Inspection Report - Southern Railway
Inspection Report - Southern Railway
Inspection Report - Southern Railway
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
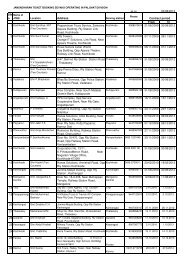
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm Tablets (India) Limited,<br />
Jhaver Centre, 72<br />
Marshalls Road,<br />
Chennai 600 008<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case Names of such product to be given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
Tablets (India) Limited,<br />
Jhaver Centre, 72<br />
Marshalls Road,<br />
Chennai 600 008<br />
Allianz Biosciences<br />
Private Limited, 55/1,2 &<br />
3, Thiruvandar Koil<br />
Street, Whirlpool Road,<br />
Pondicherry 605 102<br />
Yes<br />
Yes<br />
Yes<br />
2004-05 8,720,71 lakhs<br />
2005-06 10,556.27 lakhs<br />
2006-07 11,692.78 lakhs<br />
9 Company turn over figure for last 3 years 2004-05 8,720,71 lakhs<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2005-06 10,556.27 lakhs<br />
2006-07 11,692.78 lakhs<br />
2004-05 164.18 lakhs<br />
2005-06 213.61 lakhs<br />
2006-07 314.86 lakhs
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research<br />
products/formulations developed by firm<br />
enclosed as annexure I<br />
12 Names of products for which firm is<br />
original manufacturer<br />
enclosed as annexure I<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or by<br />
any other agency<br />
15 a) Names of imported drug items supplied<br />
by firm. List of developed countries where<br />
item is approved and supplied<br />
b) The source of manufactured raw/finished<br />
products for quality report<br />
c) Authorization letter by OEM abroad for<br />
local agent<br />
16 Other conditions<br />
No<br />
enclosed as annexure J<br />
Nil<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified staff yes<br />
g) Quality control inhouse lab/outsource yes<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
17 Date of inspection 03.11.2008<br />
18 i) Place of inspection Pondicherry<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
yes<br />
Mr Senthil,<br />
Chief Production<br />
Manager,
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market<br />
applied by firms for registgration are<br />
in the same brand name<br />
available in open market with some brand<br />
in southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr. N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) K.Satyababu,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr. A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm Baxter (India) Pvt Ltd, 2nd<br />
Floor, Tower C, Building<br />
no.8, DLF Cyber City, DLF<br />
Phase II, Gurgaon 122 002,<br />
Haryana<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
Baxter (India) Pvt Ltd, 2nd<br />
Floor, Tower C, Building<br />
no.8, DLF Cyber City, DLF<br />
Phase II, Gurgaon 122 002,<br />
Haryana<br />
Baxter (India) Pvt Ltd, 69/72,<br />
SIDCO Pharmaceuticals<br />
Complex, Alathur, 603<br />
110<br />
Yes<br />
Yes<br />
Yes<br />
2004-05 17631.89 lakhs<br />
2005-06 20315.63 lakhs<br />
2006-07 26151.65 lakhs<br />
9 Company turn over figure for last 3 years 2004-05 17631.89 lakhs<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2005-06 20315.63 lakhs<br />
2006-07 26151.65 lakhs<br />
Not available
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F. 150 to 182<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
F 185 to F 193<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
16 Other conditions<br />
No<br />
Nil<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection<br />
18 i) Place of inspection Alathur<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
yes
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr. N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) K.Satyababu,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr. A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm Tablets (India) Limited,<br />
Jhaver Centre, 72<br />
Marshalls Road,<br />
Chennai 600 008<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
Tablets (India) Limited,<br />
Jhaver Centre, 72<br />
Marshalls Road,<br />
Chennai 600 008<br />
Allianz Biosciences<br />
Private Limited, 55/1,2 &<br />
3, Thiruvandar Koil<br />
Street, Whirlpool Road,<br />
Pondicherry 605 102<br />
Yes<br />
Yes<br />
Yes<br />
2004-05 8,720,71 lakhs<br />
2005-06 10,556.27 lakhs<br />
2006-07 11,692.78 lakhs<br />
9 Company turn over figure for last 3 years 2004-05 8,720,71 lakhs<br />
Yes<br />
yes<br />
2005-06 10,556.27 lakhs<br />
2006-07 11,692.78 lakhs
Sl.No. Description Remarks<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
2004-05 164.18 lakhs<br />
2005-06 213.61 lakhs<br />
2006-07 314.86 lakhs<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research<br />
products/formulations developed by firm<br />
enclosed as annexure I<br />
12 Names of products for which firm is<br />
original manufacturer<br />
enclosed as annexure I<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or by<br />
any other agency<br />
15 a) Names of imported drug items supplied<br />
by firm. List of developed countries where<br />
item is approved and supplied<br />
b) The source of manufactured raw/finished<br />
products for quality report<br />
c) Authorization letter by OEM abroad for<br />
local agent<br />
16 Other conditions<br />
No<br />
enclosed as annexure J<br />
Nil<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified staff yes<br />
g) Quality control inhouse lab/outsource yes<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
17 Date of inspection 03.11.2008<br />
18 i) Place of inspection Pondicherry<br />
ii) Storage facility holding time for<br />
manufactured products<br />
yes
Sl.No. Description Remarks<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
Mr Senthil,<br />
Chief Production<br />
Manager,<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market<br />
applied by firms for registgration are<br />
in the same brand name<br />
available in open market with some brand<br />
in southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr. N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) K.Satyababu,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr. A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm Glaxosmithkline<br />
Pharmaceuticals Limited,<br />
262, Golden Towers (II floor),<br />
Royapettah, Chennai 600 014<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
Glaxosmithkline<br />
Pharmaceuticals Limited,<br />
262, Golden Towers (II floor),<br />
Royapettah, Chennai 600 014<br />
Orchid Healthcare (A<br />
division of Orchid Chemicals<br />
and Pharmaceuticals<br />
Limited) B 77, SIDCO<br />
Industrial Estate, Alathur<br />
Yes<br />
Yes<br />
Yes<br />
2004-05 1575.89 crores<br />
2005-06 1677.57 crores<br />
2006-07 1712.87 crores<br />
9 Company turn over figure for last 3 years 2004-05 1575.89 crores<br />
10 R&D faciRAVIKUMAR.M [ MRK ]lities<br />
with firm. Annual expenditure in R&D in<br />
last 3 years<br />
Yes<br />
yes<br />
2005-06 1677.57 crores<br />
2006-07 1712.87 crores<br />
Not available
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F 2 to 38<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
F 452<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
16 Other conditions<br />
No<br />
F 423-437<br />
Available<br />
Available<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection<br />
18 i) Place of inspection Alathur<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designatioRAVIKUMAR.M [<br />
MRK ]n of the company official<br />
accompanying for inspection<br />
yes
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr. N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) K.Satyababu,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr. A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm Micro Labs Limited<br />
27, Race Course,<br />
bangalore 560 001<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
Yes<br />
# 92, Sipcot Industrial<br />
Complex, Hosur 635 126<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
Yes<br />
Yes<br />
2004-05 533.74 crores<br />
2005-06 707.21 crores<br />
2006-07 970.10 crores<br />
9 Company turn over figure for last 3 years 2004-05 533.74 crores<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2005-06 707.21 crores<br />
2006-07 970.10 crores<br />
Not available
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F 7 to F 11<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
F 12 to F 15<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
16 Other conditions<br />
No<br />
F 423-437<br />
Available<br />
Available<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection<br />
18 i) Place of inspection Bangalore<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
yes<br />
ANANDAKUMAR.S [ SAK ]
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) Dr K.Satyababu,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm Arvind Remedies Limited<br />
190 Poonamalee High<br />
Road Chennai 600 084<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
Shed no. 38, 39 & 40 ,<br />
SIDCO Industrial Estate,<br />
Kakkalur, Thiruvallur<br />
District<br />
Pincode 602 003<br />
Yes<br />
Yes<br />
Yes<br />
2005-06 165.08 crores<br />
2006-06 179.73 crores<br />
2007-08 201.51 crores<br />
9 Company turn over figure for last 3 years 2005-06 165.08 crores<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2006-06 179.73 crores<br />
2007-08 201.51 crores<br />
Not available
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F 4 to F 43<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
16 Other conditions<br />
No<br />
F 423-437<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection 26.02.2010<br />
18 i) Place of inspection Kakkalur, Thiruvallur Dis<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
yes
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) Dr K.Satyababu,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm <strong>Southern</strong> Petrochemical<br />
Industries Corpn Ltd<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
Yes<br />
SPIC House, No.88,<br />
Mount Road, Guindy,<br />
Chennai 600 032<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
SPIC Ltd, Pharmaceuticals<br />
Division, Plot no.5, NH-7,<br />
Maraimalainagar 603209<br />
Kancheepuram District<br />
Yes<br />
Yes<br />
Yes<br />
2005-06 7926.35 lakhs<br />
2006-06 11199.46 lakhs<br />
2007-08 11145.27 lakhs<br />
9 Company turn over figure for last 3 years 2005-06 7926.35 lakhs<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2006-06 11199.46 lakhs<br />
2007-08 11145.27 lakhs<br />
Not available
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F 4 to F 43<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
1632- Other conditions<br />
No<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection 18.06.2010<br />
18 i) Place of inspection Maraimalainagar,<br />
Kancheepuram District<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
yes
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) Dr Prasannakumar<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
REPORT ON APPLICATION SUBMITTED BY <strong>Southern</strong> Petrochemical<br />
Industries Corporation Ltd (Pharmaceutical Division) FOR<br />
REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : <strong>Southern</strong> Petrochemical Industries<br />
Corporation Ltd (Pharmaceutical<br />
Division)<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: SPIC House, No.88, Mount Road,<br />
Guindy, Chennai 600 032<br />
: SPIC Limited – Pharmaceutical Divisionl<br />
Plot no.5, NH 7, Maraimalainagar-<br />
603209 – Kancheepuram District,<br />
Tamilnadu<br />
“ F 10 to 86<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
MANDATORY CONDITIONS<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
3 WHO GMP certificate Yes –<br />
available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
F 94<br />
F 1 to 5<br />
F 7 to 7D<br />
F 9<br />
F 92<br />
F 95<br />
Desirable conditions<br />
1 ISO 9000 certification Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
Yes -<br />
available<br />
F 89 to 91e<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes –<br />
available<br />
Registered in<br />
Central<br />
<strong>Railway</strong><br />
F 89 to 91e<br />
F 87-88<br />
a<br />
b<br />
c<br />
d<br />
II. For drugs manufactured abroad and prolposed for suply by the firm,<br />
the following informations are MANDATORILY REQUIRED: NOT<br />
APPLICABLE<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected<br />
b. Does the firm qualify for registration<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm M/s Orchid Health Care<br />
(A division of Orchid<br />
Chemicals and<br />
Pharmaceuticals Ltd)<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
Yes<br />
Orchid Towers, No. 313,<br />
Valluvarkottam High<br />
Road, Nungambakkam,<br />
Chennai 600 034<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
M/s Orchid Health Care<br />
B 77, SIDCO Industrial<br />
Estate, Alathur 603110,<br />
Kancheepuram District<br />
Yes<br />
Yes<br />
Yes<br />
2007-08 120287.72 lakhs<br />
2008-09 116835.64 lakhs<br />
2009-10 121385.21 lakhs<br />
9 Company turn over figure for last 3 years 2005-06 7926.35 lakhs<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2006-06 11199.46 lakhs<br />
2007-08 11145.27 lakhs
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F 21<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
1632- Other conditions<br />
No<br />
Nil<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection<br />
18 i) Place of inspection Valluvarkottam,<br />
Nungambakkam, Chennai 600<br />
034<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
yes
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr N.Kannan<br />
Chief Paediatrician, Rly Hospital,<br />
Perambur<br />
2) Dr Prasannakumar<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur<br />
3)Dr A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
REPORT ON APPLICATION SUBMITTED BY M/s Orchid Chemicals and<br />
Pharmaceuticals Ltd FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Orchid Healthcare<br />
(A division of Orchid Chemicals and<br />
Pharmaceuticals Ltd)<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Orchid Towers, # 313, Valluvarkottam<br />
High Road, Nungambakkam, Chennai<br />
600 034<br />
: M/s Orchid Healthc (A division of<br />
Orchid Chemicals and Pharmaceuticals<br />
Ltd), B 77, SIDCO Industrial Estate,<br />
Alathur 603110, Kancheepuram<br />
District, Tamilnadu<br />
“ F 26-27<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
MANDATORY CONDITIONS<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Yes –<br />
available for<br />
3 yrs<br />
Yes –<br />
available<br />
3 WHO GMP certificate Yes –<br />
available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
F 24<br />
F 33-34, 2A-6A<br />
F 11 – 12<br />
Rs. 1,21,385.21.28<br />
< 50 crores<br />
F 4<br />
F 3<br />
Desirable conditions<br />
1 ISO 9000 certification F 6-7<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Nil
-2 -<br />
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
Yes -<br />
available<br />
F 29-32<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes –<br />
available<br />
Registered in<br />
Central<br />
<strong>Railway</strong><br />
F 89 to 91<br />
F 5<br />
a<br />
III. For drugs manufactured abroad and prolposed for suply by the firm,<br />
the following informations are MANDATORILY REQUIRED: NOT<br />
APPLICABLE<br />
The source of manufactured<br />
Not applicable<br />
raw/finished products and quality<br />
report<br />
b<br />
c<br />
d<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected 11.10.2010<br />
b. Does the firm qualify for registration yes<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team
REPORT ON APPLICATION SUBMITTED BY M/s Galpha Laboratories<br />
Limited FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Galpha Laboratories Limited<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Jagdish Bhawan, Exhibition Road,<br />
Patna 800 001<br />
: M/s Galpha Laboratories Limited,<br />
Village-Thana Baddi, Dist.Solan (H>P)<br />
“ F 1-7<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
3 WHO GMP certificate Yes –<br />
available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
F 28-11<br />
F 73-91<br />
F 92-99<br />
2009-10 Rs.1,45,42,87,000<br />
2008-09 Rs.1,33,36,39,000<br />
2007-08 Rs.1,00,47,16,000<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
F 72 and 136<br />
F 137<br />
Desirable conditions<br />
1 ISO 9000 certification F 138<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
139-144
-2 -<br />
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes -<br />
available<br />
Yes –<br />
available<br />
Registered in<br />
Central<br />
<strong>Railway</strong><br />
F 145-184<br />
F 202-219<br />
F 185-189<br />
IV. For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
No<br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration yes<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Galpha Laboratories<br />
Limited FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Fourrts (India)Laboratories Pvt Ltd<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: No.1, Fourrts Avenue, Annai Indira<br />
Nagar,Okkiyum Thoraipakkam,<br />
Chennai 600 097<br />
: M/s Fourrts (India) Laboratories Pvt<br />
Ltd, Vandalur Road, Kelambakkam<br />
603 103<br />
“ Product list at 1a and 1b<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
3 WHO GMP certificate Yes –<br />
available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
F 48<br />
Yes - F-34<br />
available<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
F 11 to 16<br />
F 4 to 10<br />
F 49<br />
F 49<br />
1 ISO 9000 certification F 17<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
F-3
-2 -<br />
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes -<br />
available<br />
Yes –<br />
available<br />
Not available<br />
F 18 to 32<br />
F 18 to 32<br />
V. For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
No<br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration yes<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Indian Immunologisticals<br />
Limited FOR REGISTRATION<br />
IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Indian Immunologicals Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
: Road No. 44, Jubilee Hills,<br />
Hyderabad 500 033<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: M/s Human Biologicals Institute (A<br />
division of India Immunologicals Ltd,<br />
Kozhipannai, Pudumund Post,<br />
Uddhagamandalam 643 007<br />
“ Rabies vaccine – Human (Cell Culture) –<br />
I.P<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
3 WHO GMP certificate Folios 12 - 14<br />
Market standing o three years vide<br />
Folio 22<br />
License for the period from 2007-<br />
2011 issued by the Director of<br />
Drugs Control (I/c) and Licensing<br />
Authority, Tamilnadu and<br />
countersigned by the Central<br />
License Approving Authority and<br />
Drug Controller General (India),<br />
New Delhi – Folio 30<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
1 ISO 9000 certification<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Rs.272.89 crores for 2009-10 at<br />
Folio 11<br />
Folio 16 and 17<br />
Folio 10
-2 -<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
-<br />
-<br />
-<br />
VI. For drugs manufactured abroad and proposed for supply by the firm, the<br />
following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
Yes<br />
Kozhipannai, Pudumund Post<br />
Udhamandalam 643 007<br />
a. If yes, has the firm been inspected<br />
b. Does the firm qualify for registration<br />
The firm does not qualify on the market standing certificate is only<br />
available for three years (Item no.1 mandatory conditions)<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Centaur Pharmaceuticals<br />
Pvt Ltd FOR REGISTRATION<br />
IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Centaur Pharmaceuticals Pvt Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
: Centaur House, Near Grand Hyatt, Vakola,<br />
Santacruz (E), Mumbai 400 055<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: M/s Centaur Pharmaceuticals Pvt Ltd<br />
Plot No.3, Tivim Industrial Estate,<br />
Karaswada, Mapusa, Goa 403 526<br />
“ F. 7 to 10<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Yes - Available<br />
F.78-156<br />
Yes - Available F.14 to 76<br />
3 WHO GMP certificate Yes - Available F.116 to 170<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
Yes - Available F. 283<br />
Yes - Available F.216 & 217<br />
Yes- available<br />
1 ISO 9000 certification No – Not<br />
available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
No – Not<br />
available
-2 -<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
No – not<br />
available<br />
F.182 to 213-<br />
F.172 to 180<br />
-<br />
VII.For drugs manufactured abroad and proposed for supply by the firm, the<br />
following informations are MANDATORILY REQUIRED:NOT APPLICABLE<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
Not available<br />
a. If yes, has the firm been inspected No<br />
b. Does the firm qualify for registration Yes/No<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s BOEHRINGER INGELHEIM<br />
FOR REGISTRATION<br />
IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s BOEHRINGER INGELHEIM<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
: 11 th FLOOR,<br />
HALLMARK BUSINESS PLAZA<br />
NR.GURU NANAK HOSPITAL<br />
BANDRA (E)<br />
MUMBAI – 400 051.<br />
: M/S BOEHRINGER INGELHAIM PHARMA<br />
I&CO.<br />
KG, BIRKENDORFER STR64, 88397<br />
BIBERACH AN DER RIASS, GERMANY.<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
“ : AVAILABLE AT FOLIO 2.<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
: Available at Folio 13 & Folio 4A<br />
: Available at Folio 6-12<br />
3 WHO GMP certificate : Available Folio 1A<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
: Available Folio. 51<br />
: Available at Folio .58<br />
: Available at Folio 2<br />
Desirable conditions<br />
1 ISO 9000 certification : Not available
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
: Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering yearly<br />
requirements for the <strong>Railway</strong>/Unit<br />
: Available<br />
at<br />
Folio 48-50<br />
: Available Folio 44-47<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
: Not<br />
available<br />
-<br />
a<br />
VIII.For drugs manufactured abroad and proposed for supply by the firm, the<br />
following informations are MANDATORILY REQUIRED:<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
: M/s BOEHRINGER<br />
INGELHAIM PHARMA I&CO .<br />
KG, BIRKENDORFER STR 64,<br />
88397 BIBERACH AN DER<br />
RIASS, GERMANY.<br />
b<br />
c<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
: A WHOLLY OWNED<br />
SUBSIDIARY OF<br />
BOEHRINGER INGELHEIM<br />
INTERNATIONAL GmbH,<br />
GERMANY.<br />
: AVAILABLE IN GERMANY.<br />
d<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
: AT FOLIOS 4 & 5<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected : NOT APPLICABLE<br />
b. Does the firm qualify for registration : YES<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s HEXAGON NUTRITION PVT.<br />
LTD. FOR REGISTRATION<br />
IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s HEXAGON NUTRITION PVT. LTD.<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
: 404/a, GLOBAL CHAMBERS, ADARSH<br />
NAGAR, OFF. LINK ROAD, ANDHERI<br />
(W)MUMBAI – 422 202.<br />
: M/S HEXAGON NUTRITION PVT. LTD..<br />
PLOT NO.92, UNANDA NAGAR,<br />
LAKHMAPUR, TAL. DINDORI, DIST.<br />
NASHIK-422 202.<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
“ : AVAILABLE AT FOLIO 2.<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
: Available at Folio 7<br />
: Available at Folio 6<br />
3 WHO GMP certificate : Available at folio 5<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
: * Please see comments below<br />
: Available at Folio 3<br />
: Available at Folio 2<br />
Desirable conditions<br />
1 ISO 9000 certification : Available at<br />
folio 1<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
: Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
: Not<br />
Available
4 Cases of high value orders covering yearly<br />
requirements for the <strong>Railway</strong>/Unit<br />
: NOT<br />
AVAILABLE<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
: Not<br />
available<br />
-<br />
a<br />
IX. For drugs manufactured abroad and proposed for supply by the firm, the<br />
following informations are MANDATORILY REQUIRED:<br />
The source of manufactured<br />
: NOT APPLICABLE<br />
raw/finished products and quality<br />
report<br />
b<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
: NOT APLICABLE.<br />
c<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
: NOT APLICABLE..<br />
d<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
: NOT APLICABLE.<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected : NOT APPLICABLE<br />
b. Does the firm qualify for registration : -<br />
* Even though the annual turnover is less than Rs. 50 crores(Folio 4), the firm is<br />
manufacturing and marketing exclusively speciality food & nutrition products. It<br />
Casuais recommended for registratiion, subject to approval by competent<br />
authority.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Themis Medicare Limited<br />
FOR REGISTRATION<br />
IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Themis Medicare Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
: Sector 6A, Plot No. 16,17,18,<br />
I.I.E.SIDCUL, Haridwar,Uttarakhand<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Themis Medicare Limited<br />
Sector 6A, Plot No. 16,17,18,<br />
I.I.E.SIDCUL, Haridwar,Uttarakhand<br />
“ Available at folios 20 – 63<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Market standing three years vide<br />
Folio 67<br />
Available at folio 65<br />
3 WHO GMP certificate Available at folio 15<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at folio 14<br />
Available at folio 7<br />
Available at folio 6<br />
Desirable conditions<br />
1 ISO 9000 certification Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
-<br />
Not available -<br />
Available at folios 1-5
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
II. For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration No<br />
The firm does not qualify as the market standing certificate is only<br />
available for three years (Item no.1 mandatory conditions)<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Sandoz Private Limited<br />
FOR REGISTRATION<br />
IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Sandoz Private Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Dr.Annie Besant Road, Worli,<br />
Mumbai 400 042<br />
: M/s Sandoz Private Limited,<br />
Plot No.8-A/2 and 8-B, TTC Industrial<br />
Area, Kalwe Block, Village Dighe, Navi<br />
Mumbai 400 078<br />
“ Available at folios 52 and 53<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at folio 72<br />
Available at folio 71<br />
3 WHO GMP certificate Available at folio 68<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at folio 65<br />
Available at folio 54 and 55<br />
Available at folio 3<br />
Desirable conditions<br />
1 ISO 9000 certification Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Available at folio 2<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
-<br />
Available at folios 1 and 2A
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
II. For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected No<br />
b. Does the firm qualify for registration No<br />
The firm was registered on 06.04.2009 under the New Regulations for<br />
Registration of Firms. The firm had submitted Market Standing Certificate for 5<br />
yeaqrs then. The firm qualified for registration as they have now submitted a<br />
Market Standing Certificate for 3 years.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Tablets India Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Tablets India Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Jhaver Centre, Marshalls Road, Chennai<br />
600 008<br />
: No. 179, TH Road, Chennai 600 081<br />
“ Available at Annexure F and G<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Annexure H<br />
Available at Annexure A<br />
3 WHO GMP certificate Available at Annexure B<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
Available at Annexure D<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
Available at Annexure E<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Page 7 of Annexure F<br />
Desirable conditions<br />
1 ISO 9000 certification Available at Annexure I<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Nil<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
-<br />
Nil<br />
Available at Annexure J
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected No<br />
b. Does the firm qualify for registration Yes<br />
The firm was registered on 06.02.2009 under the New Regulations for<br />
Registration of Firms. The firm had submitted Market Standing Certificate for 5<br />
yeaqrs then. The firm now qualified for registration as they have submitted a<br />
Market Standing Certificate for 3 years. Thus the total market styanding will be<br />
or more than 5 years and hence the firm qualiies for registration.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s NIRMA LIMITED<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Nirma Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
: Nirma House Ashram Road Ahmedabad,<br />
Gujarat, India<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
: M/s Nirma Limited (Healthcare Limited)<br />
Village: Sachana, Taluka:Viramgam, Dist.<br />
Ahmedabad 382 150<br />
4 Products for which “ Available at Folio 15<br />
registration is sought<br />
strength wise<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
MANDATORY CONDITIONS<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
Available only for three years (Folio 5)<br />
(should be atleast 5 years)<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folio 3 and 4<br />
3 WHO GMP certificate Available at Folio 7<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
Available at Folio 6<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
Available at Folio 10<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retail sale in the<br />
same brand name<br />
Available at Folio 12<br />
Doies not specifically mentioned that<br />
products submitted for registgration are<br />
available in market for retain sale in the<br />
same brand name<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected No<br />
b. Does the firm qualify for registration No<br />
The firm does not qualify for the regisgtratin since the firm submitted a<br />
Market Standing Certificate is only for 3 years (Item No. 1 of Mandatory<br />
conditions)<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s TORRENT<br />
PHARMACEUTICALS<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Torrent Pharmaceuticals<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
: Torrent House, Off. Ashram Road,<br />
Ahmedabad 380 009<br />
: M/s Torrent Pharmaceuticals Limied,<br />
Villege Bhud and Makhumajra, Baddi<br />
173205, the. Nalagarh, Dist Solan<br />
(H.P)India<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
“ Available at Folio 1 to 22<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available for three years only<br />
Available at Folio 3 and 4<br />
3 WHO GMP certificate Available at Folio 7<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
Available at Folio 6<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
Available at Folio 10<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 12<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Not available
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
-<br />
Not available<br />
Available at folio 12 and 13<br />
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected No<br />
b. Does the firm qualify for registration Yes<br />
The firm does not qualify for the regisgtratin since the firm submitted a<br />
Market Standing Certificate is only for 3 years.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s AUROBINDO PHARMA LTD<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Aurobindo Pharma Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: 'Auro House', 313 Bachupally,<br />
Quthuballapur (Mandal ), RR District,<br />
Hyderabad 500 072<br />
: Plot No. 2, Matrivihar, Amerpet,<br />
Hyderabad 500 072<br />
“ Available at Folio 2<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available for three years only<br />
Available at Folio 6<br />
Available at Folio 11 and 12<br />
3 WHO GMP certificate Available at Folio 13 to 21<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
Available at Folio 114 to 120<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
Available at Folio 58 to 60<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 121<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Available at Folio 82 to 113<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
-<br />
Available at Folio 79 to 81<br />
Available at folio 75 to 78<br />
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected No<br />
b. Does the firm qualify for registration No<br />
The firm does not qualify for the regisgtratin since the firm submitted a<br />
Market Standing Certificate is only for 3 years.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Biological Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Biological Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products fo<br />
r which registration is sought<br />
strength wise<br />
: 18/1 and 3, Azamabad, Hyderabad<br />
500 020, AP<br />
: 18/1 and 3, Azamabad, Hyderabad<br />
500 020, AP<br />
“ Available at Folio 1 to 10<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available for three years only<br />
(Folio 61 to 74)<br />
Available at Folio 11 to 12<br />
3 WHO GMP certificate Not available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
Available at Folio 43<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
Available at Folio 46<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 3<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Not available<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
-<br />
Not available<br />
Available at folio 12 and 13
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not Available<br />
b. Does the firm qualify for registration Yes/No<br />
The firm was registered on 09.04.2009 under the New Regulations for<br />
Registration of Firms. The firm had submitted Market Standing Certificate for 5<br />
yeaqrs then. The firm qualified for registration as they have now submitted a<br />
Market Standing Certificate for 3 years.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Albert David Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Albert David Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: 15, Chittaranjan Avenue, Kolkata 700 072<br />
: B-12/13, Meerut Road, Industrial Area,<br />
Ghjaziabad, UP<br />
“ Available at Folio 6 to 15<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available for three years only<br />
(Folio 16 to 20)<br />
Available at Folio 1 to 5<br />
3 WHO GMP certificate Available at Folio 22 to 24<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
Available at Folio 21<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
Index Page<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Index Page<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Not available<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
-<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration Not applicable<br />
The firm was registered on 30.03.2009 under the New Regulations for<br />
Registration of Firms. The firm had submitted Market Standing Certificate for 5<br />
yeaqrs then. The firm qualified for registration as they have now submitted a<br />
Market Standing Certificate for 3 years.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Albert David Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Zee Laboratories<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Zee Laboratories, Uchani, G.T.Road,<br />
Karnal 132 001, India<br />
: Zee Laboratories<br />
47, Industrial Area, Paonta-Sahib District,<br />
Sirmour, H.P. India<br />
“ Available in Annexures<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folio 30<br />
Available at Folio 35-37<br />
3 WHO GMP certificate Available at Folio 34<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 19<br />
Available at Folio 38<br />
Available at Folio 1<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Folios 2-11<br />
Folios 12-18<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration Yes<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Albert David Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s CIPLA Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: 106 A, Alapakkam Main Road,<br />
Alapakkam, Chennai 600 116<br />
: CPO Ltd<br />
Vill. Malpur, Baddi District, Solan (H>P)<br />
“ Available between Folios 397 and 414<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available between Folios 462 and<br />
513<br />
Available between Folios 1 and 322<br />
3 WHO GMP certificate Available between Folios 323 and<br />
376<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 395<br />
Available between Folios 377 and<br />
392 and Index Page<br />
Covering Letter<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration Yes<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Albert David Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Ranbaxy Laboratories Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Sahiubzada Ajit Singh Nagar 160 055 ,<br />
Dist Ropar (Punjab)<br />
: Sahiubzada Ajit Singh Nagar 160 055 ,<br />
Dist Ropar (Punjab)<br />
“ Available between Folios 39-18<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available atFolio 17<br />
Available at Folio 321<br />
3 WHO GMP certificate Available at folio 153<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 58<br />
Available at folio 57<br />
Available at folio 1<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration Yes/No<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s SHREE KRISHNA KESHAV<br />
LABORATORIES LIMITED FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Shree Krishna Keshav Laboratories<br />
Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose products<br />
are likely to be marketed by<br />
this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Devashish 3 rd floor, Near Hotel Klasic<br />
Gold, Off C.G.Road, Ahmedabad 380 006<br />
: Shree Krishna Keshav Laboratories<br />
Limited, Old No. 69, New 141, Linghi<br />
Chetty Street, Chennai 600 001<br />
“ Available between Folios 32 to 35<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folio 26 & 26A for<br />
Three years only<br />
However the item manufactured by<br />
this firm has been procured on a<br />
regular basis for the past 5 years<br />
Available at Folio 1 & 2<br />
Licence under the process of<br />
renewal<br />
3 WHO GMP certificate Available at folio 23<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 36, Less than 50<br />
Crores for the past 3 years *<br />
Available at folio 25<br />
Available at folio 27<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being sold<br />
in USA Europe and other developed<br />
countries equi to Europeal countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration Yes/No *<br />
* We have been experiencing difficulties in procuring the following<br />
medicines from sources other than M/s Krishna Keshav Laboratories.<br />
Sl.No. Item No. Description<br />
1 90005 Normal Saline-Glass Bottle<br />
2 90033 Dextrose 100 ml<br />
3 90034 Dextrose plus Half Normal Saline<br />
4 90038 Glycine irrigation 1.5%<br />
5 90040 Isolyte E<br />
6 90120 3% Sodium Chloride 100 ml<br />
For the above items, the only source of supply for us has been (Shree<br />
Krishna Keshav Laboratories Limited). The above items come under the Vital<br />
Group and are life saving in nature. Hence, even though the annual turn over the<br />
said firm is less than 50 Crores, for the previous 3 years, the undersigned<br />
recommend the inclusion of the firm in the Suppliers List subject to extant rules<br />
and procedures, for selected vital medicines like IV fluid preparations.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s FRESNIUS KABI ONCOLOGY<br />
LIMITED FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Fresnius Kabi Oncology Limited<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Echelon Institutional Area, Plot No. 11,<br />
Sector No. 32, Gurgon 122 001<br />
: Plot No. 19, HPSIDC, Industrial Area,<br />
Baddi, Dist Solan, H.P<br />
“ Available at Folios 1 - 3<br />
MANDATORY CONDITIONS<br />
1 Firm's standing In<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folios 91-97 * (Three<br />
years certificate now enclosed. This<br />
firm was registered in 16.04.2009,<br />
duly verifying the<br />
manufacturing/market standing for<br />
three years then)<br />
Available at Folio 4 to 66<br />
3 WHO GMP certificate Available at Folios 67 to 82<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
Available at Folio 88<br />
Available at Folios 89, 90A<br />
Available at Folio 115<br />
1 ISO 9000 certificate Available at Folio 98<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
Available at Folios 107 to 110<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Registered in North Central<br />
<strong>Railway</strong>, PO of Western Central<br />
<strong>Railway</strong> at Folio 105 to 106<br />
Available at Folio 100 to 102
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
IV . If yes, has the firm been inspected<br />
Not applicable<br />
V. Does the firm qualify for registration Yes/No *<br />
* Note: The firm was registered on 16.04.2009 under the new Regulations<br />
for Registration of Firms, duly obtaining a Market Standing Certificate then. The<br />
firm now qualifies for registraton as they have submitted a Market Standing<br />
Certificate for three years, EXCEPT for the products under Folio 95 and 96,<br />
wherein the Market Standing is less than Five years.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s M/s MEDOPHARM FOR<br />
REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Medopharm<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: P.B.No. 2353, No. 25, Puliyur 2 nd Main<br />
Road, Trustpuram, Kodambakkam,<br />
Chennai 600 0243<br />
: Medopharm<br />
50 Kayarambedu Village, Guduvanchery<br />
603 203<br />
“ Available at Folio 31<br />
MANDATORY CONDITIONS<br />
1 Firm's standing In<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folios 51-54<br />
Available at Folios 32-40 and<br />
Folios 57-58<br />
3 WHO GMP certificate Available at Folios 55-56<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 5<br />
Available at Folios 28<br />
Available at Folio 30<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Available at Folios 69-88<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
IV . If yes, has the firm been inspected<br />
To be inspected<br />
V. Does the firm qualify for registration Yes/No<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s FOURRTS (INDIA)<br />
LABORATIRES LTD FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Fourrts (India)Laboratories Pvt Ltd<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: No.1, Fourrts Avenue, Annai Indira<br />
Nagar,Okkiyum Thoraipakkam,<br />
Chennai 600 097<br />
: M/s Fourrts (India) Laboratories Pvt<br />
Ltd, Vandalur Road, Kelambakkam<br />
603 103<br />
“ Product list at 1a and 1b<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
3 WHO GMP certificate Yes –<br />
available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
F 48<br />
Yes - F-34<br />
available<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
(Available for 3<br />
years)<br />
F 11 to 16<br />
F 4 to 10<br />
F 49<br />
F 49<br />
1 ISO 9000 certification F 17<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
F-3
-2 -<br />
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes -<br />
available<br />
Yes –<br />
available<br />
Not available<br />
F 18 to 32<br />
F 18 to 32<br />
X. For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
No<br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration Yes/No<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s FOURRTS (INDIA)<br />
LABORATIRES LTD FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Fourrts (India)Laboratories Pvt Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: No.1, Fourrts Avenue, Annai Indira<br />
Nagar,Okkiyum Thoraipakkam,<br />
Chennai 600 097<br />
: M/s Fourrts (India) Laboratories Pvt<br />
Ltd, Vandalur Road, Kelambakkam<br />
603 103<br />
“ Product list at 1a & 1b<br />
MANDATORY CONDITIONS<br />
1 Firm's standing In<br />
manufacturing/marketing<br />
pharmaceutical products<br />
2<br />
7<br />
(should be atleast 5 years)<br />
Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folios 51-54<br />
Available at Folios 32-40 and<br />
Folios 57-58<br />
3 WHO GMP certificate Available at Folios 55-56<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 5<br />
Available at Folios 28<br />
Available at Folio 30<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Available at Folios 69-88<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
IV . If yes, has the firm been inspected<br />
To be inspected<br />
V. Does the firm qualify for registration Yes/No<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Sr Divl Medical Officer Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm M/s Medopharm<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
P.B.No. 2353, No. 25, Puliyur<br />
2 nd Main Road, Trustpuram,<br />
Kodambakkam,<br />
Chennai 600 024<br />
Yes<br />
50, Kayarambedu Village,<br />
Guduvancherry 603 203<br />
Yes<br />
Yes<br />
Yes<br />
2008-09 12980.60 lakhs<br />
2009-10 12748.00 lakhs<br />
2010-11 13307.52 lakhs<br />
9 Company turn over figure for last 3 years 2008-09 12980.60 lakhs<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2009-10 12748.00 lakhs<br />
2010-11 13307.52 lakhs<br />
Not available
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research Available at Folio 30<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
Nil<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
16 Other conditions<br />
No<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
-<br />
Not Applicable<br />
Not Applicable<br />
Not Applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection 25 th June 2012<br />
18 i) Place of inspection Guduvancherry, Chennai<br />
ii) Storage facility holding time for<br />
manufactured products<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
Yes<br />
Mr. Abdul Hameed, Technical<br />
Director, No. 50, Kayarambedu<br />
Village, Guduvancherry, 603<br />
202, Kancheepuram District
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1) Dr N.Kannan<br />
Medical Director and Chief Paediatrician,<br />
Rly Hospital,<br />
Perambur<br />
2) Dr Prasanna Kumar,<br />
Additional Chief Health Director,<br />
Rly Hospital, Perambur<br />
3)Dr A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
REPORT ON APPLICATION SUBMITTED BY M/s FOURRTS (INDIA)<br />
LABORATIRES LTD FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Parenteral Drugs (India) Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Shree Ganesh Chambers, A.B.Road,<br />
Navlakha Crossing, Indore 452 001<br />
: M/s Parenteral Drugs (India) Ltd<br />
Village Asrawad, Post Dudhia,<br />
Nemawar Road, Dist. Indore, M.P<br />
“ Available at folio 4<br />
MANDATORY CONDITIONS<br />
1 Firm's standing In<br />
manufacturing/marketing<br />
pharmaceutical products<br />
2<br />
7<br />
(should be atleast 5 years)<br />
Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folios 1<br />
(Three years certificate now<br />
enclosed. This firm was registered<br />
in 2009, duly verifying the<br />
marketing standing for three years<br />
then)<br />
Available at Folio 2<br />
3 WHO GMP certificate Available at Folio 7<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Available at Folio 3<br />
Available at Folios 5<br />
Available at Folio 4<br />
Desirable conditions<br />
1 ISO 9000 certificate Not available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not available<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not available<br />
Not available<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
IV . If yes, has the firm been inspected<br />
Not Applicable<br />
V. Does the firm qualify for registration No<br />
The following products of the firm supplied to other <strong>Railway</strong>s were found<br />
to be substandard.<br />
1. Multiple Electrolyte and Dextrose 21.05.2012<br />
2. Dextrose IP 100 ml 12.02.2011<br />
3. Inj.Ondansetron 30.08.2011<br />
4. Tab.Hotril 2.5 mg 12.08.2011<br />
5. Inj.Artesunate 08.05.2011<br />
6. iv Dextrose 10% 22.06.2011<br />
7. Inj.iv Sodium Chloride 04.11.2011<br />
In view of the above it is recommended that the application from M/s<br />
Parenteral Drugs (India) Ltd should not be considered for renewal.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director CSS/ Lab and Admn Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
No. HQ/MD/Regn. of Firms Dt: 14.08.2012.<br />
CMD/MAS<br />
Sub: Registration of firms – New Procurement Policy<br />
Ref: Your letter no. MD.69/Registration dt. 05.07.2012<br />
On perusal of the file it is seen that M/s Sanjivani<br />
Parenteral Limited have been registered with Central <strong>Railway</strong> and<br />
Western <strong>Railway</strong> during 2007 for a initial period of one year, to be<br />
reviewed subject to the performance of the Company.<br />
It may be ascertained from both Central and Western<br />
<strong>Railway</strong> regarding the performance of the firm before deciding on<br />
the issue.<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director CSS/ Lab and Admn Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team
REPORT ON APPLICATION SUBMITTED BY M/s FOURRTS (INDIA)<br />
LABORATIRES LTD FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Fourrts (India)Laboratories Pvt Ltd<br />
2 Postal address of Head Office<br />
/ Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: No.1, Fourrts Avenue, Annai Indira<br />
Nagar,Okkiyum Thoraipakkam,<br />
Chennai 600 097<br />
: M/s Fourrts (India) Laboratories Pvt<br />
Ltd, Vandalur Road, Kelambakkam<br />
603 103<br />
“ Product list at 1a & 1b<br />
MANDATORY CONDITIONS<br />
1 Firm's standing In<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each product<br />
Available at Folio 48<br />
Available at Folios 11 to 16<br />
3 WHO GMP certificate Available at Folios 4 to 10<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should be<br />
Rs. 50 crores for previous three years)<br />
5 Firm's Declaration with regard to non<br />
conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
2005-2006 100.64 crores<br />
2006-2007 107.33 crores<br />
2007-2008 138.22 crores<br />
Available at Folio 49<br />
Available at Folio 49<br />
Desirable conditions<br />
1 ISO 9000 certificate Available at Folio 17<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Available at Folio 3<br />
3 Details of supply orders received from<br />
Institutions in the previous three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the<br />
<strong>Railway</strong>/Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Available at Folio 18 to 32<br />
Folio 18 to 32<br />
Not available
II.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad for<br />
local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
IV . If yes, has the firm been inspected<br />
To be inspected<br />
V. Does the firm qualify for registration Yes/No<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director Chief Staff Surgeon/Admn Sr Divl Medical Officer<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
REPORT ON APPLICATION SUBMITTED BY M/s Galpha Laboratories<br />
Limited FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Fourrts (India)Laboratories Pvt Ltd<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: No.1, Fourrts Avenue, Annai Indira<br />
Nagar,Okkiyum Thoraipakkam,<br />
Chennai 600 097<br />
: M/s Fourrts (India) Laboratories Pvt<br />
Ltd, Vandalur Road, Kelambakkam<br />
603 103<br />
“ Product list at 1a and 1b<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
3 WHO GMP certificate Yes –<br />
available<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
F 48<br />
Yes - F-34<br />
available<br />
Yes –<br />
available<br />
Yes –<br />
available<br />
F 11 to 16<br />
F 4 to 10<br />
F 49<br />
F 49<br />
1 ISO 9000 certification F 17<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
F-3
-2 -<br />
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Yes -<br />
available<br />
Yes –<br />
available<br />
Not available<br />
F 18 to 32<br />
F 18 to 32<br />
XI. For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
Yes<br />
a. If yes, has the firm been inspected Yes. <strong>Inspection</strong> report<br />
enclosed<br />
b. Does the firm qualify for registration yes<br />
(Dr.N.Kannan) (Dr.Prasannakumar) (Dr.A.P.Preetham)<br />
Medical Director & Sr Divl Medical Officer Sr Divl Medical Officer<br />
Chief Paediatrician<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director
Proforma to be filled up by the Inspecting Team<br />
Note: <strong>Inspection</strong> is to be done by respective Zonal <strong>Railway</strong>s in whose jurisdiction<br />
the Manufacturing Unit is located. Wherever there is inadequate space please<br />
submit information in separate sheet enclosed and marked as Annexure (Also<br />
having item no. of this proforma in reference to which Annexure is prepared)<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
1 Name of the firm M/s Fourrts (India)<br />
Laboratories Pvt Ltd<br />
2 Whether the firm is having registered<br />
office/Branch office. Give address and<br />
Telephone no in case more than one<br />
manufacturing unit - details all to be<br />
inspected<br />
In case marketing a product manufactured<br />
by other company. If 'yes' that also to be<br />
inspected<br />
3 Whether the firm is having 5 years<br />
standing marketing/manufacturing of<br />
pharmaceutical products<br />
4 Whether firm is having 3 years standing in<br />
marketing/manufacturing of each<br />
individual product to which it wishes to get<br />
Registered (in case 'No' names of such<br />
product to be given)<br />
5 Whether valid drug license for each<br />
product exists from Drug Controller (In<br />
case 'No' names of such product to be<br />
given)<br />
6 Audited annual turn over figures for each<br />
product for the last 3 years<br />
7 Whether WHO GMP certificate for<br />
production unit/s is there<br />
8 Whether any other certificate for<br />
firm/manufacturing and manufacturing<br />
process (as desired in item 4 of terms and<br />
conditions exit)<br />
Yes<br />
No.1, Fourrts Avenue,<br />
Annai Indira<br />
Nagar,Okkiyum<br />
Thoraipakkam, Chennai<br />
600 097<br />
M/s Fourrts (India)<br />
Laboratories Pvt Ltd,<br />
Vandalur Road,<br />
Kelambakkam 603 103<br />
Yes<br />
Yes<br />
Yes<br />
2008-09 145.05 crores<br />
2009-10 154.90 crores<br />
2010-11 207.79 crores<br />
9 Company turn over figure for last 3 years 2008-09 145.05 crores<br />
10 R&D facilities with firm. Annual<br />
expenditure in R&D in last 3 years<br />
Yes<br />
yes<br />
2009-10 154.90 crores<br />
2010-11 207.79 crores
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
11 Name of Original research F 4 – 10<br />
products/formulations developed by<br />
firm<br />
12 Names of products for which firm is<br />
original manufacturer<br />
Not Applicable<br />
13 Whether any punitive action has been<br />
taken or contemplated State/Central<br />
Institution/Drug Controller. If yes give<br />
details<br />
14 Relevant information for each specific<br />
product as per ORG-MARG analysis or<br />
by any other agency<br />
15 a) Names of imported drug items<br />
supplied by firm. List of developed<br />
countries where item is approved and<br />
supplied<br />
b) The source of manufactured<br />
raw/finished products for quality report<br />
c) Authorization letter by OEM abroad<br />
for local agent<br />
16 Other conditions<br />
No<br />
Nil<br />
a) Cleanliness of premises Clean<br />
b) Whether plan is manual or automatic<br />
and level of automation<br />
c)Quality of packaging and packaging<br />
system<br />
d) Availability of sterile room yes<br />
e) Degree of hand handling in<br />
manufacturing process<br />
f) Availability of technically qualified<br />
staff<br />
Not Applicable<br />
Not applicable<br />
Not applicable<br />
Automatic<br />
Excellent<br />
Minimal<br />
yes<br />
g) Quality control inhouse lab/outsource yes<br />
17 Date of inspection 02.07.2012<br />
18 i) Place of inspection Kelambakkam<br />
ii) Storage facility holding time for<br />
manufactured products<br />
yes
Sl.No. Description Remarks<br />
19 Name and designation of the company<br />
official accompanying for inspection<br />
Mr. Vanjinathan<br />
Vice President, Technical<br />
Sl.No. Description Remarks<br />
(S.No. in alphabetical list)<br />
20 Whether firm is recommended suitable for Yes<br />
registration. Whether the product being<br />
Available in open market in<br />
applied by firms for registgration are<br />
the same brand name in<br />
available in open market with some brand<br />
southern region<br />
name and the region<br />
21 Name and Designation of the Team<br />
Member<br />
Signature<br />
1.Dr.N.Kannan<br />
Medical Director and Chief Paediatrician<br />
<strong>Railway</strong> Hospital, Perambur<br />
2. Dr Prasannakumar<br />
Chief Staff Surgeon, Lab and Admn,<br />
Rly Hospital, Perambur<br />
3.Dr A.P.Preetham,<br />
Sr Divl Medical Officer, Rly Hospital,<br />
Perambur
REPORT ON APPLICATION SUBMITTED BY M/s Aventis Pharma Limited<br />
FOR REGISTRATION IN SOUTHERN RAILWAY<br />
1 Name of the firm : M/s Aventis Pharma Limited<br />
2 Postal address of Head<br />
Office / Registered Office<br />
3 Name and address of<br />
manufacturer whose<br />
products are likely to be<br />
marketed by this firm<br />
4 Products for which<br />
registration is sought<br />
strength wise<br />
: Aventis House, 54/A, Sir Mathurdas<br />
Vasanji Road, Andheri (East), Mumbai<br />
: M/s Aventis Pharma Limited, GIDC,<br />
Plot No. L 121, Phase III, Verna<br />
Industrial Estate, Verna, Nagoa, Goa<br />
403722<br />
“ Available at folio 4<br />
Amount tobe paid as Registration Fee for three years : Rs. 5,000/-<br />
1 Firm's standing in<br />
manufacturing/marketing<br />
pharmaceutical products<br />
(should be atleast 5 years)<br />
MANDATORY CONDITIONS<br />
2 Valid drug license for manufacture<br />
from Drug Controller for each<br />
product<br />
Available at folio 2A (Three years<br />
certificate now enclosed. The firm<br />
was registered in 2009, duly<br />
verifying the market standing for<br />
three years then)<br />
Available at folio 2<br />
3 WHO GMP certificate Available at folio 7<br />
4 Annual turnover of the firm from<br />
domestic market (minimum should<br />
be Rs. 50 crores for previous three<br />
years)<br />
5 Firm's Declaration with regard to<br />
non conviction of their firm<br />
6 Firm's declaration that products<br />
submitted for registratiion are<br />
available in market for retain sale in<br />
the same brand name<br />
Desirable conditions<br />
Available at folio 3<br />
Available at folio 6<br />
Available at folio 5<br />
1 ISO 9000 certification Not Available<br />
2 Market share of items as per latest<br />
ORG-MARG NIELSEN analysis or<br />
National/central Health Agencies<br />
Not Available
-2 -<br />
3 Details of supply orders received<br />
from Institutions in the previous<br />
three years<br />
4 Cases of high value orders covering<br />
yearly requirements for the <strong>Railway</strong>/<br />
Unit<br />
5 Performance <strong>Report</strong> issued by other<br />
Government Organisations<br />
Not Available<br />
Not Available<br />
Not Available<br />
XII.For drugs manufactured abroad and proposed for supply by the firm,<br />
the following informations are MANDATORILY REQUIRED:<br />
a<br />
b<br />
c<br />
d<br />
The source of manufactured<br />
raw/finished products and quality<br />
report<br />
Relation of Indian agent with the<br />
foreign company in past 3 years<br />
Whether the same product is being<br />
sold in USA Europe and other<br />
developed countries equi to Europeal<br />
countries<br />
Authorisation letter by OEM abroad<br />
for local agent<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
Not applicable<br />
III. Does the firm has manufacturing facilities<br />
situated in <strong>Southern</strong> <strong>Railway</strong><br />
a. If yes, has the firm been inspected Not applicable<br />
b. Does the firm qualify for registration yes<br />
(Dr.Prasannakumar) (Dr.R.Saravanan) (Dr.A.P.Preetham)<br />
Chief Staff Surgeon Sr Divl Medical Officer Sr Divl Medical Officer<br />
Lab & Administration (Ophthalmology) (ENT)<br />
Rly Hospital, Perambur Rly Hospital, Perambur Rly Hospital, Perambur<br />
Head of <strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team Member/<strong>Inspection</strong> Team<br />
Chief Medical Director<br />
Chief Health Director