76% 23%<br />
Al 2 O 3<br />
NO 2<br />
R<br />
NO 2<br />
H<br />
R<br />
Hwu et al. J Chem Soc Chem Commun 1994. 1425<br />
Taniguchi et al. JOC 2010. 75, 8126<br />
Ph<br />
R<br />
R<br />
Ph<br />
92%<br />
NO 2<br />
Fe(NO 3 ) 3 9H 2 O<br />
FeCl 3<br />
MeCN, reflx<br />
NO 2 BF 4<br />
MeCN<br />
Ph<br />
R<br />
NO 2<br />
Cl<br />
R NO 2<br />
80 - 90%<br />
NHAc<br />
84%<br />
NO 2<br />
alkaline nitration<br />
O 2 N<br />
O 2 N<br />
Br<br />
NO 2<br />
NO 2<br />
O 2 N<br />
2. N 2 O 4<br />
1. NaHMDS<br />
NO 2 74%<br />
1. nBuLi<br />
N<br />
N<br />
CO 2 R<br />
O 2 N<br />
NO 2<br />
2. N 2 O 4 -78 °C<br />
S<br />
S<br />
77%<br />
O 2 N<br />
NItration selectivity on arene/heteroarene<br />
Kakiuchi, et al. Synlett 1999. 901<br />
F 3 C<br />
O<br />
O<br />
O<br />
CF 3<br />
TBAN<br />
F 3 C<br />
O<br />
O<br />
Cl<br />
NO 2<br />
NO 2<br />
O 2 N<br />
NO O 2 N<br />
2<br />
KNO 3<br />
H 2 SO 4<br />
traditional<br />
[NO + 2 ]<br />
44%<br />
TBAN<br />
TFAA<br />
76%<br />
N<br />
N<br />
NO 2<br />
O 2 N NO 2<br />
NO<br />
O 2 N<br />
2 NO 2<br />
N<br />
CO 2 R<br />
N<br />
CO 2 R<br />
Cl<br />
Cl<br />
NO 2<br />
* No rxn in<br />
presence <strong>of</strong><br />
TEMPO
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
1° and 2° nitro compounds<br />
Victor Meyer rxn<br />
- alkyl chlorides too slow<br />
- only good for 1° (2° alkyl halide gives nitrate ester)<br />
- nitrate ester arises from desproportionation <strong>of</strong> silver nitrate acc. by heat/light<br />
R<br />
modified VM (alkali metal nitrites e.g. NaNO 2 ) time and solubility is VIMP<br />
F NO 2<br />
R<br />
R<br />
R N O<br />
R NO R<br />
NaNO 2<br />
X<br />
NO 2 + O<br />
NO 2<br />
fast<br />
slow<br />
R<br />
R<br />
R nitrite ester<br />
R<br />
R<br />
OH<br />
OH<br />
O<br />
H + O N<br />
R<br />
OR<br />
NO 2<br />
HO OH<br />
R<br />
phloroglucinol<br />
O O<br />
Fluorotrinitromethane<br />
NO 2<br />
NO 2 (or)<br />
NO 2<br />
NO 2<br />
NO 2<br />
O 2 N NO 2 F NO 2<br />
NO 2<br />
gem-dinitros from acids<br />
R<br />
R<br />
HNO NO 3<br />
2<br />
NO<br />
HNO 2<br />
CO 2 H<br />
3<br />
R<br />
20 - 30%<br />
MeO 2 C CO 2 H<br />
R NO 2<br />
60 % MeO 2 C NO 2<br />
oxidation <strong>of</strong> amines<br />
NH + 3 Cl -<br />
NO 2<br />
DMDO<br />
C+H 3 N<br />
Acetone<br />
O 2 N<br />
NH 3 +C 91%<br />
NO 2<br />
C+H 3 N<br />
O 2 N<br />
oxidation <strong>of</strong> isocyanates<br />
NCO<br />
NO 2 via amine thus<br />
DMDO<br />
H 2 O essential<br />
Eaton et al. JOC 1988. 5353<br />
OCN<br />
X<br />
+<br />
NaNO 2<br />
DMSO<br />
N<br />
O<br />
OAg<br />
ether<br />
Acetone/H 2 O<br />
85%<br />
+ AgX<br />
R NO 2 R O NO<br />
O 2 N<br />
Kornblum et al. JACS 1956. 78, 1497<br />
O<br />
O<br />
NO 2 H<br />
NOH<br />
1.KOtBu<br />
1. NBS<br />
amyl nitrate<br />
2. H + 2. [O]<br />
3. [H]<br />
NO 2<br />
CF 3 CO 3 H<br />
O<br />
N<br />
OH<br />
S 2 O 8 2-<br />
NO 2<br />
only useful<br />
oxidant<br />
O 2 N NO 2<br />
H +<br />
NO<br />
+ 2 O 2 N NO 2<br />
Kaplan<br />
Shechter Rxn<br />
NO<br />
Synthesis <strong>of</strong> an energetic nitrate ester O 2 NO 2<br />
ONO 2<br />
Chavez, D.E. et al. Angew. 2008, 47, 8307 O 2 NO<br />
ONO<br />
NO 2<br />
2<br />
R 1 R 2<br />
Me<br />
Me<br />
O<br />
Me<br />
NO 2<br />
N<br />
R 1 R 2<br />
N<br />
O<br />
Me<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O Na<br />
O<br />
O O<br />
NaOH N 1. NaNO 2<br />
NO 2<br />
N<br />
O<br />
NH<br />
N<br />
NH<br />
N<br />
Original Target/Route via modified Kaplan Shechter Rxn<br />
N<br />
NH<br />
N<br />
Me<br />
Me<br />
O<br />
O<br />
O<br />
N<br />
N<br />
O<br />
O<br />
O<br />
O 2 N<br />
NO 2<br />
Ag 0<br />
2. AgNO<br />
R 1 R 3 R1 2<br />
R 2<br />
Ag +<br />
nitronate<br />
Ag + Ag<br />
O O<br />
O O<br />
-Ag 0 O O<br />
O N N O<br />
O N N<br />
O N N O<br />
O<br />
R 1 R 2<br />
R<br />
R 1 R 1 R 2<br />
2<br />
N<br />
activation<br />
NH<br />
N<br />
O 2 N<br />
NO 2<br />
+<br />
NO 2<br />
(low yields)<br />
Me<br />
Me<br />
O<br />
O<br />
O<br />
N<br />
Fe<br />
O<br />
NH<br />
N<br />
N<br />
retro<br />
aldol<br />
-CH 2 O<br />
N<br />
NH<br />
[O]<br />
Me<br />
Me<br />
R<br />
R<br />
O<br />
O<br />
O<br />
N<br />
O<br />
NH<br />
N<br />
NO 2<br />
OH<br />
N<br />
NH<br />
N
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
Initial Results: homocoupled product<br />
1.cat. K 3 [Fe(CN) 6 ]<br />
Na 2 S 2 O 8<br />
Routes to O-Nitro functionality<br />
1. 2 - methoxypropene<br />
HO NO 2 cat. H +<br />
Me O O<br />
- Use <strong>of</strong> mixed acids (esterification) and nitrogen oxides described for C-Nitraion<br />
Me<br />
N<br />
2. NaOH<br />
H<br />
Key points:<br />
HO OH<br />
O O<br />
N<br />
N<br />
1. fuming (anhydrous) HNO<br />
X<br />
3 prep: dry air bubbled through anhydrous HNO 3 to<br />
H 2 N<br />
remove any oxides <strong>of</strong> nitrogen present, followed by addition <strong>of</strong> trace urea<br />
NO<br />
Me O<br />
N<br />
N 2<br />
N<br />
O<br />
to remove any nitrous acid present. AKA "white nitric acid"<br />
N N<br />
2. Urea destruction <strong>of</strong> nitrous acid important to avoid violent fume-<strong>of</strong>f<br />
O O O O Me<br />
Me<br />
NO<br />
Me O 2 NH 3. O-nitrations with mixed acids <strong>of</strong> "white nitric acid" above ambient temperatures<br />
N N<br />
O<br />
O Me<br />
NO 2<br />
Me<br />
NH is dangerous and has increase risk <strong>of</strong> explosion<br />
12%<br />
4. anhydrous HNO<br />
O<br />
3 /Ac 2 O- Acetyl nitrate is generally a weak nitrating agent but<br />
in the presence <strong>of</strong> a strong acid like HNO 3 , ionization to nitronium ion occurs<br />
O O O O<br />
NO 2<br />
NO 2<br />
NO 2<br />
Me Me<br />
Me Me<br />
O O<br />
O<br />
O O<br />
2 NO<br />
ONO 2<br />
anh HNO 3 HO<br />
OH 90% HNO<br />
N<br />
3 HO<br />
ONO 2<br />
N<br />
Me O<br />
O<br />
Ac 2 O<br />
Ac<br />
Me O<br />
O<br />
2 O<br />
activation<br />
Me<br />
Me<br />
Me<br />
Me<br />
O 2 NO<br />
ONO 2 90% HO<br />
OH 70% O 2 NO<br />
OH<br />
O<br />
O Me<br />
- CN - O<br />
O Me<br />
N<br />
N<br />
NO 2 NO<br />
O O<br />
2<br />
NO 2<br />
O O<br />
shock sensative<br />
NC CN<br />
NaO 3 S<br />
NC CN<br />
Fe<br />
NC Fe CN +CN -<br />
Transfer nitration (neutral conditions - good for acid sensative alcohols)<br />
O O<br />
NC Fe OSO 3 Na<br />
Me<br />
-NaSO<br />
-<br />
NC CN<br />
SO 3 Na<br />
4<br />
NC CN<br />
BF<br />
- 4<br />
Me<br />
NO<br />
O<br />
NO<br />
Me O 2<br />
2 NO 2<br />
ONO<br />
Me N Me<br />
Olah G.A. et al JOC, 1965. 30, 3373<br />
m.p. 86 °C<br />
2 1.HCl, MeOH<br />
O<br />
OH<br />
ONO<br />
NO 2<br />
Me<br />
Me<br />
2 eq 2<br />
Det.Temp<br />
+<br />
BF<br />
- 4<br />
O 2 NO<br />
ONO 2<br />
2. Ac 2 O/HNO<br />
works for 1°, 2°, 3° alcohols<br />
140 °C<br />
NO 3 O<br />
O Me<br />
MeCN<br />
Me N Me<br />
2<br />
NO 2<br />
OH quant yield ONO 2<br />
72% (2 steps)<br />
H<br />
65% optimized<br />
in situ halide displacement with AgNO 3<br />
ONO 2<br />
Low yields for 2°<br />
O 2 NO<br />
PPh 3 , I 2 AgNO 3 HgNO 3 can be used for 2° and<br />
R OH<br />
R I<br />
R ONO 2 3° alkyl halide displacements<br />
ONO 2<br />
O 2 NO<br />
decomposition <strong>of</strong> nitrocarbonates (very mild, rt or reflux MeCN)<br />
20,164 MPH!!<br />
comparable<br />
stability to PETN<br />
O 2 N<br />
N<br />
NO 2<br />
N<br />
RO<br />
O<br />
Cl<br />
AgNO 3<br />
Py<br />
RO<br />
O<br />
ONO 2<br />
-AgCl<br />
-CO 2<br />
ring opening <strong>of</strong> strained oxygen heterocycles<br />
R ONO 2<br />
80%<br />
H 2 O<br />
N N<br />
N 2 O 4<br />
O 2 N NO 2<br />
ONO<br />
O (or)<br />
CH 2 Cl<br />
ONO 2<br />
CHEETAH calculates<br />
2<br />
[O]<br />
as powerful as HMX<br />
O<br />
N 2 O 5<br />
HO<br />
O 2 NO<br />
ONO 2<br />
ONO 2
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
selective O-nitrations<br />
HO<br />
OH<br />
nitrodesilylation<br />
OH<br />
1 eq SOCl(NO 3 )<br />
2 eq SOCl(NO 3 )<br />
70%<br />
N 2 O 5<br />
RO SiR 3<br />
CH 2 Cl 2<br />
RO ONO 2 + O 2 NO SiR 3<br />
Routes to N-Nitro functionality<br />
65%<br />
O 2 NO<br />
3 eq SOCl(NO 3 ) O 2 NO<br />
100%<br />
ONO 2<br />
ONO 2<br />
deamination<br />
NO 2 F<br />
R NH 2<br />
MeCN<br />
R ONO 2<br />
- Compounds resulting from nitration <strong>of</strong> nitrogen are <strong>of</strong> far less use for<br />
mainstream organic synthesis. However the N-NO 2 group is an important<br />
'explosophore' and is present in many enrgetic materials<br />
HN<br />
N<br />
O<br />
O<br />
OH<br />
H +<br />
N R OH + N 2 O<br />
HO<br />
OH<br />
OH<br />
ONO 2<br />
ONO 2<br />
- Direct nitration <strong>of</strong> a 1° amine to a nitramine using HNO 3 /mixed acids is not<br />
possible due to instability <strong>of</strong> the tautomeric isonitramine in strongly acidic<br />
conditions. 2° amines are more stable and can undergo electrophilic nitration<br />
using HNO 3 /Ac 2 O<br />
2° w/ HNO 3 /Ac 2 O<br />
R<br />
NC<br />
NO 2<br />
N<br />
NO 2<br />
N<br />
CN<br />
NO 2<br />
Me N Me<br />
93% 22% 6%<br />
analines - must contain one or more nitro groups on the aromatic ring<br />
How to get around this problem??<br />
- synthesis via condensation chemistry (Mannich, 1,4 addition, etc)<br />
What about if need more direct method??<br />
-non-acidic nitrating reagents (nucleophilic nitration)<br />
R NH 2<br />
R<br />
nBuLi<br />
N<br />
O<br />
-78 °C R NHLi Et ONO 2<br />
R N N<br />
OLi<br />
1. Na<br />
-<br />
O<br />
Ar NH<br />
Ar NH 2 2 Na +<br />
Ar N N<br />
2. EtONO 2<br />
EtONO 2<br />
O<br />
H +<br />
ONa<br />
R NH<br />
NO 2<br />
H + NO 2<br />
Ar NH<br />
-chloride ion catalysis<br />
anhydrous ZnCl 2 , hydrochloride salt <strong>of</strong> amine, or dissolved HCl(g) can serve<br />
as a source <strong>of</strong> electropositive chloride under the oxidizing conditions <strong>of</strong> nitration<br />
NC<br />
2 HCl + 2HNO 3 + 3Ac 2 O 2AcOCl + N 2 O 3 + 4AcOH<br />
AcOCl + R 2 NH R 2 NCl + AcOH<br />
R 2 NCl + HNO 3 + Ac 2 O R 2 NNO 2 + AcOCl + AcOH<br />
NO 2<br />
N<br />
CN<br />
93%<br />
HNO 3 /Ac 2 O<br />
(w/o chloride)<br />
R NH 2<br />
2 HOCl<br />
NC<br />
- Nitrolysis <strong>of</strong> fully substituted nitrogen<br />
R<br />
N<br />
CN<br />
Wright et al. Can. J. Res. 1948. 26B, 294<br />
HNO 3 /Ac 2 O<br />
R = Cl - (N + )<br />
HNO 3 /Ac 2 O<br />
R = H<br />
Ease <strong>of</strong> alkyl nitrolysis depends on stability <strong>of</strong> the resulting cation:<br />
benzyl, tertiary (t-Bu), etc...<br />
X<br />
NC<br />
NO<br />
HNO<br />
R NCl 3 /Ac 2 O 2 NaHSO 3 (aq)<br />
2 R N<br />
rupture <strong>of</strong> C−N bond leading to formation on N−NO 2<br />
R 1<br />
A<br />
O<br />
N<br />
R 2<br />
Path A<br />
O 2 N<br />
N R 2<br />
R 2<br />
+<br />
Cl<br />
R 1<br />
CO 2 H<br />
R 2 Path B<br />
NO 2<br />
B<br />
R 1 N + R 2 OH<br />
R 2<br />
Nitramine formation via nitrolysis possible from:<br />
R 1<br />
R 1<br />
NO<br />
+ 2<br />
N<br />
Ph<br />
R 1<br />
R 1<br />
NO 2<br />
N<br />
Ph<br />
O<br />
R 2 N NR 2<br />
R 2 N SiR 3<br />
R 1 N<br />
R 2 N NO<br />
O<br />
R 2<br />
NO 2<br />
N<br />
70%<br />
R NHNO 2<br />
R 2<br />
CN<br />
- carbamate<br />
- urea<br />
- formamide<br />
- acetamide<br />
- sulfonamide
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
Synthesis <strong>of</strong> Hexanitrohexaazaisowurtzitane (HNIW)<br />
- Many nitramines are more powerful than aromatic C-nitro compounds and have<br />
high brisance and high chemical stability and low sensativity to impact and<br />
friction compared to nitrate ester explosives. This is why they are <strong>of</strong> interest to<br />
military applications.<br />
Bn<br />
Bn<br />
Bn Bn<br />
O<br />
Bn<br />
NH 2<br />
MeCN/H<br />
H<br />
2 O N N N<br />
N N<br />
Bn<br />
Bn<br />
N N<br />
+ H<br />
Ph<br />
cat. H +<br />
N<br />
O<br />
N N<br />
N N<br />
2 eq.<br />
Bn<br />
Bn<br />
Bn Bn<br />
Bn<br />
H<br />
Ph O<br />
2 ,<br />
NO<br />
+<br />
[O] Ph<br />
Me [Pd] X 2<br />
O Ac 2 O<br />
X<br />
N<br />
N<br />
N<br />
decomp. messy. Nitration<br />
<strong>of</strong> aromatic rings<br />
Ph O<br />
CrO3 Ph<br />
N<br />
N<br />
Ac<br />
O NO 2 N<br />
2<br />
1. N<br />
N 2 O 4<br />
N N<br />
Ac 2. HNO O 2 N<br />
N<br />
3 /H 2 SO 4<br />
N N<br />
NO 2<br />
via nitroso<br />
N 93%<br />
N N<br />
Bn<br />
O 2 N NO<br />
DANGER!<br />
2<br />
99% HNO 3<br />
Bn<br />
Bn<br />
Ac<br />
N N<br />
N<br />
Bn<br />
Bn<br />
N N H 2 , Pd(OAc) Ac 2 N<br />
Ac<br />
N N 2 O, PhBr cat.<br />
N<br />
Bn<br />
Bn 65% Bn<br />
H 2 ,<br />
O NO 2 N<br />
2<br />
[Pd]<br />
N N<br />
O 2 N<br />
Ac<br />
Ac<br />
N N NO 2<br />
Ac<br />
N N 1. HCO<br />
Ac<br />
2 H<br />
Ac<br />
Ac<br />
N N<br />
N N<br />
O 2 N NO 2. Ph, Δ<br />
2<br />
HN NH -H 2 O OHCN<br />
HNIW aka CL-20<br />
- explosive/propellant (low smoke-emission)<br />
- most powerful to date (better oxidizer-to-fuel than RDX/HMX)<br />
- first prep by Nielsen 1987 Naval Air Warefare<br />
- pilot plant in 1990 for 200 kg in China Lake facility<br />
- unmatched performance in specific impulse,<br />
burn rate, detonation velocity (9.38 km/s = 21,000 MPH!!)<br />
- highest density than any other explosive (d = 2.044 g/cm 3 )<br />
- thermally stable (250 -260 °C) but sensative to mechanical stress<br />
still greater stability than nitrocellulose, PETN and others.<br />
- 4 different polymprphs with different densities/properties<br />
N<br />
N<br />
Ac<br />
N<br />
Ac<br />
N<br />
NCHO<br />
Syntheses <strong>of</strong> some nitramine explosives<br />
NNs<br />
HO<br />
O 2 N<br />
90%<br />
NH 2 NH 2 NsCl, NNs NNs<br />
K 2 CO 3 (aq)<br />
O<br />
OH<br />
NH 2<br />
NO 2<br />
NNs<br />
F 2 NSO 3 H,<br />
HNF 2 , H 2 SO 4 ,<br />
CFCl 3<br />
OH<br />
O 2 N<br />
OH<br />
NO 2<br />
N<br />
NO 2<br />
TNAZ<br />
Ns<br />
95%<br />
1. HNO 3 /NH 4 NO 3<br />
urea, 33%<br />
2. H 2 SO 4<br />
92%<br />
O 2 N<br />
HBr-AcOH<br />
160 °C<br />
72%<br />
NO 2<br />
NNs<br />
N N Ns<br />
F 2 N NF 2<br />
Br<br />
OH<br />
O<br />
1. NaHCO 3 , NaI<br />
DMSO, 100 °C<br />
NH 3 + Br -<br />
Br<br />
NOH<br />
Br<br />
2. NaNO 2 , NaOH<br />
K 3 Fe(CN) 6 , K 2 S 2 O 8<br />
29% (2 steps)<br />
O<br />
O 2 N<br />
1. CrO 3 , AcOH<br />
2. HOCH 2 CH 2 OH<br />
TsOH<br />
NNs<br />
HNO 3 /SbF 6<br />
CF 3 SO 3 H<br />
82% (2 steps)<br />
1. O 3 , CH 2 Cl 2<br />
2. DMS<br />
3. NH 2 OH/NaOAc<br />
86% (3 steps)<br />
O 2 N<br />
O 2 N<br />
O<br />
NNs<br />
NO 2<br />
O<br />
N N NO 2<br />
F 2 N NF 2<br />
O<br />
O<br />
NNs<br />
TNFX<br />
(3,3 bis(difluoroamino)octahydro<br />
1,5,7,7 tetranitro 1,5 diazocine)<br />
NaOH, 80 °C<br />
60 mmHg<br />
CH 2 Br<br />
N<br />
NO 2<br />
N<br />
Br<br />
HNO 3 /TFAA<br />
81%<br />
NNs<br />
1. NaNO 2 (aq)<br />
2. HCl (aq)<br />
10%<br />
O 2 N<br />
N<br />
NO<br />
NNs<br />
Br Br 76%<br />
K 2 CO 3<br />
CH 2 Br
R.A. Rodriguez<br />
<strong>Chemistry</strong> <strong>of</strong> <strong>High</strong> <strong>Energy</strong> <strong>Materials</strong><br />
Baran GM<br />
2012-08-18<br />
N 2 O 5<br />
No single nitrating agent is as diverse and versatile<br />
It is considered as the future for energetic materials synthesis<br />
non-acidic nitrating reagents (neutral)<br />
1° amines/nitramines leads to deamination and formation <strong>of</strong> nitrate ester<br />
by-product but analines are successful.<br />
O O nitronium nitrate salt<br />
N 2 O 5<br />
O<br />
N<br />
O N<br />
[NO + 2 ][NO - 3 ]<br />
O<br />
sublime slightly above rt<br />
condition: chlorinated solvents<br />
- clean and selective<br />
- non-oxidizing &n non-acidic<br />
polar<br />
- adopts two structures<br />
depending on condition<br />
condition: anhyd. HNO 3<br />
- powerful but acidic and non-selective<br />
- 1st prepared over 150 years ago but due to difficult prep and low thermal<br />
stability (require -60 °C long term storage) received little attention.<br />
- Environmental restrictions and push for green chemistry sparked interest<br />
Advantage: Rxns are very clean<br />
- faster and less exothermic (due to absence <strong>of</strong> oxidation byproducts)<br />
- high yields<br />
- simple isolation<br />
- non-acidic conditions possible with this reagent (compared to mixed acids)<br />
rt<br />
N 2 O Stable for 2 weeks at -20 °C<br />
5 2 N 2 O 4 + O 2<br />
Stable for up to 1 yr at -60 °C<br />
synthesis <strong>of</strong> TNT under mild conditions<br />
Me<br />
Me<br />
NO 2<br />
N 2 O 5 /HNO 3<br />
32 °C,<br />
quant yield<br />
NO 2<br />
N-nitrations <strong>of</strong> ureas<br />
H H<br />
N N<br />
HNO 3<br />
O<br />
O<br />
H<br />
N N<br />
2 SO 4<br />
H H<br />
O-nitrations <strong>of</strong> polyols<br />
HO<br />
OH<br />
OH<br />
OH<br />
OH<br />
O 2 N<br />
O<br />
O 2 N<br />
NO 2<br />
N<br />
N<br />
H<br />
OH N 2O 5 , CCl 4<br />
0 °C<br />
quant yield<br />
NO 2<br />
H<br />
N<br />
N<br />
NO 2<br />
O 2 NO<br />
O<br />
No explosion hazard<br />
HNO 3<br />
P 2 O 5<br />
O<br />
O 2 N<br />
O 2 N<br />
N<br />
N<br />
ONO 2 ONO 2<br />
ONO 2<br />
ONO 2 ONO 2<br />
N<br />
N<br />
NO 2<br />
NO 2<br />
O<br />
Preparation <strong>of</strong> N 2 O 5 [Deville 1849]<br />
O<br />
AgNO 3 + Cl 2 (g)<br />
O N +<br />
Cl<br />
AgNO 3 N 2 O 5<br />
Dehydration <strong>of</strong> nitric acid<br />
HNO 3 + P 2 O 5 N 2 O 5 + H 3 PO 4<br />
Δ<br />
N 2 O 4<br />
- isolation by sublimation and collection trap at -78 °C<br />
- stream <strong>of</strong> ozone needed to avoid collection on N 2 O 4<br />
- if don't care about acidity, can use HNO 3 /P 2 O 5 mixture directly (no ozone stream)<br />
O 3<br />
N 2 O 5<br />
Process chemist at Defense and Evaluation <strong>Research</strong> Agency (DERA) in the UK<br />
Development <strong>of</strong> a flow process: Using commercial ozonizer to generate 5 - 10%<br />
mix <strong>of</strong> ozone in oxygen and mixed in flow with N 2 O 4. N 2 O 5 is trapped in solid<br />
condenser tubes (cooled by dry ice/acetone).<br />
Explosives in JACS: Sila explosives<br />
O 2 NO<br />
O 2 NO ONO 2<br />
- Used in WW I<br />
PETN ONO 2<br />
- Det. Velocity 8,400 m/s<br />
- d= 1.7 g/cm 3<br />
Silicon analogue <strong>of</strong> PETN<br />
O 2 NO<br />
O 2 NO Si ONO 2<br />
VERY<br />
low e - dens<br />
ONO 2<br />
Klapotke T.M. JACS 2007. 129, 6908<br />
- One <strong>of</strong> most high energy explosives known<br />
- more shock sensative than TNT. used as booster mix<br />
- Europe marketed as lentonitrat (vasodilator) like NG<br />
"<strong>The</strong> crystalline compound exploded on every<br />
occasion upon contact with Teflon spatula...<br />
Solutions in diethyl ether exploded upon the slighest<br />
evaporation <strong>of</strong> the solvent."<br />
Si(CH 2 OAc) 4 Si(CH 2 Cl) 4<br />
Why does Si-PETN have drastically increased sensativity?<br />
<strong>The</strong>oretical: electrostatic potential:<br />
1. Surface electrostatic potential, in general, is related to the sensitivity <strong>of</strong> the bulk<br />
2. <strong>The</strong> more evenly distributed the electrostatic potential is over the surface <strong>of</strong> a<br />
molecule, the more stable it is to impact.<br />
O 2 NO<br />
O 2 NO<br />
O 2 NO<br />
VERY high e -<br />
dens<br />
Si<br />
O<br />
N<br />
O<br />
O