Innate immune response to viral infection Cytokine - sepeap
Innate immune response to viral infection Cytokine - sepeap
Innate immune response to viral infection Cytokine - sepeap
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ARTICLE IN PRESS<br />
2 S. Koyama et al. / Cy<strong>to</strong>kine xxx (2008) xxx–xxx<br />
virus <strong>infection</strong>. TLR3 is expressed more widely, but is mainly expressed<br />
on conventional DCs (cDCs) [5]. The function of TLR8 is<br />
not clearly known yet.<br />
Recently endoplasmic reticulum (ER) protein UNC93B1 turned<br />
out <strong>to</strong> be essential for trafficking of TLR7 and TLR9 from ER <strong>to</strong><br />
endosome [6], but what triggers this TLR-trafficking from ER <strong>to</strong><br />
endosome before <strong>viral</strong> recognition by TLRs remains <strong>to</strong> be elucidated.<br />
A recent report suggests that TLR-sorting <strong>to</strong> the ligand<br />
may utilize au<strong>to</strong>phagy, which is a cellular process for recycling<br />
cy<strong>to</strong>solic compartments and, in the case of pDC, eliminating exogenous<br />
pathogen. Although cy<strong>to</strong>plasmic vesicular somatitis viruses<br />
(VSV) in pDC are thought <strong>to</strong> be trapped by au<strong>to</strong>phagosome<br />
expressing ATG5 and detected by TLR7 in lysosomes for a type I<br />
IFN <strong>response</strong> [7], in non-<strong>immune</strong> cells ATG5 suppresses the type<br />
I IFN <strong>response</strong> by interaction with caspase recruitment domains<br />
(CARDs) presented by RIG-I and IFN-b promoter stimula<strong>to</strong>r-1<br />
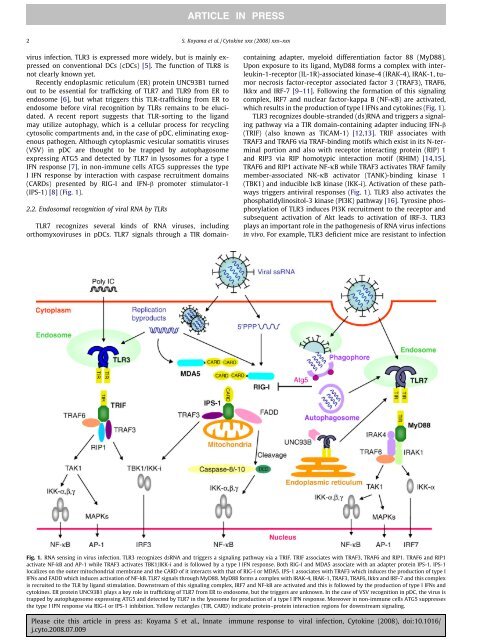
(IPS-1) [8] (Fig. 1).<br />
2.2. Endosomal recognition of <strong>viral</strong> RNA by TLRs<br />
TLR7 recognizes several kinds of RNA viruses, including<br />
orthomyxoviruses in pDCs. TLR7 signals through a TIR domaincontaining<br />
adapter, myeloid differentiation fac<strong>to</strong>r 88 (MyD88).<br />
Upon exposure <strong>to</strong> its ligand, MyD88 forms a complex with interleukin-1-recep<strong>to</strong>r<br />
(IL-1R)-associated kinase-4 (IRAK-4), IRAK-1, tumor<br />
necrosis fac<strong>to</strong>r-recep<strong>to</strong>r associated fac<strong>to</strong>r 3 (TRAF3), TRAF6,<br />
Ikka and IRF-7 [9–11]. Following the formation of this signaling<br />
complex, IRF7 and nuclear fac<strong>to</strong>r-kappa B (NF-jB) are activated,<br />
which results in the production of type I IFNs and cy<strong>to</strong>kines (Fig. 1).<br />
TLR3 recognizes double-stranded (ds)RNA and triggers a signaling<br />
pathway via a TIR domain-containing adapter inducing IFN-b<br />
(TRIF) (also known as TICAM-1) [12,13]. TRIF associates with<br />
TRAF3 and TRAF6 via TRAF-binding motifs which exist in its N-terminal<br />
portion and also with recep<strong>to</strong>r interacting protein (RIP) 1<br />
and RIP3 via RIP homotypic interaction motif (RHIM) [14,15].<br />
TRAF6 and RIP1 activate NF-jB while TRAF3 activates TRAF family<br />
member-associated NK-jB activa<strong>to</strong>r (TANK)-binding kinase 1<br />
(TBK1) and inducible IjB kinase (IKK-i). Activation of these pathways<br />
triggers anti<strong>viral</strong> <strong>response</strong>s (Fig. 1). TLR3 also activates the<br />
phosphatidylinosi<strong>to</strong>l-3 kinase (PI3K) pathway [16]. Tyrosine phosphorylation<br />
of TLR3 induces PI3K recruitment <strong>to</strong> the recep<strong>to</strong>r and<br />
subsequent activation of Akt leads <strong>to</strong> activation of IRF-3. TLR3<br />
plays an important role in the pathogenesis of RNA virus <strong>infection</strong>s<br />
in vivo. For example, TLR3 deficient mice are resistant <strong>to</strong> <strong>infection</strong><br />
Fig. 1. RNA sensing in virus <strong>infection</strong>. TLR3 recognizes dsRNA and triggers a signaling pathway via a TRIF. TRIF associates with TRAF3, TRAF6 and RIP1. TRAF6 and RIP1<br />
activate NF-kB and AP-1 while TRAF3 activates TBK1/IKK-i and is followed by a type I IFN <strong>response</strong>. Both RIG-I and MDA5 associate with an adapter protein IPS-1. IPS-1<br />
localizes on the outer mi<strong>to</strong>chondrial membrane and the CARD of it interacts with that of RIG-I or MDA5. IPS-1 associates with TRAF3 which induces the production of type I<br />
IFNs and FADD which induces activation of NF-kB. TLR7 signals through MyD88. MyD88 forms a complex with IRAK-4, IRAK-1, TRAF3, TRAF6, Ikka and IRF-7 and this complex<br />
is recruited <strong>to</strong> the TLR by ligand stimulation. Downstream of this signaling complex, IRF7 and NF-kB are activated and this is followed by the production of type I IFNs and<br />
cy<strong>to</strong>kines. ER protein UNC93B1 plays a key role in trafficking of TLR7 from ER <strong>to</strong> endosome, but the triggers are unknown. In the case of VSV recognition in pDC, the virus is<br />
trapped by au<strong>to</strong>phagosome expressing ATG5 and detected by TLR7 in the lysosome for production of a type I IFN <strong>response</strong>. Moreover in non-<strong>immune</strong> cells ATG5 suppresses<br />
the type I IFN <strong>response</strong> via RIG-I or IPS-1 inhibition. Yellow rectangles (TIR, CARD) indicate protein–protein interaction regions for downstream signaling.<br />
Please cite this article in press as: Koyama S et al., <strong>Innate</strong> <strong>immune</strong> <strong>response</strong> <strong>to</strong> <strong>viral</strong> <strong>infection</strong>, Cy<strong>to</strong>kine (2008), doi:10.1016/<br />
j.cy<strong>to</strong>.2008.07.009