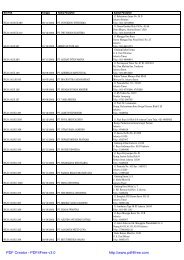
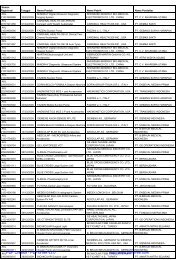
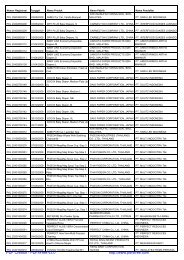
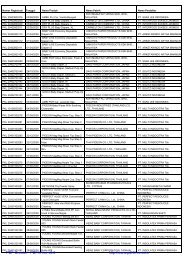
Registrasi Izin Edar ALKES Diagnostik & Reagensia Impor
Registrasi Izin Edar ALKES Diagnostik & Reagensia Impor
Registrasi Izin Edar ALKES Diagnostik & Reagensia Impor
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
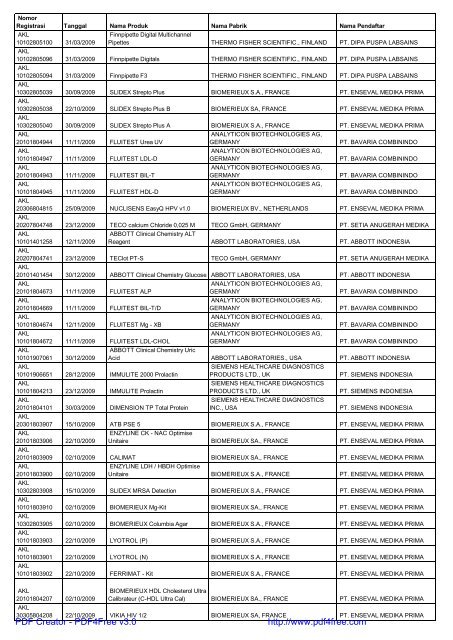
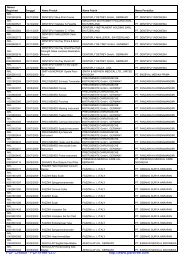
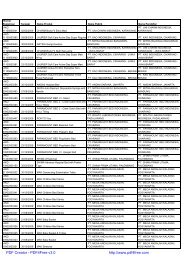
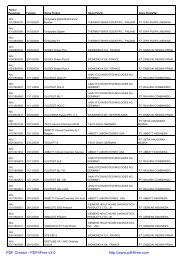
Nomor<br />
<strong>Registrasi</strong> Tanggal Nama Produk Nama Pabrik Nama Pendaftar<br />
AKL<br />
10102805100 31/03/2009<br />
Finnpipette Digital Multichannel<br />
Pipettes THERMO FISHER SCIENTIFIC., FINLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102805096 31/03/2009 Finnpipette Digitals THERMO FISHER SCIENTIFIC., FINLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102805094 31/03/2009 Finnpipette F3 THERMO FISHER SCIENTIFIC., FINLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302805039 30/09/2009 SLIDEX Strepto Plus BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302805038 22/10/2009 SLIDEX Strepto Plus B BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302805040 30/09/2009 SLIDEX Strepto Plus A BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101804944 11/11/2009 FLUITEST Urea UV<br />
AKL<br />
10101804947 11/11/2009 FLUITEST LDL-D<br />
AKL<br />
20101804943 11/11/2009 FLUITEST BIL-T<br />
AKL<br />
10101804945 11/11/2009 FLUITEST HDL-D<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
20306804815 25/09/2009 NUCLISENS EasyQ HPV v1.0 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20207804748 23/12/2009 TECO calcium Chloride 0,025 M TECO GmbH, GERMANY PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10101401258 12/11/2009<br />
ABBOTT Clinical Chemistry ALT<br />
Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20207804741 23/12/2009 TEClot PT-S TECO GmbH, GERMANY PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101401454 30/12/2009 ABBOTT Clinical Chemistry Glucose ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101804673 11/11/2009 FLUITEST ALP<br />
AKL<br />
20101804669 11/11/2009 FLUITEST BIL-T/D<br />
AKL<br />
10101804674 12/11/2009 FLUITEST Mg - XB<br />
AKL<br />
10101804672 11/11/2009 FLUITEST LDL-CHOL<br />
AKL<br />
10101907061 30/12/2009<br />
AKL<br />
10101906651 28/12/2009 IMMULITE 2000 Prolactin<br />
AKL<br />
10101804213 23/12/2009 IMMULITE Prolactin<br />
AKL<br />
20101804101 30/03/2009 DIMENSION TP Total Protein<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
ABBOTT Clinical Chemistry Uric<br />
Acid ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301803907 15/10/2009 ATB PSE 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101803906 22/10/2009<br />
ENZYLINE CK - NAC Optimise<br />
Unitaire BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101803909 02/10/2009 CALIMAT BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101803900 02/10/2009<br />
ENZYLINE LDH / HBDH Optimise<br />
Unitaire BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803908 15/10/2009 SLIDEX MRSA Detection BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101803910 02/10/2009 BIOMERIEUX Mg-Kit BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803905 02/10/2009 BIOMERIEUX Columbia Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101803903 22/10/2009 LYOTROL (P) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101803901 22/10/2009 LYOTROL (N) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101803902 22/10/2009 FERRIMAT - Kit BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101804207 02/10/2009<br />
AKL<br />
BIOMERIEUX HDL Cholesterol Ultra<br />
Calibrateur (C-HDL Ultra Cal) BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
30305804208 22/10/2009 VIKIA HIV 1/2 BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302803740 02/10/2009<br />
AKL<br />
10302803739 02/10/2009<br />
BIOMERIEUX Rinsing Solution<br />
(Fluid A) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX Thioglycolate with<br />
Resazurin - Broth BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803738 02/10/2009 BIOMERIEUX Trypcase Soy Broth BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101803717 12/11/2009 FLUITEST CREA<br />
AKL<br />
20101803718 11/11/2009 FLUITEST GLU<br />
AKL<br />
20101803714 12/11/2009 FLUITEST ALB - BCG<br />
AKL<br />
10101803719 11/11/2009 FLUITEST CHOL<br />
AKL<br />
10101803712 11/11/2009<br />
CONTRONORM AND<br />
CONTROPATH<br />
AKL<br />
20101803402 11/11/2009 FLUITEST CK-MB<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
10101803335 03/07/2009 HUMAN Gamma GT Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101803259 31/12/2009 IMMULITE 1000 Folic Acid<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302904942 05/10/2009 BIOMERIEUX VPA + VPB BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803240 09/10/2009 BIOMERIEUX Mueller Hinton 2 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803246 02/10/2009 BIOMERIEUX ZYM A + ZYM B BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803243 02/10/2009<br />
BIOMERIEUX Mac Conkey without<br />
Chrystal Violet Agar (MC - D) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803241 09/10/2009 BIOMERIEUX Mycoline BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803244 02/10/2009 BIOMERIEUX Uriline BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803239 09/10/2009 BIOMERIEUX Gonoline Duo BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803238 09/10/2009 BIOMERIEUX NIN BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803236 15/10/2009 BIOMERIEUX API M Medium BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803237 15/10/2009 BIOMERIEUX API Of Medium BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302803235 15/10/2009 BIOMERIEUX ATB UR 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803100 15/10/2009 BIODISCS Ciprofloxacin - 5 ug BIOMERIEUX S.A.,FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803101 15/10/2009 BIODISCS Cefuroxin - 360 ug BIOMERIEUX S.A.,FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803099 15/10/2009 BIODISCS Nefilmicin - 30 ug BIOMERIEUX S.A.,FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803098 15/10/2009 BIODISCS Vancomycin - 30 ug BIOMERIEUX S.A.,FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803097 15/10/2009 BIODISCS Polymixin B (PB) - 300 BIOMERIEUX S.A.,FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803095 15/10/2009 BIODISCS Colistin - 50 ug BIOMERIEUX S.A.,FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803096 25/09/2009 BIODISCS Tetracyclin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803094 25/09/2009<br />
BIODISCS Norfloxacin - 10 ug NOR<br />
10 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803093 25/09/2009 BIODISCS Oxacillin - 5 ug OX 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803092 25/09/2009 BIODISCS Ofloxacin - 5 ug OFX 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803091 25/09/2009<br />
AKL<br />
20301803090 25/09/2009<br />
AKL<br />
20301803087 25/09/2009<br />
AKL<br />
BIODISCS Ticarcillin + Clavulanic<br />
Acid (75 + 10) ug TIM 85 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIODISCS Tobramycin - 10 ug NN<br />
10 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIODISCS Cefoperazone - 30 ug<br />
CFP 30 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
20301803089 25/09/2009 BIODISCS Penicillin G-10 U/IE P10 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301803088 25/09/2009<br />
BIODISCS Amoxilllin + Clavulanic<br />
Acid (20+10) ug AMC 30 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301803086 25/09/2009 BIODISCS Ceftriaxone - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303802564 04/05/2009 IMMULITE Rubella Quantitative IgG<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD., UK<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302802473 09/10/2009 BIOMERIEUX GenBox Anaer BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802471 26/08/2009 BIOMERIEUX McFarland Standard BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802472 26/08/2009 BIOMERIEUX VP1 - VP2 Reagents BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802465 26/08/2009 BIOMERIEUX EHR BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802477 09/10/2009 BIOMERIEUX SM ID-Medium BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802468 26/08/2009 BIOMERIEUX XYL L (Xylene) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802476 09/10/2009 BIOMERIEUX FB BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802475 09/10/2009 BIOMERIEUX Color Gram 2 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802470 26/08/2009 BIOMERIEUX M. Broth BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802469 26/08/2009 BIOMERIEUX James BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802466 26/08/2009 BIOMERIEUX TDA BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802467 26/08/2009 BIOMERIEUX Nit 1 + Nit 2 Reagent BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802463 26/08/2009 BIOMERIEUX BCP Agar BIO MERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802464 26/08/2009 BIOMERIEUX Coli ID Medium BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306801602 15/10/2009 VIDAS CA 125 ll BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306801601 15/10/2009 VIDAS CA 15-3 / 153 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303801598 15/10/2009 VIDAS CMV AVIDITY / CMVU BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801600 15/10/2009 VIDAS Progesterone PRG BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303801044 28/05/2009 HEXAGEN Malaria HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10302800504 25/09/2009 BACT/ALERT i NST BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302800503 25/09/2009 BACT/ALERT i LYM BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302800502 25/09/2009 BACT/ALERT i AST BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306904945 05/10/2009 VIDAS CEA (S) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305800393 30/09/2009 VIDAS Multi Adjustor A-IC1 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10305800401 30/09/2009 VIDIA Multi Control C-INF2 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10305800399 30/09/2009 VIDIA Multi Control C-IC1 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10305800400 30/09/2009 VIDIA LUM BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101800394 22/10/2009<br />
AKL<br />
20303600203 09/11/2009<br />
AKL<br />
20305500015 09/11/2009 Procleix Ultrio Assay<br />
BIOMERIEUX HDL Cholesterol Ultra<br />
Direct (C-HDL Ultra) BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
ABBOTT AXSYM System Rubella<br />
IgM Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
GEN-PROBE IN CORPORATED, USA untuk<br />
NOVARTIS VACCINES and DIAGNOSTICS<br />
INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20102904756 01/10/2009<br />
VIDIA Fully Automated<br />
Immunoanalyzer and Accessories BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305703155 30/09/2009 VIKIA HBsAg BIOMERIEUX S.A., Brasil PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305703154 02/10/2009 VIDAS B.R.A.H.M.S PCT BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101702917 28/05/2009 HUMAN Lipose Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305702873 03/07/2009 HUMAN Ferritin Reagent Kit Human GmbH, Germany PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205702542 22/05/2009<br />
ORTHO Autovue Innova and<br />
Accessories<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
DIVISION JOHNSON & JOHNSON<br />
COMPANY., USA<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20305702544 25/09/2009 NUCLISENS EasyQ Basic BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20102904755 01/10/2009 VIDIA FT4 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20102904541 25/09/2009 VIDIA FT3 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303701752 02/10/2009 VIDIA TOXO IgG BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305701766 30/09/2009 VIDIA Ferritin BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303701749 02/10/2009 VIDIA RUB IgM BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303701750 02/10/2009 VIDIA RUB IgG BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303701751 02/10/2009 VIDIA TOXO IgM BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305701765 25/09/2009 VIDIA TSH BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10305701767 30/09/2009 VIDIA TPSA BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102903953 26/08/2009 NucliSens Easy MAG BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302701005 30/09/2009 ChromID VRE Agar (VRE) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305701000 25/09/2009<br />
NUCLISENS Easy MAG Magnetic<br />
Silica BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302701006 30/09/2009 ChromID Candida Agar (CAN 2) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204903862 21/08/2009<br />
AKL<br />
10204903861 21/08/2009<br />
NucliSens Easy MAG Extraction<br />
Buffer 2 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
NucliSens Easy MAG Extraction<br />
Buffer 1 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204903952 26/08/2009 NucliSens Easy MAG Lysis Buffer BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204701001 25/09/2009<br />
NUCLISENS Easy MAG Extraction<br />
Buffer 3 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302700875 30/09/2009 ChromID MRSA Agar BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302700876 30/09/2009 ChromID ESBL Agar BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302700873 15/10/2009<br />
AKL<br />
10302700874 30/09/2009<br />
AKL<br />
20305700061 11/11/2009<br />
AKL<br />
20101700063 11/11/2009<br />
AKL<br />
20101700058 11/11/2009<br />
AKL<br />
20101700052 11/11/2009<br />
AKL<br />
10101700064 11/11/2009<br />
BIOMERIEUX Strepto B ID Agar<br />
(STRB) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX Bouillon Todd Hewitt<br />
+ Antibiotiques (TODD H-T) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
ROCHE CRPLX cobas c111 C-<br />
reactive Protein (Latex) ROCHE DIAGNOSTICS GmbH, GERMANY PT. ROCHE INDONESIA<br />
ROCHE CKL cobas c111 Creatinine<br />
Kinase ROCHE DIAGNOSTICS GmbH, GERMANY PT. ROCHE INDONESIA<br />
ROCHE CREP2 cobas cIII<br />
Creatinine Plus Ver.2 ROCHE DIAGNOSTICS GmbH, GERMANY PT. ROCHE INDONESIA<br />
ROCHE AMYL2 cobas c11 a-<br />
Amylase EPS Ver.2 ROCHE DIAGNOSTIC GmbH., GERMANY PT. ROCHE INDONESIA<br />
ROCHE GGT-2 cobas c111<br />
Glutamyltransferase Ver.2 ROCHE DIAGNOSTICS GMBH., Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101904944 05/10/2009 VIDAS Cortisol S BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101904946 05/10/2009 VIDAS Troponin I Ultra (TNIU) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102604486 31/03/2009 Finnpipette Novus Multichannel THERMO FISHER SCIENTIFIC., FINLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302604122 22/10/2009<br />
PDF Creator - PDF4Free v3.0<br />
DAVINCI QUATTRO Automated<br />
Microtiter Plate Processor +<br />
Accessories BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
http://www.pdf4free.com
AKL<br />
20303603599 30/03/2009 SD BIOLINE Syphilis 3.0 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305402295 22/05/2009<br />
AKL<br />
30305602180 30/03/2009<br />
ABBOTT Axsym System HBe 2.0<br />
Quantitative Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
INTEC One Step Anti-HIV (1 & 2) Tri<br />
Line Test Card INTEC PRODUCTS INC., CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20101904948 05/10/2009 VIDAS TSH3 (TSH3) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301601927 30/12/2009<br />
OXOID Diagnostics Sensitive Test<br />
Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601931 30/12/2009 OXOID Hoyle Medium Base OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601924 30/12/2009 OXOID Buffered Peptone Water OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601922 31/12/2009 OXOID Salmonella Shigela Agar OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601920 30/12/2009 OXOID Lactose Broth OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601845 31/12/2009 OXOID DRBC Agar Base OXOID LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906995 30/12/2009<br />
OXOID Staph-Strep Selective<br />
Supplement OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906997 30/12/2009 OXOID GN Anaerob Supplement OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906985 30/12/2009 OXOID V.C.N Selective Supplement OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21106906567 23/12/2009 TPC Injection Pipettes (LISL)<br />
AKL<br />
20103600349 31/12/2009<br />
DIMA One Step Benzodiazepine<br />
Test<br />
AKL<br />
21106906568 23/12/2009 TPC Injection Pipettes (ZISL)<br />
AKL<br />
21106906561 23/12/2009 TPC Holding Pipettes (ZHS)<br />
AKL<br />
21106906566 23/12/2009 TPC Injection Pipettes (LISR)<br />
AKL<br />
21106906562 23/12/2009 TPC Embryo Biopsy Pipettes (LBSL)<br />
THE PIPETTE COMPANY (TPC),<br />
AUSTRALIA<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
THE PIPETTE COMPANY (TPC),<br />
AUSTRALIA<br />
THE PIPETTE COMPANY (TPC),<br />
AUSTRALIA<br />
THE PIPETTE COMPANY (TPC),<br />
AUSTRALIA<br />
THE PIPETTE COMPANY (TPC),<br />
AUSTRALIA<br />
AKL<br />
THE PIPETTE COMPANY (TPC),<br />
21106906558 23/12/2009 TPC Embryo Biopsy Pipettes (LBSR) AUSTRALIA<br />
AKL<br />
10302601470 30/12/2009<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
OXOID Perfringens (SFP) Selective<br />
Supplement OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601464 30/12/2009 OXOID Nutrient Gelatin OXOID LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906266 16/12/2009 OXOID Carbenicillin CAR100 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906269 16/12/2009 OXOID Cefamandole MA30 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302907072 30/12/2009 OXOID Malt Extract Dessicated OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906649 28/12/2009 OXOID Antibiotic Medium No. 3 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601472 30/12/2009 OXOID Trytose Blood Agar Base OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601466 30/12/2009 OXOID MLGA OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601462 31/12/2009 OXOID Sabouraud Liquid Medium OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601463 30/12/2009 OXOID An Indent Discs OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21106906564 23/12/2009 The Stripper Tips-150um MID-ATLANTIC DIAGNOSTICS INC., USA<br />
AKL<br />
10302601461 30/12/2009<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
OXOID C.D.M.N Selective<br />
Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21106906565 23/12/2009 The Stripper Micropipette MID-ATLANTIC DIAGNOSTICS INC., USA<br />
AKL<br />
21106906560 23/12/2009 The Stripper-CC Tips MID-ATLANTIC DIAGNOSTIC INC., USA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20101601341 22/10/2009 BIOMERIEUX Ca - OCP BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302601300 30/12/2009 OXOID Todd Hewitt Broth OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301907070 30/12/2009<br />
OXOID Haemophilus Test (HTM)<br />
Supplement OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305505137 28/12/2009 COBAS Taqman HBV Test ROCHE MOLECULAR SYSTEM, INC, USA PT. ROCHE INDONESIA<br />
AKL<br />
20207505124 30/09/2009<br />
HELENA Activated Partial<br />
Thromboplastin Time (APPT)<br />
Reagent Kit<br />
HELENA LABORATORIES., USA<br />
AKL<br />
20207505123 30/09/2009 HELENA Thromboplastin Reagent HELENA LABORATORIES., USA<br />
AKL<br />
20206505136 30/09/2009 COLO CARE HELENA LABORATORIES., USA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20301905478 16/10/2009 BIODISCS Spiramycin - 100 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10208504982 31/12/2009 ABX Lysebio HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504983 31/12/2009 ABX PENTRA LDH CP HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208504950 28/05/2009<br />
AKL<br />
10101504954 13/11/2009<br />
AKL<br />
20305904031 26/08/2009<br />
AKL<br />
20101504955 30/09/2009<br />
AKL<br />
10101504953 16/10/2009<br />
AKL<br />
10101504949 03/07/2009<br />
AKL<br />
20103403599 19/05/2009<br />
AKL<br />
20103403602 19/05/2009<br />
VITROS Chemistry Products FS<br />
Diluent Pack 1 (Apo Diluent/UED)<br />
VITROS Chemistry Products dHDL<br />
Slide<br />
VITROS Chemistry Products<br />
Calibrator 17<br />
VITROS Chemixtry Products<br />
Calibrator Kit 25<br />
VITROS Chemistry Products dHDL<br />
Reagent<br />
VITROS Chemistry Products dL DL<br />
Reagent<br />
NARCO Check MOP<br />
(Morphine/Heroin)<br />
NARCO Check MET<br />
(Methamphetamine)<br />
AKL<br />
20103403600 19/05/2009 NARCO Check COC (Cocaine)<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
DIVISION of JOHNSON & JOHNSON<br />
COMPANY., USA<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY CO.,<br />
USA<br />
IND DIAGNOSTIC., CANADA for CYNERGEN<br />
HEALTH LLC., USA<br />
IND DIAGNOSTIC., CANADA for CYNERGEN<br />
HEALTH LLC., USA<br />
IND DIAGNOSTIC., CANADA for CYNERGEN<br />
HEALTH LLC., USA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
AKL<br />
10204907000 30/12/2009 ABX PENTRA Clean-Chem pp CP HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20301504930 16/10/2009 OXOID Ceftibuten CFT 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905809 23/10/2009 OXOID Simmons Citrate Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905618 20/10/2009 OXOID K-F Streptococcus Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905032 08/10/2009<br />
OXOID Sodium Chloride<br />
Bacteriological OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905619 20/10/2009 OXOID Peptone Special OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301904995 08/10/2009 OXOID Antibiotic Medium N0. 1 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504895 16/10/2009 OXOID Ampicillin 10 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504896 16/10/2009 OXOID Amoxy / Clav Aciid OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504898 16/10/2009 OXOID Furazolidone OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905616 20/10/2009<br />
OXOID Aeromonas Medium Base<br />
(Ryan) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504894 16/10/2009 OXOID Sulbactam 20 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905617 20/10/2009 OXOID Heart Infusion Broth OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101905865 26/10/2009<br />
RAPIDLAB 1200 Blood Gas<br />
Analyzer + Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302904998 08/10/2009 OXOID Chromogenic Listeria Agar OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905815 23/10/2009 OXOID Wort Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302904999 08/10/2009 OXOID Buffered Peptone Water OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905812 23/10/2009<br />
OXOID Thioglycollate medium<br />
(Brewer) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302904997 08/10/2009 OXOID Skim Milk Powder OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101403265 07/08/2009 PREGCY Check Test Cassette<br />
AKL<br />
20101504761 31/12/2009<br />
GEM 3000 Cartridge BG / HCT /<br />
Electrolytes / Gluc<br />
AKL<br />
20101504760 30/12/2009 GEM CVP<br />
AKL<br />
20101504747 23/12/2009 lL Test Urea<br />
AKL<br />
20101504746 23/12/2009 lL Test AST/GOT<br />
AKL<br />
20101504749 23/12/2009 lL Test Gluc (Glucose Oxidase)<br />
AKL<br />
10101504751 30/12/2009 lL Test LDH-P<br />
AKL<br />
20101504742 30/12/2009 lL Test Calcium<br />
AKL<br />
10101504743 23/12/2009 lL Test Triglycerides<br />
AKL<br />
20101504728 23/12/2009 IL TEST Alkaline Phosphatase<br />
AKL<br />
20101504727 23/12/2009 ILTEST Total Protein<br />
AKL<br />
20101504716 30/12/2009 IL TEST Total Bilirubin<br />
AKL<br />
20101504720 30/12/2009 IL Test Albumin<br />
AKL<br />
10101504725 23/12/2009 IL TEST Uric Acid<br />
AKL<br />
10101504723 23/12/2009 ILTEST HDL Cholesterol<br />
AKL<br />
10101504719 30/12/2009 IL Test y-GT<br />
AKL<br />
20101504703 12/11/2009<br />
AEROSET/c8000 Plasma Protein<br />
Cal<br />
AKL<br />
10101504711 12/11/2009 AEROSET/c8000 Immuno Control 1<br />
AKL<br />
10101504712 12/11/2009<br />
AKL<br />
10101504702 12/11/2009<br />
AEROSET/c8000 Immuno Control<br />
Set(2 Levels)<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
INSTRUMENTATION LABORATORY APA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY APA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY APA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY APA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
PT. UNITED DICO CITAS<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AEROSET/c8000 Quantia Proteins<br />
Control BIOKIT S.A., SPAIN PT. ABBOTT INDONESIA<br />
AKL<br />
10101504704 12/11/2009 AEROSET/c8000 Immuno Control 2<br />
AKL<br />
20305504706 12/11/2009<br />
AKL<br />
20305504707 15/10/2009<br />
AKL<br />
20205504701 09/11/2009<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Immunoglobulin M ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Transferin TRF ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT Cell-Dyn Saphire Analyzer<br />
and Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20205504658 04/06/2009 HUMACOUNT 5 HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10305504705 09/11/2009<br />
ABBOTT Clinical Chemistry<br />
Prealbumin PAlb ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20205504645 31/12/2009 ABX Micros 60 OT HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504663 07/07/2009 ONETOUCH Test Strip<br />
LIFESCAN INC., A JOHNSON & JOHNSON<br />
COMPANY, USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20205504646 31/12/2009 ABX PENTRA 60 OT HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504653 31/12/2009 ABX PENTRA Pottasium - E HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504656 31/12/2009 ABX PENTRA Reference - E HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504655 31/12/2009 ABX PENTRA Reference HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504650 31/12/2009 ABX PENTRA Sodium - E HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504649 31/12/2009 ABX PENTRA Chloride - E HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10204504648 23/12/2009 ABX PENTRA Quali test Solution HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208504654 23/12/2009 ABX Leucodiff HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101905833 23/10/2009<br />
AEROSET/c8000 Clin Chem Control<br />
2<br />
AKL<br />
20101504526 12/11/2009 AEROSET/c8000 Clin Chem Cal<br />
AKL<br />
20101504527 09/11/2009 AEROSET/c8000 CKMB Calibrator<br />
AKL<br />
10101905884 26/10/2009<br />
AKL<br />
20207504402 28/05/2009<br />
AEROSET/c8000 Clin Chem Control<br />
1<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH. s.r.l., ITALY untuk ABBOTT<br />
GmbH & CO. KG., GERMANY<br />
SENTINEL CH.SPA, ITALY for ABBOTT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
hHUMAN HbA1C % Liquidirect<br />
Calibrator HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101504398 28/05/2009 AUTOCAL HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303504401 28/05/2009 HEXAGEN Strep A HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10208504397 03/07/2009 HUMAN HC - Lyse CF HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10204504403 03/07/2009 HUMAN HC - Cleaner HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10208504400 03/07/2009 HUMAN HC - Diluent HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205504394 28/12/2009 DIALAB DIACheck C1 DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305504391 28/12/2009 High Pure Viral Nucleic Acid Kit<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10102504396 30/12/2009 DIALAB Diareader DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10204906972 30/12/2009 DIALAB Diawasher II DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10302504322 04/06/2009 HUMATHERM HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102504327 04/06/2009 HUMAHELP HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205504320 04/06/2009 HUMACLOT Junior HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102504321 04/06/2009 HUMASTEP HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205504328 04/06/2009 HUMACOUNT PLUS HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20102504309 16/12/2009<br />
AKL<br />
20205504283 12/11/2009<br />
SENSITIF Strip Uji Kehamilan<br />
Pribadi<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PT. DANPAC PHARMA<br />
BALL Coagulometer MC 1,<br />
Accessories and Spare Parts MERLIN MEDICAL GmbH, GERMANY PT. RIOCA MEDICA<br />
AKL<br />
10303504271 17/07/2009 SD BIOLINE Influenza Antigen STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20207300966 23/12/2009 BIO - TP BIOLABO SA, FRANCE<br />
AKL<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
20101504201 12/11/2009 CAROLINA CALCIUM (CA) Reagent USA<br />
AKL<br />
10204903905 25/08/2009<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. RIOCA MEDICA<br />
CAROLINA Wash Concentrate II<br />
Reagent CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
20101504191 12/11/2009 CAROLINA BUN Reagent<br />
AKL<br />
20101504193 12/11/2009 CAROLINA CA Calcium Reagent<br />
AKL<br />
20101504196 12/11/2009<br />
AKL<br />
20101504195 24/08/2009<br />
AKL<br />
20101903903 25/08/2009<br />
AKL<br />
20101903902 25/08/2009<br />
AKL<br />
10204903906 25/08/2009<br />
AKL<br />
10101504192 12/11/2009<br />
AKL<br />
10204903901 25/08/2009<br />
AKL<br />
10204903900 25/08/2009<br />
PDF Creator - PDF4Free v3.0<br />
CAROLINA CALIBRATOR<br />
STANDARD I dan II Reagent<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
PT. RIOCA MEDICA<br />
PT. RIOCA MEDICA<br />
PT. RIOCA MEDICA<br />
CAROLINA Calibrator 1, 2 dan 3<br />
Synchron CX System CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
CAROLINA Homocysteine (HCY)<br />
Reagent CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
CAROLINA Homocysteine Reagent<br />
Kit CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
CAROLINA Wash Concentrate I<br />
Reagent CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
CAROLINA Alanine Transaminase<br />
Reagent (ALT)<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
PT. RIOCA MEDICA<br />
CAROLINA ISE Electrolyte Buffer<br />
Reagent CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
CAROLINA ISE Electrolyte<br />
Reference CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
http://www.pdf4free.com
AKL<br />
10102504187 30/12/2009 DIALAB Photometer DTN 410 Dialab Ges.M.B.H, Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305504164 09/11/2009<br />
AKL<br />
20101504158 09/11/2009<br />
AKL<br />
20103504157 13/11/2009<br />
AKL<br />
20103905887 26/10/2009<br />
AKL<br />
10101905728 21/10/2009<br />
ABBOTT IMX HbsAg (V2) Reagent<br />
Pack ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT Axsym Valproic Acid<br />
Standard Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Axsym Gentamycin<br />
Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Valproic Acid<br />
Reagent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
BIOMERIEUX Phosphore UV<br />
(PHOS UV) BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301504074 16/10/2009 OXOID Cephalexin 30 ug - CL 30 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504075 21/08/2009 OXOID Ceflactor 30 ug - CEC 30 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504073 21/08/2009 OXOID Ciprofloxacin 1ug - CIP 1 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504076 21/08/2009<br />
AKL<br />
20301504070 21/08/2009<br />
OXOID Phosphate Buffered Saline<br />
Tablets OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Spectinomycin 25 ug - SH<br />
25 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504071 16/10/2009 OXOID Enrofloxacin 5 ug - ENR 5 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504072 28/07/2009 Oxoid Ciprofloxacin - CIP10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101504077 09/11/2009 DSI Trulab L<br />
AKL<br />
20305504048 09/11/2009 CRP Control HS<br />
AKL<br />
20305504047 09/11/2009<br />
AEROSET/C8000 Quantia B2-<br />
Microglobulin Standard<br />
AKL<br />
20305504045 09/11/2009 CRP Calibrator HS<br />
AKL<br />
20305504043 12/11/2009 ARCHITECT Ceruloplasmin<br />
AKL<br />
20305504046 09/11/2009<br />
AEROSET/C8000 CRP Calibrator<br />
Set<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
SENTINEL CH SpA, ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
BIOKIT S.A., SPAIN for ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH SpA, ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
SENTINEL CH, SPA., ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
SENTINEL CH Spa., ITALY for ABBOTT<br />
LABORATORIES., USA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101504044 24/08/2009 AEROSEL C 8000 CK-MB Control SENTINEL CH Srl, ITALY PT. ABBOTT INDONESIA<br />
AKL<br />
20301905622 20/10/2009 OXOID Novobiocin 5 ug - NV 5 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905350 14/10/2009 OXOID Nalidixic Acid NA 30 OXOID Limited, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905620 20/10/2009 OXOID Apramycin 15 ug - APR 15 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905351 14/10/2009 OXOID Penicillin G 1 unit P1 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301903884 21/08/2009 OXOID Rifamcipin 2 ug - RD 2 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301903885 21/08/2009 OXOID Spectinomycin 100 ug OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302503036 21/08/2009<br />
OXOID Brilliant Green Agar<br />
(Modified) OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101503015 30/12/2009 DIALAB Alpha - HBDH DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10302905175 12/10/2009 API ZYM BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303904030 26/08/2009<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV Total Reagent<br />
Pack<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO., USA<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20303904032 26/08/2009<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV Total Controls<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO., UK<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20305502959 28/05/2009<br />
AKL<br />
20101502963 22/05/2009<br />
VITROS Immunodiagnostics<br />
Products Myoglobin Reagent Pack<br />
VITROS Immunodiagnostics<br />
Products Total B-hCG Calibrators<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
DIVISION JOHNSON & JOHNSON<br />
COMPANY., USA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20303904033 26/08/2009<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV Total Calibrator<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO., UK<br />
http://www.pdf4free.com<br />
PT. TAWADA HEALTHCARE
AKL<br />
20101502958 28/05/2009<br />
VITROS Immunodiagnostics<br />
Products Folate Reagent Pack 1/2<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20305901986 28/05/2009 IMTEC-CCP-Antibodies HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205502953 28/12/2009<br />
ERMA PCE-210 Hematology Auto<br />
Analyzer<br />
ERMA INC., JAPAN<br />
AKL<br />
20303502976 08/10/2009 FOKUS RPR Carbon Antigen PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20103502927 24/08/2009<br />
AKL<br />
20103502936 24/08/2009<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
ABBOTT Axsym Phenobarbital<br />
Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Axsym Phenobarbital<br />
Standard Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10103905663 20/10/2009 ABOTT AXSYM Tobramycin Control ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20103502922 09/11/2009<br />
AKL<br />
20103502921 09/11/2009<br />
AKL<br />
20103502920 10/06/2009<br />
AKL<br />
20103502918 10/06/2009<br />
AKL<br />
20103502908 10/06/2009<br />
ABBOTT Axsym Tobramycin<br />
Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Tobramycin<br />
Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System<br />
Vancomycin II Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Vancomycin II<br />
Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System<br />
Vancomycin II Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20303905834 23/10/2009 ARCHITECT Toxo IgM Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20103905664 20/10/2009<br />
ABBOTT AXSYM Quinidine<br />
Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10103502924 09/11/2009 ABBOTT AXSYM Quinidine Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20103905665 20/10/2009<br />
AKL<br />
10204903899 25/08/2009<br />
AKL<br />
20101502930 11/11/2009 Bio-Cal<br />
ABBOTT AXSYM Quinidine Reagent<br />
Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT IMX Probe Cleaning<br />
Solution ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
20101502897 23/12/2009 DIALAB Lp (a) Calibrator DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101502896 23/12/2009 DIALAB LP (a) Control DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10102502850 13/07/2009 VITRON 5,1 Fs Chemistry System<br />
AKL<br />
20305502854 01/09/2009<br />
AKL<br />
20305502856 01/09/2009<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
DIVISION OF JOHNSON & JOHNSON<br />
COMPANY., USA<br />
PT. TAWADA HEALTHCARE<br />
SYSMEX C4 Reagent ND (C4<br />
Immuno Reagent-S) SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
SYSMEX IgG Reagent ND (IgG<br />
Immuno Reagent-S) SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101502860 01/09/2009 SYSMEX TP Standard SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101502859 01/09/2009 SYSMEX m-ALB Standard SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101502858 01/09/2009 SYSMEX m-TP Standard SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20305502853 01/09/2009<br />
AKL<br />
20303502848 14/10/2009<br />
AKL<br />
20101600199 15/10/2009<br />
AKL<br />
20303502846 15/10/2009<br />
AKL<br />
20305502857 01/09/2009<br />
AKL<br />
20305502855 01/09/2009<br />
SYSMEX IgG Reagent ND (IgM<br />
Immuno Reagent-S) SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AMPLICOR HPV Controls Kit (HPV<br />
CTL)<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
ABBOTT AXSYM System LH<br />
Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AMPLICOR HPV Detection Kit (HPV<br />
MW PDK)<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
SYSMEX C3 Reagent ND (C3<br />
Immuno Reagent-S) SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
SYSMEX IgG Reagent ND (IgA<br />
ImmunoReagent-S) SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20306905883 26/10/2009 ARCHITECT CA 15-3 Controls<br />
AKL<br />
20306905832 23/10/2009 ARCHITECT CA 15-3 Calibrators<br />
PDF Creator - PDF4Free v3.0<br />
FUJIREBIO DIAGNOSTICS INC, USA, untuk<br />
ABBOTT LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTICS INC, USA, untuk<br />
ABBOTT LABORATORIES, USA<br />
http://www.pdf4free.com<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA
AKL<br />
20306905831 23/10/2009 ARCHITECT CA 15-3 Reagent Kit<br />
AKL<br />
20303502849 15/10/2009<br />
AKL<br />
20101502824 07/08/2009<br />
AKL<br />
20101502825 07/08/2009<br />
AMPLICOR HPV Amplification Kit<br />
(HPV AMP)<br />
FUJIREBIO DIAGNOSTICS INC, USA, untuk<br />
ABBOTT LABORATORIES, USA<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
ROCHE BILTS - Total Bilirubin<br />
Special Cobas Integra Cobas c<br />
systems ROCHE DIAGNOSTICS GmbH., Germany PT. ROCHE INDONESIA<br />
ROCHE/HITACHI Total Bilirubin -<br />
TBILI ROCHE DIAGNOSTICS GmbH., Germany PT. ROCHE INDONESIA<br />
AKL<br />
10101905081 09/10/2009<br />
AKL<br />
20101905080 09/10/2009<br />
AKL<br />
10101502827 15/10/2009<br />
ROCHE Ammonia/Ethanol/CO2<br />
Control Abnormal Roche Systems ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
ROCHE Ammonia/Ethanol/CO2<br />
CAlibrator Roche System ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
ROCHE<br />
Ammonia/Ethanol/CO2Control<br />
Normal Roche Systems ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10302903883 21/08/2009 OXOID Dextrose Bacteriological OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305502774 25/09/2009 VIDAS Anti - HBc Total II (HBCT) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905348 14/10/2009 OXOID Pefloxacin PEF 5 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301502771 21/08/2009 OXOID Ceftazidime 30 ug - CA2 30 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905355 14/10/2009 OXOID Vencomycin VA 30 OXOID Limited, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301502773 16/10/2009 OXOID Nitrofurantoin 300 ug - F300 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301505807 23/10/2009 OXOID Polymyxin B-PB 300 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301505352 14/10/2009<br />
AKL<br />
10102904970 08/10/2009<br />
OXOID Piperacillin/Tazobactam TZP<br />
110 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
HOSPITEK Nephelometer and<br />
Accessories HOSPITEK DIAGNOSTICS S.R.L., JAKARTA PT. GRAHA ISMAYA LTD<br />
AKL<br />
20301502767 16/10/2009 OXOID Doxycycline 30 ug - DO 30 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301502766 09/11/2009 OXOID Levofloxacin 5 ug - LEV 5 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905621 20/10/2009 OXOID Metronidazole 5 ug - MTZ 5 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301502764 21/08/2009 OXOID Cephradine 30 ug CE - 30 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905349 14/10/2009 OXOID OXACILLIN OX 5 OXOID Limited, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301502758 09/11/2009<br />
OXOID Moxifloxacin susceptibility<br />
test disc OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301502759 16/10/2009 OXOID Netilmicin 30ug - NET 30 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303502752 28/12/2009<br />
PRO DIAGNOSTICS Salmonella<br />
Typhi H Antigen<br />
AKL<br />
20305502754 05/10/2009 PRO Diagnostics CRP Latex Test<br />
AKL<br />
20305502756 05/11/2009 PRO Diagnostics RA Latex Test Kit<br />
AKL<br />
20303502751 28/12/2009<br />
AKL<br />
20303502753 28/12/2009<br />
AKL<br />
20303502746 28/12/2009<br />
AKL<br />
20303502747 28/12/2009<br />
PRO DIAGNOSTICS Salmonella H<br />
Paratyphi A Antigen<br />
PRO DIAGNOSTICS Salmonella H<br />
Paratyphi C Antigen<br />
PRO DIAGNOSTICS Salmonella O<br />
Paratyphi B Antigen<br />
PRO DIAGNOSTICS Salmonella O<br />
Paratyphi A Antigen<br />
AKL<br />
20303502749 05/11/2009 PRO Diagnostics RPR Test Kit<br />
AKL<br />
20303502748 23/12/2009<br />
AKL<br />
20303502750 23/12/2009<br />
AKL<br />
20303502742 28/12/2009<br />
PRO Diagnostics RPR Test Kit<br />
Special<br />
PRO Diagnostics VDRL Carbon<br />
Antigen<br />
PRO DIAGNOSTICS Salmonella H<br />
Paratyphi B Antigen<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303502745 16/12/2009 PRO Diagnostics TPHA Test Kit<br />
AKL<br />
20303502741 28/12/2009<br />
AKL<br />
20303502740 28/12/2009<br />
PRO DIAGNOSTICS Salmonella<br />
Typhi O Antigen<br />
PRO DIAGNOSTICS Salmonella O<br />
Paratyphi C Antigen<br />
AKL<br />
20209906265 16/12/2009 PRO Diagnostics Anti B Monoclonal<br />
AKL<br />
20209502739 23/12/2009 PRO Diagnostics Anti A Monoclonal<br />
AKL<br />
20209502744 23/12/2009 PRO Diagnostics Anti D Monoclonal<br />
AKL<br />
20303502738 16/12/2009 PRO Diagnostics Anti B Monoclonal<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LIMITED, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
AKL<br />
20305502715 08/10/2009 FOKUS CRP Latex Check Test PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20305502706 08/10/2009 FOKUS RF Latex Test PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20103502713 08/10/2009<br />
AKL<br />
20103502716 08/10/2009<br />
AKL<br />
20103502714 08/10/2009<br />
FOKUS Marijuana Check Test<br />
Device<br />
FOKUS Morphine Check Test<br />
Device<br />
FOKUS Amphetamine Check Test<br />
Device<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20103502707 14/10/2009 FOKUS URI Check 2K PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20103502709 08/10/2009<br />
FOKUS Benzodiazephine Check<br />
Test Device<br />
AKL<br />
20101502657 11/11/2009 FLUITEST Ca-A-III<br />
AKL<br />
10101502647 07/08/2009 MENO Check Test Midstream<br />
AKL<br />
20101502648 07/08/2009 PREGGY Check Test Midstream<br />
AKL<br />
10101502642 07/08/2009 MENO Check Test Cassette<br />
AKL<br />
10101502644 07/08/2009 OVU Check Test Midstream<br />
AKL<br />
10101502643 07/08/2009 OVU Check Test Strip<br />
AKL<br />
10101502646 07/08/2009 MENO Check Test Strip<br />
AKL<br />
10101502645 07/08/2009 OVU Check Test Cassette<br />
PULSE SCIENTIFIC INC., CANADA<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
AKL<br />
PLASMATEC LABORATORY PRODUCTS<br />
20303502490 08/06/2009 PLASMATEC VDRL Carbon Antigen LTD., UK<br />
AKL<br />
20101502482 04/06/2009<br />
AKL<br />
10101502495 09/11/2009<br />
AKL<br />
10101502491 11/11/2009<br />
AKL<br />
10101502489 11/11/2009<br />
PLASMATEC Pregnancy Latex Test<br />
Kit<br />
LIQUICHECK Urinalysis Control<br />
Level I<br />
LIQUICHEK Urinalysis Control<br />
Bilevel<br />
LIQUICHEK Urinalysis Control Level<br />
II<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. UNITED DICO CITAS<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20101502488 22/12/2009 QUANTASE Neonatal TSH BIORAD LABORATORIES, USA PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10102502437 11/11/2009 COBAS u411 and Accessories<br />
AKL<br />
20307502442 09/11/2009 QUANTIA D-Dimer Reagent<br />
AKL<br />
20207904771 01/10/2009 MULTIGENT HbA1c<br />
ROCHE DIAGNOSTICS LTD,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GMBH., GERMANY<br />
BIOKIT SA, SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
SERADYN INC., USA untuk ABBOTT<br />
LABORATORIES, USA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10302905475 16/10/2009<br />
AKL<br />
20305904968 08/10/2009 QUANTIA RF Standard Reagent<br />
BIOMERIEUX Count-Tact<br />
Sabouraud Dextrose Chloramphenicol<br />
Neutralizers Irradiated Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOKIT S.A., SPAIN for ABBOT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303904977 08/10/2009 QUANTIA ASO Standard Reagent<br />
AKL<br />
20208905880 26/10/2009 QUANTIA D-Dimer Standard<br />
AKL<br />
20305904978 08/10/2009 QUANTIA RF Reagent<br />
AKL<br />
10303502446 12/10/2009 QUANTIA ASO Reagents<br />
AKL<br />
20208905879 26/10/2009 QUANTIA D-Dimer Control<br />
AKL<br />
10101502440 09/11/2009 QUANTIA Lp(a) Control<br />
AKL<br />
20101905881 26/10/2009 QUANTIA Lp(a) Standard<br />
AKL<br />
10101502438 12/10/2009 QUANTIA Lp (a) Reagent<br />
AKL<br />
10101502453 09/11/2009<br />
AKL<br />
10101502452 12/11/2009<br />
ABBOTT Clinical Chemistry<br />
Apolipoprotein B<br />
BIOKIT S.A., SPAIN for ABBOT<br />
LABORATORIES, USA<br />
BIOKIT SA., SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT S.A., SPAIN for ABBOT<br />
LABORATORIES, USA<br />
BIOKIT S.A., SPAIN for ABBOT<br />
LABORATORIES, USA<br />
BIOKIT SA., SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT SA, SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT SA., SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT S.A., SPAIN for ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT SA, SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Apolipoprotein A1 ApoA ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20102906998 30/12/2009 ARKRAY Aution Jet Aj-4270 ARKRAY FACTORY INC., JAPAN<br />
AKL<br />
10103502335 26/08/2009 ABBOTT Cholinesterase<br />
AKL<br />
10101502340 26/08/2009<br />
AKL<br />
20305904770 01/10/2009<br />
AKL<br />
10101502339 30/09/2009<br />
AKL<br />
20101904028 26/08/2009<br />
AKL<br />
20101110210 26/08/2009<br />
SENTINEL CH., SpA., ITALY for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABBOTT INDONESIA<br />
AEROSET/C8000 Cholesterol HDL<br />
Liquid SENTINEL CH S.rl., ITALY PT. ABBOTT INDONESIA<br />
AEROSET/C8000 CRP Ultra<br />
Reagent<br />
AEROSET/C8000 Cholesterol LDL<br />
Liquid<br />
SENTINEL CH S.r.l., ITALY untuk ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH S.r.l., ITALY untuk ABBOTT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AEROSET/C8000 HDL/LDL<br />
Calibrator SENTINEL CH s.r.l., ITALY PT. ABBOTT INDONESIA<br />
AEROSET/ARCHITECT CK-MB<br />
Liquid<br />
SENTINEL CH SpA, ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101502338 26/08/2009 AEROSET/C8000 Lipids Control SENTINEL CH S.rl., ITALY PT. ABBOTT INDONESIA<br />
AKL<br />
20101502183 17/07/2009 SD UroColor 4 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101502184 17/07/2009 SD UroColor 3A STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101502182 17/07/2009 SD UroColor 2K STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101502185 17/07/2009 SD Urocolor 2 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10101502181 17/07/2009 SD UroColor 1P STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20306502223 19/05/2009<br />
AKL<br />
20306502216 19/05/2009<br />
AKL<br />
20101502220 22/05/2009<br />
GBC Prostate Specific Antigen GENERAL BIOLOGICALS CORPORATION.,<br />
(PSA) Enzyme Immunoassay Test Kit TAIWAN R.O.C<br />
GB Cancer Antigen CA 19-9 Enzyme<br />
Immunoassay Test Kit<br />
GB Triiodothyronine (T3) Enzyme<br />
Immunoassay Test Kit<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101502219 22/05/2009<br />
AKL<br />
20101502215 22/05/2009<br />
AKL<br />
20101502218 22/05/2009<br />
AKL<br />
20101502202 26/08/2009<br />
AKL<br />
20101904029 26/08/2009<br />
GB Thyroid Stimulating Hormone GENERAL BIOLOGICALS CORPORATION.,<br />
(TSH) Enzyme Immunoassay Test Kit TAIWAN R.O.C<br />
GB Total Thyroxine (T4) Enzyme<br />
Immunoassay Test Kit<br />
GBC Free Triiodothyronine (FT3)<br />
Enzyme Immunoassay Test Kit<br />
ABBOTT AXSYM System BNP<br />
Standard Calibrator<br />
ABBOTT AXSYM System BNP<br />
Reagent Pack<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT LABORATORIES., USA<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT LABORATORIES., USA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305502137 01/09/2009<br />
ELECSYS Preci Control HIV Combi<br />
Elecsys and Cobas e Analyzer ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101502132 26/08/2009 TOUCH-IN Blood Glucose Test Strip APEX BIOTECHNOLOGY COPR., R.O.C PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
10101502133 01/10/2009 UA Sure Blood Uric Acid Test Strip<br />
AKL<br />
UA Sure Blood Uric Acid Monitoring<br />
10101502134 01/10/2009 System<br />
PDF Creator - PDF4Free v3.0<br />
APEX BIOTECHNOLOGY CORP, TAIWAN,<br />
R.O.C<br />
APEX BIOTECHNOLOGY CORP, TAIWAN,<br />
R.O.C<br />
http://www.pdf4free.com<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA
AKL<br />
20101502135 01/10/2009<br />
GLUCOSURE PLUS Blood Glucose<br />
Monitoring System<br />
APEX BIOTECHNOLOGY CORP, TAIWAN,<br />
R.O.C<br />
PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
10203502082 04/06/2009 HUMASCOPE HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305502089 16/12/2009 PRO - HBsAg Serum Test<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., CHINA<br />
PT. PILAR REJEKI OPTIMA<br />
AKL<br />
20305502088 16/12/2009 PRO HCV Elisa ALFA SCIENTIFIC DESIGNS INC., USA PT. PILAR REJEKI OPTIMA<br />
AKL<br />
20101502090 16/12/2009 PRO HCG Urine Test<br />
AKL<br />
20305502079 13/07/2009<br />
AKL<br />
20305502078 13/07/2009<br />
ABBOTT AXSYM Anti-Tg Standard<br />
Calibrators<br />
ABBOTT Axsym Anti-Tg Reagent<br />
Pack<br />
AKL<br />
20305502073 13/07/2009 ABBOTT Axsym Anti-Tg Controls<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., CHINA<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK for<br />
ABBOTT LABORATORIES., USA<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK for<br />
ABBOTT LABORATORIES., USA<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK for<br />
ABBOTT LABORATORIES., USA<br />
AKL<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
20305502077 26/08/2009 ABBOTT AXSYM Anti-TPO Controls ABBOTT LABORATORIES., USA<br />
AKL<br />
20305502071 26/08/2009<br />
AKL<br />
10101502074 26/08/2009<br />
ABBOTT AXSYM System Anti-TPO<br />
Standard Calibrator<br />
ABBOTT AXSYM System BNP<br />
Controls<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT LABORATORIES., USA<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT LABORATORIES., USA<br />
PT. PILAR REJEKI OPTIMA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101502018 17/07/2009 SD UroColor 3 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101502019 17/07/2009 SD UroColor 4K STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101502017 17/07/2009 SD UroColor 11 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101502016 17/07/2009 SD UroColor 10 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305501985 13/07/2009 ABBOTT Imx HBs Ag Controls ABBOTT Gmbh & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305501983 13/07/2009<br />
ABBOTT ARCHITECT Anti-HBc<br />
IgM Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305501984 13/07/2009 ABBOTT IMX HBsAG Confirmatory ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305501982 13/07/2009<br />
AKL<br />
20305501979 10/06/2009<br />
AKL<br />
20305501978 13/07/2009<br />
AKL<br />
20305501980 10/06/2009<br />
AKL<br />
20305501977 10/06/2009<br />
AKL<br />
20305501974 13/07/2009<br />
AKL<br />
20305501976 10/06/2009<br />
ABBOTT ARCHITECT Anti-Hbe<br />
Reagent Pack ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HBeAg<br />
Calibrator ABBOTT GmbH., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT Anti-Hbe<br />
Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT Anti-Hbc IgM<br />
Reagent Kit ABBOTT GmbH., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HBeAg<br />
Controls ABBOTT GmbH., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT Anti HBe<br />
Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HBeAg<br />
Reagent Pack ABBOTT GmbH., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
10201905044 08/10/2009 SYSMEX Urinocatch (UCH-700 A) SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101501940 09/10/2009 SYSMEX UF Check SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10201905042 08/10/2009 SYSMEX Urinosearch SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20208501937 30/09/2009 SYSMEX Ca Cal-S SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10204903904 25/08/2009<br />
CAROLINA Probe Rinse<br />
Concentrate CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
20101501892 13/11/2009 FOKUS hCG Check Strip GENIX TECHNOLOGY INC., CANADA<br />
AKL<br />
20101501893 13/11/2009 FOKUS hCG Check Device GENIX TECHNOLOGY INC., CANADA<br />
AKL<br />
20303904976 08/10/2009<br />
FOKUS Dengue IgG/IgM Check<br />
Device<br />
PULSED SCIENTIFIC Inc., CANADA<br />
AKL<br />
20305501891 13/11/2009 FOKUS Anti HBs Check Device GENIX TECHNOLOGY INC., CANADA<br />
AKL<br />
20305501894 13/11/2009 FOKUS HbsAg Check Strip GENIX TECHNOLOGI INC., CANADA<br />
AKL<br />
20305501885 13/11/2009 FOKUS HBsAg Check Device GENIX TECHNOLOGI INC., CANADA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305501879 13/11/2009 FOKUS Anti-HCV Check Strip GENIX TECHNOLOGI INC., CANADA<br />
AKL<br />
20305501883 13/11/2009 FOKUS Anti HBs Check Strip GENIX TECHNOLOGI INC., CANADA<br />
AKL<br />
20103401735 28/05/2009<br />
AKL<br />
20101501662 12/11/2009<br />
AKL<br />
20101501668 12/11/2009<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
ABBOTT AXSYM System<br />
Theophyllin II Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
CAROLINA ALP Alkaline<br />
Phophatase Reagent<br />
CAROLINA CREA Creatinine<br />
Reagent<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
PT. RIOCA MEDICA<br />
PT. RIOCA MEDICA<br />
AKL<br />
10101501667 21/08/2009 CAROLINA Uric Acid Reagent (Uric) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
20101501659 12/11/2009<br />
AKL<br />
20101501670 26/08/2009<br />
AKL<br />
10101501660 12/11/2009<br />
AKL<br />
20101501663 24/08/2009<br />
CAROLINA AST Aspartate<br />
Transaminase Reagent<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
PT. RIOCA MEDICA<br />
CAROLINA Total Protein Reagent<br />
(TP) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
CAROLINA CHOL Cholesterol<br />
Reagent<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
PT. RIOCA MEDICA<br />
CAROLINA Direct Bilirubin Reagent<br />
(DBIL) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
20101501665 24/08/2009 CAROLINA Glucose Reagent (GLU) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
10101501661 24/08/2009<br />
CAROLINA Phosphorus Reagent<br />
(PO4) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
20101501658 12/11/2009 CAROLINA ALB Albumin Reagent<br />
AKL<br />
10101501669 21/08/2009<br />
CAROLINA LIQUID CHEMISTRIES Corp.,<br />
USA<br />
PT. RIOCA MEDICA<br />
CAROLINA Triglycerides Reagent<br />
(TG) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
AKL<br />
20101501677 17/07/2009 SD UroColor 6 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501678 17/07/2009 SD UroColor 1G STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501672 17/07/2009 SD UroColor 9 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501675 17/07/2009 SD UroColor 7 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501674 17/07/2009 SD UroColor 4S STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501676 17/07/2009 SD UroColor 8 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501673 17/07/2009 SD UroColor 5 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10101501634 01/09/2009 SYSMEX LDL-C P.KL SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20103501613 08/06/2009 dBest One Step MET Strip Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101501618 08/06/2009 dBest One Step HCG Strip Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20206501616 04/06/2009<br />
dBest One Step Ocult Blood Strip<br />
Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101501617 04/06/2009 dBest One Step HCG Card Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103501612 04/06/2009 dBest One Step MET Card Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10101501614 04/06/2009<br />
dBest One Step LH Ovulation Strip<br />
Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20208501633 21/08/2009 RADIOMETER Calibrating Solution I RADIOMETER MEDICAL ApS, DENMARK<br />
AKL<br />
20101501611 28/09/2009 ARKRAY Glucocard X Meter ARKRAY INC., JAPAN<br />
AKL<br />
20306501340 22/05/2009<br />
AKL<br />
20306501341 19/05/2009<br />
AKL<br />
20101501342 22/05/2009<br />
GB Breast Cancer CA 15-3 Enzyme<br />
Immunoassay Test Kit<br />
GB Cancer Antigen CA 125 Enzyme<br />
Immunoassay Test Kit<br />
GBC Free Thyroxine (FT4) Enzyme<br />
Immunoassay Test Kit<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
GENERAL BIOLOGICALS CORPORATION.,<br />
TAIWAN R.O.C<br />
AKL<br />
10102501286 01/09/2009 Accumax Fix FINE CARE CORPORATION, INDIA<br />
AKL<br />
20101501588 10/06/2009<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
ABBOTT AXSYM Total T3 Master<br />
Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101501597 28/05/2009 ABBOTT AXSYM total T3 Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101501585 28/05/2009<br />
AKL<br />
10204501591 28/05/2009<br />
AKL<br />
20303501595 10/06/2009<br />
ABBOTT AXSYM Total T3 Standard<br />
Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Probe Cleaning<br />
Solution ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Toxo IgG<br />
Master Calibrators ABBOTT GmbH CO & KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
10102501285 01/09/2009 Accumax Variable Volume Pipette FINE CARE CORPORATION, INDIA<br />
AKL<br />
10101501561 21/08/2009<br />
AKL<br />
20101501560 21/08/2009<br />
QUICK CHECK One Step Ovulation<br />
Test Device<br />
QUICK CHECK One Step<br />
Pregnancy Test Strip<br />
ACON BIOTECH (HANGZHOU) Co., Ltd.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) Co., Ltd.,<br />
CHINA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305501236 24/08/2009 ABX PENTRA Orosomucoid ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305501228 17/07/2009 ABX PENTRA Haptoglobin HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305501229 31/12/2009 ABX PENTRA Alpha1 - Antitrypsin HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101501231 17/07/2009 ABX PENTRA CO2 Cal HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20207903272 17/07/2009 ABX PENTRA Fructosamine HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305501227 03/07/2009 ABX Pentra Ceruloplasmin HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101501230 17/07/2009 ABX PENTRA CO2 Control HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101501233 17/07/2009<br />
ABX PENTRA Fructo Control P dan<br />
N HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101501226 17/07/2009 ABX PENTRA CO2 RTU HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20208903270 17/07/2009 ABX PENTRA Fructo Cal HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20103504156 12/11/2009<br />
AKL<br />
10101504159 12/11/2009<br />
ABBOTT Axsym Gentamycin<br />
Standard Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Valproic<br />
Acid Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10202903866 21/08/2009 RADIOMETER Salt-Bridge Solution RADIOMETER MEDICAL ApS, DENMARK<br />
AKL<br />
10204904775 01/10/2009 HELENA Clear Aid HELENA LABORATORIES., USA<br />
AKL<br />
20208501559 21/08/2009 RADIOMETER Calibrating Solution RADIOMETER MEDICAL ApS, DENMARK<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20101501502 12/06/2009 HUMAN Auto-Bilirubin D Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101501501 12/06/2009 HUMAN Auto-Bilirubin T Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101501500 12/06/2009 HUMAN Auto-Creatinine Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10205501513 12/06/2009 HUMAN HUMASED 40 HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102501497 12/06/2009 HUMAN Combilyzer Plus HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102501499 21/08/2009<br />
HUMAN Elisys Quattro +<br />
Accessories & Spare Part HUMAN GmbH, GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102501503 12/06/2009 HUMAN HUMA Star HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102501496 30/10/2009<br />
AKL<br />
20103501183 28/05/2009<br />
HUMAN Auto Compylizer and<br />
Accessories HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
ABBOTT AXSYM System<br />
Theophylline II Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10303501481 14/09/2009 MICROSCREEN Staph MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20303501482 14/09/2009 MICROSCREEN Salmonella MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20303904774 01/10/2009 MICROSCREEN Legionella MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20101501107 28/05/2009 SYSMEX CK - MB - L.R1 & R2 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101807467 12/11/2009 DIASYS Total Protein UC FS<br />
AKL<br />
20305501463 28/05/2009<br />
DIASYS DIAGNOSTICS SYSTEM GmbH &<br />
CO. KG., GERMANY<br />
PT. MULTIREDJEKI KITA<br />
ABBOTT ARCHITECT Anti-HCV<br />
Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305501465 28/05/2009<br />
AKL<br />
20305501464 28/05/2009<br />
ABBOTT ARCHITECT Anti-HCV<br />
Reagent Pack ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT Anti-HCV<br />
Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20101501057 01/09/2009 QCA GOT / AST U.V(IFCC) QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101501055 14/08/2009 QCA Total Proteins QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101501053 14/08/2009 QCA Alkaline Phospatase (P-NPP) QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101501051 14/08/2009 QCA Albumin QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101501044 14/08/2009 QCA Gamma - GT QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20205501445 04/05/2009<br />
MINDRAY Automated Hematology<br />
Analyzer<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
AKL<br />
20101500987 12/06/2009<br />
ONE TOUCH ULTRA Blood<br />
Glucose Monitoring System<br />
JOHNSON & JOHNSON MEDICAL (CHINA)<br />
LTD., CHINA for LIFESCAN INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101501434 14/08/2009 QCA Bilirubin QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101501441 01/09/2009 QCA GPT / ALT U.V (IFCC) QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101501427 06/04/2009 QCA HDL Cholesterol Direct Quimica Clinica Aplicada S.A., Spain PT. DUTA INDO OMNITRON<br />
AKL<br />
10101501426 01/09/2009 QCA TGL Liquid QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20207501000 11/11/2009 DSI Hemoglobin FS<br />
AKL<br />
10102906116 20/11/2009 BOECO Micropipettes Fixed Volume<br />
AKL<br />
20101500920 16/06/2009<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH,<br />
GERMANY<br />
BIOHIT OYJ, FINLAND untuk BOECKEL +<br />
Co. KG., GERMANY<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. BAVARIA COMBININDO<br />
ABBOTT AXSYM System Estradiol<br />
Master Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10303500854 30/09/2009 MICROGEN Listeria Latex Kit MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302500856 25/09/2009 MICROGEN GN - ID Reagent Set D MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302500855 14/09/2009 MICROGEN GN - ID A Panel MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20305500775 24/08/2009 ABX PENTRA Kappa ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305500776 24/08/2009 ABX PENTRA Lambda ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305500770 24/08/2009 ABX Pentra IgM ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305500769 24/08/2009 ABX Pentra IgG ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101500773 24/08/2009 ABX PENTRA Prealbumin ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305500768 24/08/2009 ABX PENTRA IgA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101500766 13/07/2009 ABX PENTRA LDL Cal HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101500767 13/07/2009 ABX PENTRA Lipase CP HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101500774 13/07/2009 ABX Pentra HDL Cal HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101907139 31/12/2009<br />
BD Vacutainer Sodium Flouride 5<br />
mg/ Potassium Oxalate 4 mg (FX) /<br />
Plus Blood Collection Tubes<br />
AKL<br />
10101500655 09/11/2009 LIQUICHECK Lipids Control Level II<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20207500653 22/12/2009 D-10 Hemoglobin A1 Program BIORAD LABORATORIES, USA PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10101500654 09/11/2009<br />
LIQUCHECK Urine Chemistry<br />
Control Level I<br />
AKL<br />
10101500651 09/11/2009 LIQUICHECK Lipids Control Level I<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101500652 11/11/2009<br />
AKL<br />
20205500656 16/12/2009<br />
LIQUICHECK Urine Chemistry<br />
Control Level II<br />
BIORAD LABORATORIES, USA atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
D-10 Hemoglobin Testing System<br />
With Accessories BIO-RAD LABORATORIES INC., USA PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20208500688 16/06/2009 SYSMEX Manoresh SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20103500632 21/08/2009<br />
ACON One Step Methadone Test<br />
Device<br />
AKL<br />
20305904966 08/10/2009 QUANTIA ASO-RF Control II<br />
AKL<br />
10302905498 19/10/2009<br />
ACON BIOTECH (HANGZHOU) Co., Ltd.,<br />
CHINA<br />
BIOKIT S.A., SPAIN for ABBOTT<br />
LABORATORIES, USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ABBOTT INDONESIA<br />
BIOMERIEUX Kovacs Reagent<br />
(Kovacs) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101500589 22/05/2009 URISCAN 10 SGL YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500591 22/05/2009 URISCAN 5 Hemoketo GPH Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500585 22/05/2009 URISCAN 2 Gluketo Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500582 22/05/2009 URISCAN 9 SG Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500586 22/05/2009 URISCAN 8 Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
10101500593 22/05/2009 URITROL Level 1,2,3 YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500583 22/05/2009 URISCAN 7 Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
10101500587 22/05/2009 URITROL Level 2 YD DIAGNOSTICS., KOREA<br />
AKL<br />
10101500592 22/05/2009 URISCAN 1 protein Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
10101500588 22/05/2009 URITROL Level 3 YD DIAGNOSTICS., KOREA<br />
AKL<br />
10101500590 22/05/2009 URISCAN 1 Ketones Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
10101500581 22/05/2009 Uritrol Level 1 YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500584 22/05/2009 URISCAN GP 2 YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101500524 22/05/2009 VITROS DT Calibrator Kit<br />
AKL<br />
20101500525 30/06/2009<br />
VITROS Immunodiagnostic Products<br />
TSH Calibrators<br />
AKL<br />
10101500518 22/05/2009 VITROS Uric DT Slides<br />
AKL<br />
20101500519 13/11/2009<br />
AKL<br />
10101500517 30/06/2009<br />
AKL<br />
20101500523 30/06/2009<br />
AKL<br />
10101500521 30/06/2009<br />
VITROS Chemistry Products DT<br />
Reference Fluid<br />
VITROS Chemistry Products NBIL<br />
DT Slides<br />
VITROS Chemistry Products Na DT<br />
Slides<br />
VITROS Immunodiagnostic Products<br />
Anemia Controls<br />
AKL<br />
10204403103 19/05/2009 MINDRAY M-30R Rinse<br />
AKL<br />
20101500527 30/06/2009<br />
AKL<br />
20101502964 18/05/2009<br />
VITROS Immunodiagnostic Products<br />
Cortisol Calibrators<br />
VITROS Immunodiagnostics<br />
Products Vitamin B12/Folate Reagent<br />
Pack 3<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
DIVISION JOHNSON & JOHNSON<br />
COMPANY., USA<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
DIVISION JOHNSON & JOHNSON<br />
COMPANY., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
DIVISION A JOHNSON & JOHNSON<br />
COMPANY., USA<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
DIVISION JOHNSON & JOHNSON<br />
COMPANY., UK<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
DIVISION JOHNSON & JOHNSON<br />
COMPANY., UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20208500513 14/10/2009 SYSMEX SE-Check N, L, H SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10208500509 14/10/2009 SYSMEX Stromatolyser WL (II) SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10208500510 04/06/2009 SYSMEX CellPack - 3D (ll) SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10208500505 01/06/2009 SYSMEX Cellsheath SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208500508 14/10/2009 SYSMEX stromatolyser = 3D (II) SSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10102500511 09/10/2009<br />
SYSMEX Urine Analyzer,<br />
Accessories and Spare Parts SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10208500506 14/10/2009 SYSMEX Stromatolyser - NR SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20205900634 25/03/2009 CHRONO - PAR Collagen CHRONO-LOG CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
AKL<br />
10101900630 25/03/2009 MEDIA HDL PEG 6000 MEDIA IVD., ITALY<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
AKL<br />
20205900631 25/03/2009 CHRONO - PAR Epinephrine CHRONO-LOG CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20205900632 25/03/2009<br />
AKL<br />
20205900633 25/03/2009<br />
CHRONO - LOG Aggregometer and<br />
Accessories CHRONO-LOG CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
CHRONO - LOG Aggregometer and<br />
Accessories CHRONO-LOG CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
AKL<br />
10305403665 19/05/2009 ABX Pentra RF CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10204403099 19/05/2009 MINDRAY M-30P Probe Cleanser<br />
AKL<br />
20305500415 28/05/2009<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
SYSMEX CRP (Y) Immuno Reagent -<br />
HA SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500411 28/05/2009 SYSMEX CRE - S R2 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500413 09/10/2009 SYSMEX CRE-S-R1 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500410 09/10/2009 SYSMEX LD (L)- L.R2 & PL.R2 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500407 09/10/2009 SYSMEX CRE-S-R2 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500408 28/05/2009 SYSMEX CRE - Buffer R1 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101500406 16/06/2009 SYSMEX Mg Reagent L-H SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500390 13/07/2009 ABX Pentra ALP CP HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305500395 03/07/2009 ABX Pentra C4c HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305500393 09/10/2009 ABX PENTRA Transferrin ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20207500398 17/07/2009<br />
ABX PENTRA HbA1c WB<br />
Hemolysis Reagent HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305400394 03/07/2009 ABX Pentra C3c HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101500399 09/10/2009 ABX PENTRA Sample Diluent CP ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20205500372 28/05/2009<br />
ORPHEE Mythic 18 Haematology<br />
Analyser Automated ORPHEE S.A., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10208500369 28/05/2009 ORPHEE Enzymatic Cleaner ORPHEE S.A., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10208500370 28/05/2009 ORPHEE Lytic Reagent ORPHEE S.A., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10208500371 28/05/2009 ORPHEE Diluent ORPHEE S.A., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20101500291 30/06/2009<br />
VITROS Chemistry Products LDH<br />
Slides<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20101500084 04/06/2009 SYSMEX Bili - Trol SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20305500079 01/06/2009 SYSMEX CRP Standard SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101500081 04/06/2009 SYSMEX Lipid Calibrator SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101500082 04/06/2009 SYSMEX M-Trol 1 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101500080 13/11/2009 SYSMEX Sero - trol - X dan II - X SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101500083 04/06/2009 SYSMEX M - Trol 2 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101403679 06/04/2009 AMP Triglyccerides AMP Medizintechnik Handels GmbH, Austria PT. SETIA GUNA MEDIKA<br />
AKL<br />
20103403601 19/05/2009 NARCO Check THC (Marijuana)<br />
IND DIAGNOSTIC., CANADA for CYNERGEN<br />
HEALTH LLC., USA<br />
PT. UNITED DICO CITAS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101403267 07/08/2009 PREGCY Check Test Strip<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
CYNERGEN HEALTH LLC., USA<br />
PT. UNITED DICO CITAS<br />
AKL<br />
30305905488 16/10/2009 NUCLISENS EasyQ HIV-1 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204903859 21/08/2009<br />
AKL<br />
10101804109 30/03/2009 DIMENSION CHOL Cholesterol<br />
AKL<br />
10204904540 25/09/2009<br />
NucliSens Magnetic Extraction<br />
Reagents BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
NUCLISENS Reader Cleaning<br />
Solution BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204904542 25/09/2009 NUCLISENS Reader Assay Buffer BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303402654 03/08/2009 BIOCON ASOslide<br />
AKL<br />
20101402660 03/08/2009 BIOCON ACP/ACP-P<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
10207402537 14/10/2009 Merck Leucognost POX MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10207402542 14/10/2009 MERCK Leucognost Basic Kit MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201402545 14/10/2009 Merck Leucognost EST MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10207904987 08/10/2009 Merck Leucognost NASDCL MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201402544 14/10/2009 Merck Leucognost PAS MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10207904989 08/10/2009 MERCK Leucognost ALPA MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201402540 14/10/2009 MERCK Hemacolor MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201402541 14/10/2009 MERCK Leucognost SP MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302905499 19/10/2009<br />
AKL<br />
20101402571 26/06/2009 Dsi Alpha HBDH FS<br />
BIOMERIEUX Colestos Medium<br />
(Colestos-T) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
20101402517 06/04/2009 Dima Genesis Pregnancy Test DIMA Ges.f. DIAGNOSTIKA mbH., Germany PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20101402484 21/08/2009 RANDOC - Calibrator Serum Level II RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20305402354 26/06/2009 NOVA LITE Hep-2 ANA Kit INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20305402353 26/06/2009 QUANTA LITE ANA ELISA INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20103904295 03/09/2009 EMIT II Plus Amphetamines Assay<br />
AKL<br />
20101402317 31/03/2009<br />
AKL<br />
20101402316 31/03/2009<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
RADIOMETER Calibrating Solution<br />
2 RADIOMETER MEDICAL ApS., DENMARK<br />
RADIOMETER Calibrating Solution<br />
1 Radiometer Medical A/S, Dermark<br />
AKL<br />
10204402324 31/03/2009 RADIOMETER Hypochlorite Solution RADIOMETER MEDICAL ApS., DENMARK<br />
AKL<br />
10204402320 31/03/2009 RADIOMETER Cleaning Solution RADIOMETER MEDICAL ApS., DENMARK<br />
AKL<br />
10204402319 31/03/2009 RADIOMETER Cleaning Solution RADIOMETER MEDICAL ApS., DENMARK<br />
AKL<br />
10202402318 31/03/2009 RADIOMETER Salt - Bridge Solution RADIOMETER MEDICAL ApS., DENMARK<br />
AKL<br />
20101402339 23/12/2009<br />
AKL<br />
20101402340 23/12/2009<br />
BIOLABO Uree Methode<br />
Colorimetrique<br />
BIOLABO Bilirubine Totale & Directe<br />
Methode D.M.S.O<br />
BIOLABO SA, FRANCE<br />
BIOLABO SA, FRANCE<br />
AKL<br />
20101402338 23/12/2009 BIOLABO AST (GOT) IFCC BIALABO SA, FRANCE<br />
AKL<br />
20101402335 23/12/2009<br />
BIOLABO Albumine Methode AU<br />
BCG<br />
BIOLABO SA, FRANCE<br />
AKL<br />
10101402333 11/11/2009 BIOLABO Amylase CNPG3 BIOLABO S.A., FRANCE<br />
AKL<br />
20101402331 28/12/2009 BIOLABO Creatinine BIOLABO SA., FRANCE<br />
AKL<br />
20101402311 23/12/2009<br />
BIOLABO Proteines Totales<br />
Methode Biuret<br />
BIOLABO SA, FRANCE<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. SIEMENS INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101402315 11/11/2009 BIOLABO Glucose GOD-PAP BIOLABO S.A., FRANCE<br />
AKL<br />
10101402342 11/11/2009 BIOLABO ALT/GPT (IFCC) BIOLABO S.A., FRANCE<br />
AKL<br />
10101402336 09/11/2009 BIOLABO Triglycerides GPO BIOLABO S.A., FRANCE<br />
AKL<br />
10101402334 11/11/2009 BIOLABO Uric Acid BIOLABO S.A., FRANCE<br />
AKL<br />
10101402332 11/11/2009 BIOLABO Cholesterol CHDD-PAP BIOLABO S.A., FRANCE<br />
AKL<br />
10101402330 11/11/2009 BIOLABO Cholesterol-HDL (PTA) BIOLABO S.A., FRANCE<br />
AKL<br />
10101402313 11/11/2009 BILABO Exatrol-N BIOLABO S.A., FRANCE<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20303600202 09/11/2009 ABBOTT AXSYM System CMV IgG ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20303600201 09/11/2009<br />
AKL<br />
20303600205 09/11/2009<br />
ABBOTT AXSYM System CMV IgG<br />
Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Rubella<br />
IgG Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20305401314 26/06/2009 QUANTA LITE ds DNA ELISA INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20305401310 26/06/2009 QUANTA LITE ACA IgM lll INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20305401311 26/06/2009 QUANTA LITE B2 GPI IgM ELISA INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20207903956 26/08/2009<br />
AKL<br />
20101401193 26/08/2009<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
RANDOX Haemoglobin (Drabkins<br />
Reagent) RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
RANDOX LDH opt (Pyruvate Lactate<br />
Dehidrogenase) RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20101906654 28/12/2009 BD Vacutainer Plus SST II Tube<br />
AKL<br />
20101900768 30/03/2009 DIMENSION ABS Absorbance<br />
AKL<br />
20305401022 18/05/2009<br />
AKL<br />
20305401020 18/05/2009<br />
AKL<br />
20305401021 18/05/2009<br />
AKL<br />
20305401019 18/05/2009<br />
AKL<br />
20103401015 18/05/2009<br />
AKL<br />
20103401014 18/05/2009<br />
AKL<br />
20103401012 18/05/2009<br />
AKL<br />
20103401010 18/05/2009<br />
AKL<br />
20103401009 18/05/2009<br />
AKL<br />
20101401017 18/05/2009<br />
Inst-Answer HBaAg One Step<br />
Hepatitis B Surface Antigen Test<br />
Device<br />
Inst-Answer HCV Rapid Hepatitis C<br />
Virus Test Strip<br />
Inst-Answer HBaAg One Steap<br />
Hepatitis B Surface Antigen Test<br />
Strip<br />
Inst-Answer HCV Rapid Hepatitis C<br />
Virus Test Device<br />
Inst-Answer THC One Step<br />
Marijuana Test Strip<br />
Inst-Answer MOP One Step<br />
Morphine Test Strip<br />
Inst-Answer AMP One Step<br />
Amphetamine Test Strip<br />
Inst-AnswerMET One Step<br />
Methamphetamine Test Strip<br />
Inst-Answer MET One Step<br />
Methamphetamine Test Device<br />
Inst-Answer hCG One Step<br />
Pregnancy Test Strip<br />
BECTON DICKINSON CO, UK melalui<br />
BECTON DICKINSON CO, SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SIEMENS INDONESIA<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
AKL<br />
20101400985 21/08/2009 RANDOX - CK NAC RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
10101400984 26/08/2009<br />
RANDOX Ransel (Glutathione<br />
Peroxidase) RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20101400557 21/08/2009 HUMAN CK-MB Liqui UV HUMAN GmbH, GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10302905177 12/10/2009 RADIPEC Staph BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303600204 15/10/2009<br />
ABBOTT AXSYM Rubella IgM<br />
Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101600197 15/10/2009 ABBOTT AXSYM System Total T4 ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101301647 12/11/2009<br />
AKL<br />
ANTEC Direct Pregnancy Latex Test<br />
Kit200iu/L<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCT, UK<br />
20101901322 31/03/2009 SPOTCHEM II Creatinine ARKRAY FACTORY INC., JAPAN<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ABADINUSA<br />
USAHASEMESTA
AKL<br />
20103805178 30/03/2009<br />
AKL<br />
20103901464 01/04/2009<br />
AKL<br />
10204901323 31/03/2009 DIESSE washing Buffer<br />
INTEC One Step Metamhetamine<br />
Test Card INTEC PRODUCTS INC., CHINA PT. MITRASAMAYA SEJATI<br />
Synthron QuickScreen Cup Drug<br />
Test (AMP, THC, MOR, MET, COC,<br />
BZD) SYNTHRON BIORESEARCH., USA PT. MEDIHOP<br />
DIEESE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
AKL<br />
10101901321 31/03/2009 SPOTCHEM II GGT ARKRAY FACTORY INC., JAPAN<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
10302805420 30/09/2009 RAPID ID 32 E BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302805421 30/09/2009 RAPID 20 E BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302805430 25/09/2009 OPTOCHIN Test BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302805422 30/09/2009 RAPIDEC ur BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805425 25/09/2009 SLIDEX Meningite Strepto B BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805424 30/09/2009 SLIDEX Pneumo - Kit BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805427 25/09/2009 SLIDEX Strepto - Kit BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805428 25/09/2009 SLIDEX Staph Plus BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805429 25/09/2009 RABBIT Plasma BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805431 25/09/2009 VIKIA Rota - Adeno BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805407 25/09/2009 VIDAS CK - MB BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303805432 25/09/2009 SLIDEX Rota - Kit 2 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805413 25/09/2009 VIDAS T4 (T4) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301805418 30/09/2009 RAPID ATB G BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805416 22/10/2009 DENSICHEK Calibration Standard BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301805417 30/09/2009 RAPID ATB UR BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301805419 30/09/2009 RAPID ATB E BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303805408 25/09/2009 VIDAS CMV IgG (CMVG) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303805426 25/09/2009 SLIDEX Meningite Kit 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805405 25/09/2009 VIDAS Anti - HBs Total (HBST) BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805404 25/09/2009 VIDAS Total IgE BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805414 25/09/2009 VIDAS HAV IgM (HAVM) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805406 25/09/2009 VIDAS Anti - HAV Total (HAVT) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306805409 25/09/2009 VIDAS TPSA (TPSA) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306805410 25/09/2009 VIDAS CEA (CEA) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306805412 25/09/2009 VIDAS AFP (AFP) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20302805450 25/09/2009 RAPID ID 32 A BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10301903511 03/08/2009 ATB Medium BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301805449 25/09/2009 RAPID ATB Staph BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101902210 29/05/2009 INDEC GTP/ALT<br />
AUDIT DIAGNOSTICS, IRELAND dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
PT. INDEC DIAGNOSTICS<br />
AKL<br />
10302903504 03/08/2009 ID 32 GN BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301903503 03/08/2009 ATB G-5 BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301903507 03/08/2009 ATB Staph 5 BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301903510 03/08/2009 ATB NH BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301903512 03/08/2009 ATB VET BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301903505 03/08/2009 ATB ANA BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301903509 03/08/2009 ATB Fungus BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301903508 03/08/2009 ATB Strep 5 BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102805689 31/03/2009 Finnpipette Focus Multichannel Plus THERMO FISHER SCIENTIFIC., FINLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102805688 31/03/2009 FINNPIPETTE Digital Fixed Volume THERMO FISHER SCIENTIFIC., FINLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101805806 02/10/2009<br />
BIOMERIEUX HDL Cholesterol<br />
Direct BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102805793 15/10/2009 BIOMERIEUX Washer 470 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101805815 22/10/2009 ENZYLINE y GT 6S, 10S, 20S BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101805813 22/10/2009 ENZYLINE Lipase Color BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101805816 22/10/2009<br />
BIOMERIEUX LDL Cholesterol<br />
Direct BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102805792 15/10/2009 BIOMERIEUX Reader 270 BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10304805797 15/10/2009<br />
VIDAS PC Configuration +<br />
Accessories BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10304805796 25/09/2009 VIDAS Analyzer and Accessories BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302903506 03/08/2009 API NaCI 0.85 % Medium BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302903660 07/08/2009 API 20 E BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805800 04/09/2009 VIDAS Cortisol CORT BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805804 04/09/2009 VIDAS hcG BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805808 02/10/2009<br />
BIOMERIEUX LDL Cholesterol<br />
Direct Calibrateur BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805807 02/10/2009 BIOMERIEUX Albumine Kit BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805809 02/10/2009<br />
AKL<br />
20101805810 02/10/2009<br />
ENZYLINE ASAT/GOT 6.20 & 50<br />
Monocreactif BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX HDL Cholesterol<br />
Direct Calibrateur BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20207805801 04/09/2009 VIDAS vWF BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805814 22/10/2009 ENZYLINE a Amylase RTU BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101805811 02/10/2009<br />
BIOMERIEUX Urea Kit S-180 & S-<br />
1000 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805794 25/09/2009 VIDAS HBC lgM (HBCM) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303805795 25/09/2009 VIDAS RUB lgM (RBM) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805798 25/09/2009 VIDAS Anti-HBs Total Quick (HBST) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303805805 04/09/2009 VIDAS TOXO lgG ll BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805799 25/09/2009 VIDAS HBsAg Ultra Confirmatory BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305805802 04/09/2009 VIDAS Protein C BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806006 02/10/2009 BIOMERIEUX Zn BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806005 02/10/2009 BIOMERIEUX FVB BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302806008 02/10/2009 Scheadler Agar + 5 % Sheep Blood BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806007 02/10/2009 P Y Z BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806009 02/10/2009 SCHAEDLER - Broth BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806010 02/10/2009 Baird Parker + RPF Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806011 02/10/2009 CLED - Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806012 02/10/2009<br />
Scheadler Neo Vanco + 5 % Sheep<br />
Blood (SOS) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806089 22/10/2009 SMAC - Agar Mac Conkey Sorbitol BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806090 02/10/2009 Cefixime-Tellurite Mixture (CT-M) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806094 02/10/2009 BIOMERIEUX XLD Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806092 22/10/2009 SM ID 2 Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806093 02/10/2009<br />
AKL<br />
10302806314 02/10/2009<br />
Glucose Chloramphenicol-Agar<br />
(YGC) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX Sabouraud<br />
Chloramphenicol Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806313 02/10/2009 BIOMERIEUX O157:H7 ID Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806315 02/10/2009 BIOMERIEUX Genbox CO2 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806316 02/10/2009<br />
BIOMERIEUX Kliger Medium (Iron<br />
Agar) BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302806318 02/10/2009 BIOMERIEUX Hektoen Agar BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103806321 11/11/2009<br />
AKL<br />
20305806434 21/08/2009 ASAN Easy Test HBs<br />
AKL<br />
20305806433 21/08/2009 ASAN Easy Test Anti-HBs<br />
ROCHE ETOH2 cobas c111 ethanol<br />
Gen.2 ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
ASAN PHARMACEUTICAL CO., LTD.,<br />
KOREA<br />
ASAN PHARMACEUTICAL CO., LTD.,<br />
KOREA<br />
PT. SEMERU PERKASA PERMAI<br />
PT. SEMERU PERKASA PERMAI<br />
AKL<br />
20207807099 16/12/2009 Dia-TT DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20207807098 16/12/2009 Dia-CaCl2 DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101807213 11/11/2009 FLUITEST CK-Nac<br />
AKL<br />
10101807220 12/11/2009 FLUITEST CHE<br />
AKL<br />
10101807224 21/08/2009 DIASYS Triglycerides FS<br />
AKL<br />
10101807226 19/10/2009 ECOLINE HDL Precipitant<br />
AKL<br />
10101807227 19/10/2009 ECOLINE S + Triglycerides FS<br />
AKL<br />
10101807233 19/10/2009 DIASYS Uric Acid FS TBHBA<br />
AKL<br />
20101807232 21/08/2009 DIASYS Urea CT FS<br />
AKL<br />
20101807228 21/08/2009 DIASYS Urea FS<br />
AKL<br />
20101807882 19/10/2009 DIASYS total Protein FS<br />
AKL<br />
20101807857 19/10/2009<br />
DIASYS albumin in Urine / CSF FS<br />
(Microalbumin)<br />
AKL<br />
20101807867 13/11/2009 DIASYS Creatinine PAP FS<br />
AKL<br />
20101807868 11/11/2009<br />
DIASYS Alkaline Phosphatase FS<br />
IFCC 37 0 C<br />
AKL<br />
20101807869 19/10/2009 DIASYS Creatinine Standard FS<br />
AKL<br />
20101807981 13/11/2009 DIASYS Glucose Hexokinase FS<br />
AKL<br />
20101807755 19/10/2009 ECOLINE S+ Creatinine PAP FS<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DYASYS DIAGNOSTIC SYSTEMS GmbH &<br />
Co KG, GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DYASYS DIAGNOSTIC SYSTEMS GmbH &<br />
Co KG, GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101807768 19/10/2009 ECOLINE S+ Cholesterol FS<br />
AKL<br />
20101807769 19/10/2009<br />
ECOLINE S+ ASAT (GOT) FS<br />
(IFCC Mod)<br />
AKL<br />
20101807771 19/10/2009 ECOLINE S+ Total Protein FS<br />
AKL<br />
20101807773 15/10/2009 ECOLINE S+ Urea FS<br />
AKL<br />
20101808185 21/08/2009 DIASY Bilirubin Auto Direct FS<br />
AKL<br />
10101808186 21/08/2009<br />
AKL<br />
20101808193 09/10/2009<br />
AKL<br />
20101808201 15/10/2009<br />
AKL<br />
20101808202 30/09/2009<br />
DIASYS ALAT (GPT) FS (IFCC<br />
Mod)<br />
ECOLINE S+ Bilirubin Auto Direct<br />
FS<br />
ECOLINE S+ Urea CT FS<br />
(Berthelot)<br />
ECOLINE S + Bilirubin Auto Total<br />
FS<br />
AKL<br />
10303808203 21/08/2009 ASAN Helicobacter Test<br />
AKL<br />
10302808145 02/10/2009<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
ASAN PHARMACEUTICAL CO., LTD.,<br />
KOREA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. SEMERU PERKASA PERMAI<br />
BIOMERIEUX Lowenstein - Jensen<br />
Medium (LJ-T) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302808146 02/10/2009 BIOMERIEUX TSI Agar (TSI-D) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302808150 02/10/2009<br />
AKL<br />
10302808153 15/10/2009<br />
BIOMERIEUX Chocolate Agar +<br />
PolyViteX (PVX) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIO MERIEUX Urea - Arginine LYO<br />
2 BIO MERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302808154 15/10/2009 BIO MERIEUX Mycoplasma IST 2 BIO MERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302808155 15/10/2009<br />
AKL<br />
10101808157 16/10/2009 ECOLINE S+ Uric Acid TOOS<br />
AKL<br />
20101807925 30/09/2009<br />
BIO MERIEUX Campylosel Agar<br />
(CAM) BIO MERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
ECOLINE S+ Bilirubin Jendrassik -<br />
Grof FS<br />
AKL<br />
20101807928 09/10/2009 ECOLINE St Albumin FS<br />
AKL<br />
20101807929 21/08/2009 DIASYS Bilirubin Auto Total FS<br />
AKL<br />
10101807930 19/10/2009 DIASYS Uric Acid FS TOOS<br />
AKL<br />
20101807931 21/08/2009<br />
DIASYS ASAT (GOT) FS (IFCC<br />
Mod)<br />
AKL<br />
20101807933 13/11/2009 DIASYS Creatinine FS<br />
AKL<br />
20101807940 19/10/2009 DIASYS Total Protein Standard FS<br />
AKL<br />
20101807941 13/11/2009 DIASYS Glucose Standard FS<br />
AKL<br />
10101807942 19/10/2009 DIASYS Cholesterol Standard FS<br />
AKL<br />
10101807945 19/10/2009 DIASYS Uric Acid Standard FS<br />
AKL<br />
20205808221 19/10/2009 ECOLINE Hemoglobin FS<br />
AKL<br />
20101808239 21/08/2009 DIASYS Albumin FS<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH &<br />
CO. KG., GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DYASYS DIAGNOSTIC SYSTEMS GmbH &<br />
Co KG, GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DYASYS DIAGNOSTIC SYSTEMS GmbH &<br />
Co KG, GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
AKL<br />
10302808166 15/10/2009<br />
AKL<br />
20205900008 22/01/2009<br />
AKL<br />
10101808160 19/10/2009 DIASYS Cholesterol FS<br />
AKL<br />
10101808173 21/08/2009 DIASYS Triglycerides FS<br />
AKL<br />
10101808174 19/10/2009 DIASYS HDL - C Immuno FS<br />
AKL<br />
10101808176 19/10/2009<br />
BIO MERIEUX Count-Tact<br />
Sabouraud Dextore Chloramphenicol<br />
Neutralizer Agar BIO MERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
DIASPECT HEMOGLOBIN System<br />
and Accessories DIASPECT MEDICAL AB., SWEDEN PT. MEDQUEST JAYA GLOBAL<br />
ECOLINE S+ALAT (GPT) FS (IFCC<br />
Mod)<br />
DIASYS DIAGNOSTICS SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS SIAGNOSTICS SYSTEM GmbH,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH.,<br />
GERMANY<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305900023 16/02/2009 CHORUS Beta 2 - Glycoprotein - G<br />
AKL<br />
20305900035 16/02/2009 CHORUS Beta 2 - Glycoprotein - M<br />
AKL<br />
20303900036 16/02/2009<br />
AKL<br />
30305900011 16/02/2009<br />
AKL<br />
20101900012 16/02/2009<br />
AKL<br />
20305900014 16/02/2009<br />
AKL<br />
20101900015 16/02/2009<br />
AKL<br />
20305900017 16/02/2009<br />
CHORUS Cytomegalovirus Ig G<br />
Avidity<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
ABBOTT ARCHITECT HIV Ag / Ab<br />
Combo Reagent Kit ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Total T4 Master<br />
Calibrators<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION, USA<br />
PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HIV Ag / Ab<br />
Combo Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT System Total B - hCG<br />
Specimen Diluent<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION, USA<br />
PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HIV Ag / Ab<br />
Combo Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20306900022 16/02/2009 ABBOTT CEA Calibrators ABBOTT JAPAN CO. LTD., JAPAN PT. ABBOTT INDONESIA<br />
AKL<br />
20305900031 16/02/2009<br />
ABBOTT MUREX Anti - HBs<br />
Reagent Pack<br />
MUREX BIOTECH LIMITED., UNITED<br />
KINGDOM<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20306900032 16/02/2009 ABBOTT CEA Controls ABBOTT JAPAN CO. LTD., JAPAN PT. ABBOTT INDONESIA<br />
AKL<br />
30305900033 16/02/2009<br />
ABBOTT AXSYM HIV Ag / Ab<br />
Combo Reagent Pack ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20207900020 16/02/2009 DIAGON DIA - PT 10 DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20207900021 16/02/2009 DIAGON DIA - FIB DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20303900018 16/02/2009 IMMUTREP Carbon Antigen<br />
AKL<br />
20101808436 13/11/2009 DIASYS Glucose GOD FS<br />
AKL<br />
20101808435 12/11/2009<br />
DIASYS Alkaline Phosphatase<br />
DGKC FS<br />
AKL<br />
30305808438 14/10/2009 SERODIA HIV 1/2 Mix FUJIBERIO., JAPAN<br />
AKL<br />
20101900365 10/03/2009<br />
AKL<br />
10304900366 10/03/2009<br />
AKL<br />
10101900367 10/03/2009<br />
AKL<br />
20101900370 10/03/2009<br />
AKL<br />
20101900371 10/03/2009<br />
OMEGA DIAGNOSTICS LTD., UNITED<br />
KINGDOM<br />
DYASYS DIAGNOSTIC SYSTEMS GmbH &<br />
Co KG, GERMANY<br />
DIASYS DIAGNOSTICS SYSTEM GmbH &<br />
CO. KG., GERMANY<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. USAHA KARYATAMA<br />
MANDIRI<br />
ABBOTT Clinical Chemistry<br />
Neonatal Bilirubin ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT PRISM SYSTEM,<br />
Accessories and Spare Part ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM T-Uptake Reagent<br />
Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM T-Uptake Master<br />
Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM T-Uptake<br />
Standard Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20205900373 10/03/2009 ABBOTT DAINASCREEN HEMO ABBOTT JAPAN CO. LTD., JAPAN PT. ABBOTT INDONESIA<br />
AKL<br />
20101900375 10/03/2009<br />
JIM KEMP Sodium Citrate Tube<br />
(1:4) JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900376 10/03/2009 JIM KEMP EDTA K3 Vacuum Tubes JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900377 10/03/2009<br />
JIM KEMP Sodium Citrate Tube<br />
(1:4) JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900378 10/03/2009 JIM KEMP No-Adhesive Tube JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900379 10/03/2009 JIM KEMP No-Adhesive Tube JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900380 10/03/2009<br />
JIM KEMP Sodium Citrate Tube<br />
(1:9) JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900381 10/03/2009 JIM KEMP EDTA K3 Vacuum Tube JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900382 10/03/2009 JIM KEMP Lithium Heparin JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900383 10/03/2009 JIM KEMP Lithium Heparin JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900384 10/03/2009<br />
JIM KEMP Sodium Citrate Tube<br />
(1:9) JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20101900385 10/03/2009 JIM KEMP Clot Activator JIM KEMP MEDICAL CO. LTD., CHINA PT. MRK DIAGNOSTICS<br />
AKL<br />
20208900281 04/03/2009<br />
ROCHE HbA1C Control P and<br />
Control N ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101900446 17/03/2009<br />
AKL<br />
20101900447 17/03/2009<br />
AKL<br />
20101900448 17/03/2009<br />
AKL<br />
20101900449 17/03/2009<br />
AKL<br />
20101900450 17/03/2009<br />
IDLAB Vacuum Tube EDTA K3<br />
Plastic<br />
IDLAB Vacuum Tube Sodium Citrate<br />
Plastic<br />
IDLAB Vacuum Tube Red Plain<br />
Glass<br />
IDLAB Vacuum Tube EDTA K3<br />
Glass<br />
IDLAB Vacuum Tube Gel Clot<br />
Activator Plastic<br />
AKL<br />
20101900451 17/03/2009 IDLAB Vacuum Tube ESR Plastic<br />
AKL<br />
20101900452 17/03/2009<br />
AKL<br />
20101900453 17/03/2009<br />
AKL<br />
20101900454 17/03/2009<br />
IDLAB Vacuum Tube EDTA K2<br />
Plastic<br />
IDLAB Vacuum Tube Red Plain<br />
Plastic<br />
IDLAB Vacuum Tube Heparin<br />
Lithium Plastic<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., P.R. CHINA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
PT. INDOLAB ARTHA MEDIKA<br />
AKL<br />
20101900472 17/03/2009 ELECSYS Folate ll CalSet ll ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20205900635 25/03/2009 CHRONO - PAR ADP Reagent CHRONO-LOG CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20205900636 25/03/2009 CHRONO - PAR Arachidonic Acid CHRONO-LOG CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
AKL<br />
10101900637 25/03/2009<br />
LIQUICHEK Immunoassay Plus<br />
Control Level 1<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10101900638 25/03/2009<br />
LIQUICHEK Immunoassay Plus<br />
Control Level 2<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10101900639 25/03/2009<br />
LIQUICHEK Immunoassay Plus<br />
Control Level 3<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10101900640 25/03/2009<br />
LYPOCHEK Urine Toxicology<br />
Control<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20101900217 26/06/2009 PICCOLO Basic Metabolyte Panel ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900221 26/06/2009 PICCOLO Hepatic Function Panel ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900222 26/06/2009 PICCOLO Renal Function Panel ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900205 23/02/2009<br />
B. BRAUN OMNITEST PLUS Blood<br />
Glucose Test Strips<br />
INFOPIA CO. LTD., KOREA untuk B. BRAUN<br />
MELSUNGEN AG OPM., GERMANY<br />
PT. B.BRAUN MEDICAL<br />
INDONESIA<br />
AKL<br />
20101900206 23/02/2009<br />
B. BRAUN OMNITEST PLUS<br />
Glucose Monitor<br />
INFOPIA CO. LTD., KOREA untuk B. BRAUN<br />
MELSUNGEN AG OPM., GERMANY<br />
PT. B.BRAUN MEDICAL<br />
INDONESIA<br />
AKL<br />
10101900207 23/02/2009<br />
B. BRAUN OMNITEST PLUS<br />
Control M<br />
AKL<br />
20303900542 23/03/2009 CHORUS Rubella IgG<br />
AKL<br />
20303900543 23/03/2009 CHORUS Rubella IgM<br />
AKL<br />
20303900544 23/03/2009 CHORUS Toxoplasma IgG<br />
AKL<br />
20303900545 23/03/2009 CHORUS Toxoplasma IgM<br />
AKL<br />
20303900546 23/03/2009 CHORUS Herpes Simplex 1+2 IgM<br />
AKL<br />
20303900547 23/03/2009 CHORUS HSV 2 Screen<br />
AKL<br />
20303900548 23/03/2009 CHORUS HSV 1 Screen<br />
AKL<br />
20305900549 23/03/2009 CHORUS Cardiolipin-M<br />
AKL<br />
20305900550 23/03/2009 CHORUS ANA-8 S<br />
AKL<br />
20305900551 23/03/2009 CHORUS dsDNA-G Reagent<br />
AKL<br />
20305900552 23/03/2009 CHORUS Cardiolipin-G<br />
PDF Creator - PDF4Free v3.0<br />
INFOPIA CO. LTD., KOREA untuk B. BRAUN<br />
MELSUNGEN AG OPM., GERMANY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
DIESSE DIAGNOSTIC SENESE S.p.a.,<br />
ITALY<br />
PT. B.BRAUN MEDICAL<br />
INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
http://www.pdf4free.com
AKL<br />
20305900298 05/03/2009 MUREX Anti HBc (Total) MUREX BIOTECH LTD., ENGLAND PT. ABBOTT INDONESIA<br />
AKL<br />
20103900295 05/03/2009<br />
AKL<br />
20103900296 05/03/2009<br />
SPEEDYTEST - DOA 4 Drugs<br />
Panel Test (MOP/THC/AMP/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST - DOA 4 Drugs<br />
Panel Test (MOP/THC/MET/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20101900284 26/06/2009 PICCOLO Metabolic Panel Plus ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900285 26/06/2009 PICCOLO Electrolyte Panel ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900287 26/06/2009 PICCOLO General Chemistry 6 ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20305900293 05/03/2009 DIASYS Immunoglobulin G FS<br />
AKL<br />
20103900290 05/03/2009<br />
AKL<br />
20103900291 05/03/2009<br />
AKL<br />
20103900294 05/03/2009<br />
AKL<br />
10102900297 05/03/2009<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
GERMANY<br />
PT. MULTIREDJEKI KITA<br />
ONCOPROBE Multi Drug Abuse 3<br />
in 1 Rapid Test CDI / BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
ONCOPROBE Barbiturate Rapid<br />
Test CDI / BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
ONCOPROBE Barbiturate Rapid<br />
Test CDI / BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AWARENESS STAT FAX 303 Plus<br />
Microstrip Reader<br />
AWARENESS TECHNOLOGY INC., USA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
20303900283 05/03/2009 FLAVICHECK HCV QUALPRO DIAGNOSTICS., INDIA PT. SUPRAMEDIKA PRIMA<br />
AKL<br />
10304900752 30/03/2009<br />
ABBOTT PRISM SYSTEM and<br />
Accessories & Spare Part ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20305900775 30/03/2009 ABX CRP Trol l / Trol ll HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101900776 30/03/2009 ABX PENTRA Creatinine 120 CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101900777 30/03/2009 ABX PENTRA Total Protein 100 CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305900778 30/03/2009 ABX CRP Std HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305900779 30/03/2009 ABX MINOTROL CRP Level 1 / 2 / 3 HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305900780 30/03/2009 ABX CRP Rea HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20209900641 25/03/2009 HEMATEST A1, A2, B, O DIAGAST., FRANCE PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303900642 25/03/2009 WAMPOLE Sm ELISA ll<br />
AKL<br />
20305900643 25/03/2009 WAMPOLE RF ELISA ll<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209900644 25/03/2009 HEMATEST A1, B DIAGAST., FRANCE PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305900645 25/03/2009 WAMPOLE ds DNA ELISA ll<br />
AKL<br />
20305900646 25/03/2009 WAMPOLE SCL - 70 ELISA ll<br />
AKL<br />
20305900647 25/03/2009 WAMPOLE Gliadin IgA ELISA ll<br />
AKL<br />
20305900648 25/03/2009 WAMPOLE SS - B ELISA ll<br />
AKL<br />
20305900649 25/03/2009 WAMPOLE Sm / RNP ELISA ll<br />
AKL<br />
20305900650 25/03/2009<br />
WAMPOLE Thyroid Microsomal<br />
ELISA ll<br />
AKL<br />
20305900651 25/03/2009 WAMPOLE Gliadin IgG ELISA ll<br />
AKL<br />
20305900652 25/03/2009 WAMPOLE Jo - 1 ELISA ll<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305900653 25/03/2009 WAMPOLE SS - A ELISA ll<br />
AKL<br />
20303900654 25/03/2009 WAMPOLE HSV - 1 IgG ELISA ll<br />
AKL<br />
20303900655 25/03/2009 WAMPOLE HSV - 2 IgG ELISA ll<br />
AKL<br />
10303900656 25/03/2009 WAMPOLE Mumps IgG ELISA ll<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE INC., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERIC<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101900769 30/03/2009 TRIAGE Profiler S.O.B Control Set<br />
BIOSITE INCORPORATED, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101900770 30/03/2009<br />
TRIAGE TOX Drug Screen Controls<br />
Set, Control 1<br />
BIOSITE INCORPORATED, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101900771 30/03/2009<br />
TRIAGE TOX Drug Screen Controls<br />
Set, Control 2<br />
BIOSITE INCORPORATED, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101900772 30/03/2009<br />
TRIAGE Profiler S.O.B Calibration<br />
Verification Controls<br />
BIOSITE INCORPORATED, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101900773 30/03/2009<br />
TRIAGE CardioProfillER Calibration<br />
Verification Control<br />
BIOSITE INCORPORATED, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101900774 30/03/2009 TRIAGE CardioProfillER Control Set<br />
AKL<br />
20101900657 25/03/2009 CBC URS-2K<br />
AKL<br />
10102900658 25/03/2009 CBC Urine Analyzer<br />
AKL<br />
20101900659 25/03/2009 CBC URS-10<br />
AKL<br />
20101900660 25/03/2009 CBC URS-3<br />
AKL<br />
20101900661 25/03/2009 CBC URS-1<br />
AKL<br />
20101900662 25/03/2009 CBC URS-5K<br />
AKL<br />
20101900663 25/03/2009 CBC URS-4<br />
AKL<br />
20101900664 25/03/2009 CBC URS-2P<br />
BIOSITE INCORPORATED, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC., USA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
CBC MEDICAL SUPPLY CO. LTD., P. R.<br />
CHINA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
AKL<br />
10302900757 30/03/2009 LAB M Mannitol Salt Agar LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
20301900758 30/03/2009 LAB M Mueller Hinton Agar ll LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
10302900759 30/03/2009<br />
LAB M Mac Conkey Agar (Without<br />
Salt) LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
10302900760 30/03/2009 LAB M Mac Conkey Agar No. 3 LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
10302900761 30/03/2009 LAB M Blood Agar Base LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
20301900762 30/03/2009 LAB M Mueller Hinton Broth LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
10302900763 30/03/2009 LAB M Blood Agar Base No. 2 LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
10302900764 30/03/2009 LAB M Mac Conkey Agar (With Salt) LAB M LIMITED., UNITED KINGDOM PT. PYRIDAM FARMA<br />
AKL<br />
20209900753 30/03/2009 VacuBest Serum Tube<br />
AKL<br />
20209900754 30/03/2009 VacuBest Citrate Tube<br />
WEIHAI HONGYU MEDICAL DEVICE CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICE CO.<br />
LTD., CHINA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20209900755 30/03/2009 VacuBest EDTA Tube<br />
AKL<br />
20209900756 30/03/2009 VacuBest Multi Sample Needle<br />
AKL<br />
20101900767 30/03/2009<br />
DIMENSION ALP Alkaline<br />
Phosphatase<br />
AKL<br />
10101900766 30/03/2009 DIMENSION IRN Iron<br />
AKL<br />
10101900765 30/03/2009 DIMENSION TGL Triglycerides<br />
AKL<br />
20101901072 30/03/2009<br />
GREEN Vac-Tube Serum Gel<br />
Praperation (SGS)<br />
AKL<br />
20101901073 30/03/2009 GREEN Vac-Tube EDTA-K3<br />
AKL<br />
20101901071 30/03/2009 GREEN Vac-Tube Serum PLAIN<br />
AKL<br />
20101901070 30/03/2009 GREEN Vac-Tube EDTA-K2<br />
AKL<br />
20101901069 30/03/2009 GREEN Vac-Tube Coagulation<br />
AKL<br />
20303300421 06/04/2009<br />
AKL<br />
20303300420 06/04/2009<br />
AKL<br />
20303301986 06/04/2009<br />
AKL<br />
20303301985 06/04/2009<br />
AKL<br />
20303300422 06/04/2009<br />
AKL<br />
20303300419 06/04/2009<br />
AKL<br />
20303300424 06/04/2009<br />
AKL<br />
20303301988 06/04/2009<br />
ZEUS SCIENTIFIC Rubella IgG<br />
ELISA Kit<br />
ZEUS SCIENTIFIC Herpes<br />
Simp0lex Virus-1 IgM ELISA Kit<br />
ZEUS SCIENTIFIC Cytomegalovirus<br />
IgM ELISA Kit<br />
ZEUS SCIENTIFIC Cytomegalovirus<br />
IgG ELISA Kit<br />
ZEUS SCIENTIFIC Rubella IgM<br />
ELISA Kit<br />
ZEUS SCIENTIFIC Herpes Simplex<br />
Virus-2 IgM ELISA Kit<br />
ZEUS SCIENTIFIC Herpes Simplex<br />
Virus-2 IgG ELISA Kit<br />
ZEUS SCIENTIFIC Toxoplasma IgG<br />
ELISA Kit<br />
AKL<br />
20101403680 06/04/2009 AMP Protein (Total) Biuret<br />
AKL<br />
20101403378 06/04/2009 AMP Glucose HK<br />
AKL<br />
20207403376 06/04/2009 AMP Hemoglobin<br />
AKL<br />
10101403379 06/04/2009 AMP Gamma GT<br />
AKL<br />
10101403681 06/04/2009 AMP Cholesterol<br />
AKL<br />
20101403384 06/04/2009 AMP Creatinine<br />
AKL<br />
20303901328 31/03/2009 CHORUS Herpes Simplex 1+2 IgG<br />
AKL<br />
20303901327 31/03/2009 CHORUS Rubella IgG Avidity<br />
AKL<br />
10204901326 31/03/2009 CHORUS Cleaning Solution 2000 X<br />
AKL<br />
10204901325 31/03/2009<br />
CHORUS Washing Buffer<br />
Autoimmunity<br />
AKL<br />
20303901324 31/03/2009 CHORUS Toxoplasma IgG Avidity<br />
WEIHAI HONGYU MEDICAL DEVICE CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICE CO.<br />
LTD., CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
DIESSE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
AKL<br />
20205901346 31/03/2009 COADATA 501 Coagulometers HELENA BIOSCIENCES EUROPE., UK<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. NEXTWAY ELECTRONICS<br />
PT. NEXTWAY ELECTRONICS<br />
PT. NEXTWAY ELECTRONICS<br />
PT. NEXTWAY ELECTRONICS<br />
PT. NEXTWAY ELECTRONICS<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20101901347 31/03/2009<br />
IRMA TRUPOINT Blood Analysis<br />
System Capillary Collection Device INTERNATIONAL TECHNIDYNE., USA PT. DEMKA SAKTI<br />
AKL<br />
21106700708 31/03/2009 G - FreezeKit Blast VITROLIFE AB., SWEDEN<br />
AKL<br />
20101300550 06/04/2009<br />
AKL<br />
10101300552 06/04/2009<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
QCA BIODIAGNOSTIC GOT/AST<br />
U.V QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
QCA BIODIAGNOSTIC<br />
Triglycerides QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20205901368 01/04/2009 One Lab Auto Hematology Analyzer<br />
AKL<br />
20205901367 01/04/2009 One Lab Hematology Analyzer<br />
PDF Creator - PDF4Free v3.0<br />
SHENZHEN MINDRAY BIO - MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
SHENZHEN MINDRAY BIO - MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
http://www.pdf4free.com<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI
AKL<br />
20209901361 01/04/2009 Alyx Red Kit FENWAL INC., USA PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303901362 01/04/2009<br />
AKL<br />
20303901366 01/04/2009<br />
WAMPOLE Cytomegalovirus IgG<br />
ELISA II<br />
WAMPOLE Cytomegalovirus IgM<br />
ELISA II<br />
AKL<br />
20305901365 01/04/2009 WAMPOLE Thyroglobulin ELISA II<br />
AKL<br />
20303901364 01/04/2009 WAMPOLE Rubella IgG ELISA II<br />
AKL<br />
20303901363 01/04/2009 WAMPOLE Measles IgG ELISA II<br />
ZEUS SCIENTIFIC INC., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC INC., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC INC., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC INC., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC INC., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
AKL<br />
20101901355 31/03/2009 SPINREACT Glucose LQ SPINREACT., SPAIN<br />
AKL<br />
10101901356 31/03/2009 SPINREACT LDL Cholesterol-D SPINREACT., SPAIN<br />
AKL<br />
10101901357 31/03/2009 SPINREACT HDL Cholesterol SPINREACT., SPAIN<br />
AKL<br />
10101901358 31/03/2009 SPINREACT Uric Acid LQ SPINREACT., SPAIN<br />
AKL<br />
20101901074 30/03/2009 GREEN Vac-Tube Plasma<br />
AKL<br />
20101901068 30/03/2009 GREEN Vac-Tube Glucose<br />
AKL<br />
20101901067 30/03/2009<br />
AKL<br />
20303301987 06/04/2009<br />
AKL<br />
20303300423 06/04/2009<br />
AKL<br />
20303901338 31/03/2009<br />
AKL<br />
20209901337 31/03/2009<br />
AKL<br />
20303901331 31/03/2009<br />
AKL<br />
20303901330 31/03/2009<br />
AKL<br />
20303901329 31/03/2009<br />
AKL<br />
20209901334 31/03/2009<br />
AKL<br />
20303901335 31/03/2009<br />
AKL<br />
20209901336 31/03/2009<br />
AKL<br />
20303901332 31/03/2009<br />
AKL<br />
20209901333 31/03/2009<br />
GREEN Vac-Tube Sodium Citrate<br />
(ESR)<br />
ZEUS SCIENTIFIC Toxoplasma IgM<br />
ELISA Kit<br />
ZEUS SCIENTIFIC Herpes Simplex<br />
Virus-1 IgG ELISA Kit<br />
REIGED Diagnostic Salmonella<br />
Paratyphi CH<br />
REIGED Diagnostic Anti AB<br />
Monoclonal<br />
REIGED Diagnostics Salmonella<br />
Typhi O<br />
REIGED Diagnostics Salmonella<br />
Typhi H<br />
REIGED Diagnostics Salmonella<br />
Typhi AH<br />
REIGED Diagnostics Anti D<br />
Monoclonal<br />
REIGED Diagnostics Salmonella<br />
Paratyphi BH<br />
REIGED Diagnostics Anti B<br />
Monoclonal<br />
REIGED Diagnostics Salmonella<br />
Paratyphi AO<br />
REIGED Diagnostics Anti A<br />
Monoclonal<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
STANDARD PLUS MEDICAL CO. LTD.,<br />
KOREA<br />
ZEUS SCIENTIFIC INC., USA<br />
ZEUS SCIENTIFIC INC., USA<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
PT. NEXTWAY ELECTRONICS<br />
PT. NEXTWAY ELECTRONICS<br />
PT. NEXTWAY ELECTRONICS<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
AKL<br />
20103901380 01/04/2009<br />
FAST Multi Drug One Step Multi Line<br />
7 Drug Screen Test Panel with<br />
Integrated E-Z Split Key Cup (Urin<br />
AKL<br />
20101403380 06/04/2009 AMP Alkaline Phosphatase<br />
AKL<br />
10101403385 06/04/2009 AMP HDL Cholesterol Direct<br />
AKL<br />
20101403683 06/04/2009 AMP Bilirubin (Total/Direct)<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDELS GmbH.,<br />
AUSTRIA<br />
PT. SAPTA LARONA MUDA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
AKL<br />
10101901354 31/03/2009 FirstSign LH UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20306901348 31/03/2009 FirstSign Semi Q PSA UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
AKL<br />
20101901349 31/03/2009<br />
FirstSign Midstream Pregnancy Card<br />
Test UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
AKL<br />
20305901350 31/03/2009 FirstSign Myoglobin Card Test UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
AKL<br />
20101901351 31/03/2009 FirstSign HCG Duo Test UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
AKL<br />
20101901352 31/03/2009 FirstSign HCG UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
AKL<br />
10305901359 31/03/2009<br />
AKL<br />
20103901487 06/04/2009<br />
AKL<br />
20103901488 06/04/2009<br />
FirstSign Semi Q Troponin I Card<br />
Test UNIMED BIOSYSTEM PVT. LTD., INDIA PT. SURYA DELTA MAJU<br />
INST-ANSWER Multi Drug One<br />
Step 5 Drug Screen Test Panel<br />
INST-ANSWER Multi Drug One<br />
Step 3 Drug Screen Test Panel<br />
(Urine)<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
AKL<br />
20205901486 01/04/2009 CliniMACS CD 14 Reagent MILTENYI BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20205901360 01/04/2009 CliniMACS CD 4 Reagent MILTENYI BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20101901320 31/03/2009 SPOTCHEM II CPK ARKRAY FACTORY INC., JAPAN<br />
AKL<br />
10101901319 31/03/2009 SPOTCHEM II GPT / ALT ARKRAY FACTORY INC., JAPAN<br />
AKL<br />
20205901318 31/03/2009 SPOTCHEM II Hemoglobin ARKRAY FACTORY INC., JAPAN<br />
AKL<br />
20101901317 31/03/2009 SPOTCHEM II ALP ARKRAY FACTORY INC., JAPAN<br />
AKL<br />
10204901316 31/03/2009 CHORUS Sanitizing Solution 1000 X<br />
DIEESE DIAGNOSTICA SENESE SPA.,<br />
ITALY<br />
AKL<br />
21106700941 31/03/2009 G - PGD VITROLIFE AB., SWEDEN<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
10101403669 06/04/2009 ABX Pentra Immuno II Control L/H HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101901477 01/04/2009 One Lab Electrolyte Analyzer<br />
AKL<br />
10102901476 01/04/2009 One Lab Auto Chemistry Analyzer<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
SHENZHEN MINDRAY BIO - MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20101901474 01/04/2009 Gluco - M I - SENS INC., R.D. KOREA PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20103901498 06/04/2009<br />
AKL<br />
20103901499 06/04/2009<br />
AKL<br />
20103901500 06/04/2009<br />
AKL<br />
20103901501 06/04/2009<br />
AKL<br />
20103901502 06/04/2009<br />
SPEEDYTEST DOA 4 Drugs Panel<br />
Test (MOP/THC/MET/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 5 Drugs Panel<br />
Test (MOP/COC/MET/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 4 Drugs Panel<br />
Test (MOP/THC/MET/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 3 Drugs Panel<br />
Test (THC/AMP/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 3 Drugs Panel<br />
Test (MOP/THC/MET) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103901503 06/04/2009<br />
SPEEDYTEST DOA 5 Drugs Panel<br />
Test (MOP/THC/MET/MDMA/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103901504 06/04/2009<br />
AKL<br />
20103901505 06/04/2009<br />
AKL<br />
20103901506 06/04/2009<br />
AKL<br />
20103901507 06/04/2009<br />
AKL<br />
20208901315 31/03/2009<br />
AKL<br />
20208901314 31/03/2009<br />
AKL<br />
20205901312 31/03/2009<br />
AKL<br />
20205901310 31/03/2009<br />
PDF Creator - PDF4Free v3.0<br />
SPEEDYTEST DOA 5 Drugs Panel<br />
Test (MOP/THC/AMP/MET/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 6 Drugs Panel<br />
Test<br />
(MOP/COC/THC/AMP/MET/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 6 Drugs Panel<br />
Test<br />
(MOP/COC/THC/MET/MDMA/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST DOA 3 Drugs Panel<br />
Test (THC/BZD) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
Point NOW Reagent - CD4NOW<br />
Control Low & Control Normal POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
Point NOW Reagent - CDCNOW<br />
Control Low & Control Normal POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
Point NOW Reagent - CD4NOW<br />
Liquipack (Clean) POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
Point NOW Reagent - CD4NOW<br />
Goldpack (Gold Reagent) POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
http://www.pdf4free.com
AKL<br />
20205901309 31/03/2009<br />
AKL<br />
20205901311 31/03/2009<br />
Point NOW Reagent - CD4NOW<br />
Goldpack (Reconstitution Fluid) POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
PointCare NOW Reagent -<br />
CD4NOW GoldPack (Accelerant) POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20205901308 31/03/2009 PointCare NOW Reagent Kit 100 POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20303901343 31/03/2009<br />
AKL<br />
20305805171 31/03/2009<br />
AKL<br />
30305805361 31/03/2009<br />
INTEC Advanced Quality One Step<br />
Syphilis (TP) Test Card<br />
INTEC Advanced Quality Rapid HCV<br />
Test Strip<br />
INTEC Advanced Quality Rapid HIV<br />
Test Strip<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20101901339 31/03/2009 OPTI CCA / CCA-TS cassette E-Ca OPTI MEDICAL SYSTEM INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
20101902340 31/03/2009 OPTI R Cassette E-Ca 50 OPTI MEDICAL SYSTEM INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
20101901341 31/03/2009<br />
OPTI CCA / CCA-TS cassettes E-<br />
CL OPTI MEDICAL SYSTEM INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
20101901342 31/03/2009 OPTI LION Cassettes E-Plus OPTI MEDICAL SYSTEM INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
10102403410 31/03/2009<br />
BIOLYTE 2000 Electrolyte Analyzer,<br />
Accessories and Spare Parts<br />
BIOCARE CORPORATION., ROC<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
AKL<br />
20205901304 31/03/2009 CliniMACS CD 34 Reagent MILTENYI BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20205901306 31/03/2009 CliniMACS CD 133 Reagent MILTENYI BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20205901305 31/03/2009 CliniMACS CD 8 Reagent MILTENYI BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
21106901722 11/05/2009 THE PIPETTE Embryo Biopsy THE PIPETTE COMPANY., AUSTRALIA<br />
AKL<br />
21106901723 11/05/2009 THE PIPETTE Zona Driling THE PIPETTE COMPANY., AUSTRALIA<br />
AKL<br />
21106901724 11/05/2009 THE PIPETTE Zona Driling THE PIPETTE COMPANY., AUSTRALIA<br />
AKL<br />
21106901725 11/05/2009 THE PIPETTE Embryo Biopsy THE PIPETTE COMPANY., AUSTRALIA<br />
AKL<br />
20303901726 11/05/2009<br />
AKL<br />
20303901727 11/05/2009<br />
AKL<br />
20101901728 11/05/2009<br />
AKL<br />
20103901730 11/05/2009<br />
AKL<br />
20103901820 12/05/2009<br />
AKL<br />
20101801800 04/05/2009<br />
AKL<br />
20101801801 04/05/2009<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
REMEL Salmonella Agglutinating<br />
Serum (b-H) REMEL INC., USA untuk REMEL INC., UK PT. DIPA PUSPA LABSAINS<br />
REMEL Salmonella Agglutinating<br />
Serum (a-H) REMEL INC., USA untuk REMEL INC., UK PT. DIPA PUSPA LABSAINS<br />
GONGDONG Vacuum Tube Sterile<br />
SST with Gel Yellow Cap<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Pro Multi-Drug<br />
Test for AMP, BZD, MET, OPI, THC<br />
Test Panel PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
INST-ANSWER Multi Drug One<br />
Step 6 Drug Screen Test Panel<br />
AKL<br />
10204403100 04/05/2009 MINDRAY M-30E E-Z Cleanser<br />
AKL<br />
10208403102 04/05/2009 MINDRAY M-30 CFL Lyse<br />
AKL<br />
10208403101 04/05/2009 MINDRAY M-30D DIF Diluent<br />
AKL<br />
20205403104 04/05/2009<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PT. TIRTA MEDILAB<br />
INTEC hCG Pregnancy Urine Test<br />
Card INTEC PRODUCTS INC (XIAMEN)., CHINA PT. MITRASAMAYA SEJATI<br />
INTEC hCG Pregnancy Urine Test<br />
Strip INTEC PRODUCTS INC (XIAMEN)., CHINA PT. MITRASAMAYA SEJATI<br />
MINDRAY Automated Hematology<br />
Analyzer<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., CHINA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
AKL<br />
10101403670 06/04/2009 ABX Pentra Immuno I Control L/H HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305403664 06/04/2009 ABX Pentra CRP Cal HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101403792 06/04/2009 ABX Pentra Protein Cal HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305403673 06/04/2009 ABX Pentra CRP CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10209901490 06/04/2009<br />
KOKUSAN Multipurpose Floor<br />
Refrigerated Centrifuge H-80 R<br />
KOKUSAN CORPORATION., JAPAN melalui<br />
ORIENTAL INSTRUMENT LTD., JAPAN<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10209901491 06/04/2009 KOKUSAN table Top Centrifuge<br />
KOKUSAN CORPORATION., JAPAN melalui<br />
ORIENTAL INSTRUMENT LTD., JAPAN<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10209901492 06/04/2009<br />
KOKUSAN Small Size Centrifuge H-<br />
19F<br />
KOKUSAN CORPORATION., JAPAN melalui<br />
ORIENTAL INSTRUMENT LTD., JAPAN<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10209901493 06/04/2009<br />
KOKUSAN Centrifuge for Cell<br />
Washing HS-12<br />
KOKUSAN CORPORATION., JAPAN melalui<br />
ORIENTAL INSTRUMENT LTD., JAPAN<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20205901494 06/04/2009<br />
AKL<br />
20101901495 06/04/2009<br />
KOKUSAN Hematocrit Centrifuge H-<br />
1200F<br />
KOKUSAN CORPORATION., JAPAN melalui<br />
ORIENTAL INSTRUMENT LTD., JAPAN<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PICCOLO Kidney Check - Reagent<br />
Disc ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101901496 06/04/2009 PICCOLO Metlyte 8 - Reagent Disc ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101901497 06/04/2009<br />
AKL<br />
20305901987 28/05/2009<br />
PICCOLO Liver Panel Plus -<br />
Reagent Disc ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
IMTEC-ANA/ENA COMBI Screen<br />
(CUT-OFF) HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305901988 28/05/2009 IMTEC RA-33 Antibodies HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305901989 28/05/2009 IMTEC-LIVER LIA S HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305901990 28/05/2009<br />
IMTEC-Phosphatidylethanolamine-<br />
Antibodies Screen HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305901991 28/05/2009 IMTEC-Liver Screen S HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305901992 28/05/2009 IMTEC-ANA Screen (CUT-OFF) HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305901993 28/05/2009 IMTEC-SLA Antibodies (CUT-OFF) HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20309901973 28/05/2009<br />
CLOSED SYSTEM APHERESIS<br />
KIT with PL 3014 Plastic Platelet<br />
Storage Containers FENWAL INC., USA PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20309901974 28/05/2009 PLASMACELL C Disposable Set FENWAL INC., USA PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10302901966 28/05/2009 BacT / ALERT BPN BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302901968 28/05/2009 BacT / ALERT BPA Culture Bottle BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203901967 28/05/2009 PREVI Color Gram BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305901969 28/05/2009 MONO One Step HBsAg cassette<br />
AKL<br />
10101901970 28/05/2009<br />
AKL<br />
10101901971 28/05/2009<br />
AKL<br />
10101901972 28/05/2009<br />
AKL<br />
20101901975 28/05/2009<br />
AKL<br />
20303901976 28/05/2009<br />
AKL<br />
20303901977 28/05/2009<br />
VITROS Chemistry Product Liquid<br />
Performance Verifier II<br />
VITROS Chemistry Product Liquid<br />
Performance Verifier I<br />
VITROS Chemistry Product<br />
Isoenzyme Performance Verifier I<br />
REIGED DIAGNOSTICS HCG<br />
Pregnancy Latex<br />
REIGED DIAGNOSTICS VDRL<br />
Carbon Antigen<br />
REIGED DIAGNOSTICS TPHA<br />
Latex<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
AKL<br />
PLASMATEC LABORATORY PRODUCTS<br />
10303901978 28/05/2009 REIGED DIAGNOSTICS ASO Latex LTD., UK<br />
AKL<br />
20303901979 28/05/2009<br />
REIGED DIAGNOSTICS RPR<br />
Carbon Antigen<br />
AKL<br />
20305901980 28/05/2009 REIGED DIAGNOSTICS RA Latex<br />
AKL<br />
10101901981 28/05/2009<br />
AKL<br />
10101901982 28/05/2009<br />
AKL<br />
20303901983 28/05/2009<br />
AKL<br />
20303901984 28/05/2009<br />
PDF Creator - PDF4Free v3.0<br />
REIGED DIAGNOSTICS Negative<br />
Polyvalent Febrile Control<br />
REIGED DIAGNOSTICS Positive<br />
Polyvalent Febrile Control<br />
REIGED DIAGNOSTICS Salmonella<br />
Paratyphi O<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
REIGED DIAGNOSTICS Salmonella PLASMATEC LABORATORY PRODUCTS<br />
Paratyphi BO<br />
LTD., UK<br />
http://www.pdf4free.com<br />
PT. BINTANG MONO INDONESIA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM
AKL<br />
20305901985 28/05/2009<br />
PLASMATEC LABORATORY PRODUCTS<br />
REIGED DIAGNOSTICS CRP Latex LTD., UK<br />
PT. RAJA ERBA INDOCHEM<br />
AKL<br />
10303403666 22/05/2009 ABX Pentra ASO CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101402601 22/05/2009<br />
AKL<br />
20101901884 22/05/2009<br />
AKL<br />
10101901885 22/05/2009<br />
GB Human Chorionic Gonadotropin GENERAL BIOLOGICALS CORPORATION.,<br />
(hCG) Enzyme Immunoassay Test Kit TAIWAN R.O.C<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
GIESSE DIAGNOSTICS Total<br />
Bilirubin (B) GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS<br />
Magnesium GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
AKL<br />
10101901886 22/05/2009 GIESSE DIAGNOSTICS Lipase GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
AKL<br />
10101901887 22/05/2009<br />
AKL<br />
20101901888 22/05/2009<br />
GIESSE DIAGNOSTICS HDL<br />
Cholesterol Precipitant GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Calibrator<br />
Clinical Chemistry GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
AKL<br />
20101901889 22/05/2009 GIESSE DIAGNOSTICS Chloride GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
AKL<br />
20101901890 22/05/2009<br />
AKL<br />
20101901891 22/05/2009<br />
AKL<br />
20101901892 22/05/2009<br />
AKL<br />
10101901893 22/05/2009<br />
AKL<br />
20305901921 26/05/2009<br />
AKL<br />
10304901922 26/05/2009<br />
GIESSE DIAGNOSTICS Alpha<br />
Amylase SL GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Calibrator<br />
Chol. HDL/LDL Direct GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Urea UV<br />
SL GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS<br />
Phosphorus UV GIESSE DIAGNOSTICS SRL., ITALY PT. BETA PRIMA<br />
AEROSET / ARCHITECT Quantia<br />
IgE Standard<br />
VITROS ECIQ Immunodiagnostic<br />
System<br />
BIOKIT SA., SPAIN untuk ABBOTT<br />
LABORATORIES., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
PT. ABBOTT INDONESIA<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
10204901923 26/05/2009<br />
AKL<br />
10304901924 26/05/2009<br />
VITROS Immunodiagnostic Product<br />
System Cleaning Solution<br />
VITROS ECI Immunodiagnostic<br />
System<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20303901925 26/05/2009 SD Bioline Dengue IgG/IgM WB STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20208901926 26/05/2009 ABX Pentra HbA1C WB Cal HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10102901938 26/05/2009<br />
AKL<br />
10102901939 26/05/2009<br />
AKL<br />
20102901940 26/05/2009<br />
AKL<br />
10102901941 26/05/2009<br />
AKL<br />
10101901942 26/05/2009<br />
AKL<br />
10201901943 26/05/2009<br />
REIGED ELISA Washer - RD<br />
Washer W 600<br />
REIGED Semi Automatic Chemistry<br />
Analyzer RD-60<br />
REIGED Electrolyte Analyzer RD-<br />
Lyte<br />
REIGED Auto Biochemistry Analyzer<br />
RD-140<br />
SIEMENS DIMENSION PHOS<br />
Phosphorus<br />
ADVIA Autoslide May Grunwald<br />
Giemsa Buffer<br />
AKL<br />
10101901944 26/05/2009 SIEMENS Check Stix Combo Pak<br />
AKL<br />
10201901945 26/05/2009<br />
ADVIA Autoslide May Grunwald<br />
Stain<br />
AKL<br />
10101901946 26/05/2009 SIEMENS DIMENSION LIP Lipase<br />
AKL<br />
20101901947 26/05/2009<br />
SIEMENS DIMENSION CREA<br />
Creatinine<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101901927 26/05/2009<br />
GONGDONG Vacuum Tube Sterile<br />
Plain (No Additive) Red Cap<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103901930 26/05/2009<br />
DIMA Phencyclidine One Step Drug<br />
Abuse Test<br />
MEIKANG BIOTECH (SHANGHAI) CO. LTD.,<br />
CHINA untuk DIMA Ges.f DIAGNOSTIKA<br />
mbH., GERMANY<br />
PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20103901931 26/05/2009<br />
DIMA Multiline 6 One Step Drug<br />
Screen Test<br />
MEIKANG BIOTECH (SHANGHAI) CO. LTD.,<br />
CHINA untuk DIMA Ges.f DIAGNOSTIKA<br />
mbH., GERMANY<br />
PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20103901932 26/05/2009<br />
DIMA Multiline 5 One Step Drug<br />
Screen Test<br />
MEIKANG BIOTECH (SHANGHAI) CO. LTD.,<br />
CHINA untuk DIMA Ges.f DIAGNOSTIKA<br />
mbH., GERMANY<br />
PT. ISOTEKINDO INTERTAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103901933 26/05/2009<br />
DIMA Multiline 3-1 One Step Drug<br />
Screen Test<br />
AKL<br />
20205901934 26/05/2009 REIGED Coagulometer RD-COAG<br />
AKL<br />
10102901935 26/05/2009 REIGED ELISA Reader ER-500<br />
AKL<br />
20205901936 26/05/2009<br />
AKL<br />
10102901937 26/05/2009<br />
REIGED Hematology Analyzer RD-<br />
7021<br />
REIGED Fully Automatic<br />
Biochemistry Analyzer RD-240<br />
MEIKANG BIOTECH (SHANGHAI) CO. LTD.,<br />
CHINA untuk DIMA Ges.f DIAGNOSTIKA<br />
mbH., GERMANY<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
SINOWA MEDICAL SCIENCE &<br />
TECHNOLOGY., CHINA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
PT. RAJA ERBA INDOCHEM<br />
AKL<br />
20103902138 28/05/2009<br />
AKL<br />
20101902214 29/05/2009<br />
ABBOTT AXSYM System<br />
Theophyllin II Standard Calibrators ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
EASYTOUCH GCU Blood<br />
Glucose/Cholesterol/Uric Acid Multi-<br />
Function Monitoring System<br />
BIOPTIK TECHNOLOGY INC., REPUBLIC<br />
OF CHINA<br />
PT. DAYA AGUNG MANDIRI<br />
AKL<br />
20101902215 29/05/2009 EASYTOUCH Blood Glucose Strip BIOPTIK TECHNOLOGY INC., TAIWAN PT. DAYA AGUNG MANDIRI<br />
AKL<br />
10101902216 29/05/2009<br />
AKL<br />
20101902217 29/05/2009<br />
EASYTOUCH GCU Blood<br />
Cholesterol Strip<br />
BIOLAND Blood Glucose Monitoring<br />
System<br />
AKL<br />
20101902218 29/05/2009 BIOLAND Blood Glucose Strip<br />
AKL<br />
10101902221 29/05/2009 EASY TOUCH Blood Uric Acid Strip<br />
AKL<br />
21102902219 29/05/2009 BESTMAN Doppler FHR Detector<br />
AKL<br />
21102902220 29/05/2009<br />
BESTMAN Doppler Fetal Hearth<br />
Rate Detector<br />
BIOPTIK TECHNOLOGY INC., REPUBLIC<br />
OF CHINA<br />
BIOPTIK TECHNOLOGY (SHENZHEN) CO.<br />
LTD., P. R. CHINA<br />
BIOPTIK TECHNOLOGY (SHENZHEN) CO.<br />
LTD., P. R. CHINA<br />
BIOPTIK TECHNOLOGY INC., REPUBLIC<br />
OF CHINA<br />
SHENZHEN BESTMAN INSTRUMENT CO.<br />
LTD., P.R. CHINA<br />
SHENZHEN BESTMAN INSTRUMENT CO.<br />
LTD., P.R. CHINA<br />
AKL<br />
10102403529 28/05/2009 URISCAN OPTIMA II YD-DIAGNOSTICS., KOREA<br />
AKL<br />
10102403530 28/05/2009 URISCAN PRO II YD-DIAGNOSTICS., KOREA<br />
PT. DAYA AGUNG MANDIRI<br />
PT. DAYA AGUNG MANDIRI<br />
PT. DAYA AGUNG MANDIRI<br />
PT. DAYA AGUNG MANDIRI<br />
PT. DAYA AGUNG MANDIRI<br />
PT. DAYA AGUNG MANDIRI<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
20208902139 28/05/2009 ABX Pentra HbA1C WB Control HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10202902208 29/05/2009 NUCLISENS EasyQ Analyzer BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302902209 29/05/2009 NUCLISENS EasyQ Incubator II BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20205902206 29/05/2009 FULLY AUTO Hematology Analyzer<br />
AKL<br />
20205902207 29/05/2009 FULLY AUTO Hematology Analyzer<br />
AKL<br />
10303902212 29/05/2009 COBAS Taq Man MTB Test<br />
AKL<br />
20305902213 29/05/2009 LIGHT CYCLER 2.0 + Accessories<br />
SHENZEN PROCAN ELECTRONICS INC.,<br />
CHINA<br />
SHENZEN PROCAN ELECTRONICS INC.,<br />
CHINA<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS Gmbh.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305303421 28/05/2009 HEXAGEN HCV HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303902137 28/05/2009 HEXAGON malaria Combi HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305304572 28/05/2009 HUMAN Hepatitis B latex Quick Test HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101304578 28/05/2009 HUMAN HUMAPREG Single Serum HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101902140 28/05/2009<br />
AKL<br />
20101301853 28/05/2009<br />
AKL<br />
20101301834 28/05/2009<br />
AKL<br />
20101401018 28/05/2009<br />
AKL<br />
20103401011 28/05/2009<br />
AKL<br />
20103401016 28/05/2009<br />
AKL<br />
20103401013 28/05/2009<br />
PDF Creator - PDF4Free v3.0<br />
GONGDONG Vacuum Tube Sterile<br />
ESR Black Cap<br />
VITROS Chemistry Products CK<br />
Slides<br />
VITROS Chemistry Products AMYL<br />
Slides<br />
INST-ANSWER Hcg One Step<br />
Pregrancy Test Device<br />
INST-ANSWER AMP One Step<br />
Amphetamine Test Device<br />
INST-ANSWER THC One Step<br />
Marijuana Test Device<br />
INST-ANSWER MOP Step Morphin<br />
Test Device<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., CHINA<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ORTHO - CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
P.R. CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
P.R. CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
P.R. CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
P.R. CHINA<br />
http://www.pdf4free.com<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB
AKL<br />
20103401007 28/05/2009<br />
INST-ANSWER MDMA One Step<br />
Ecstasy Test Device<br />
AKL<br />
10101902625 08/06/2009 BRAHMS PCT Sensitive K-QC<br />
AKL<br />
20101902626 08/06/2009 BRAHMS PCT Sensitive K-CAL<br />
AKL<br />
20101902627 08/06/2009 BRAHMS PCT Sensitive K-050<br />
AKL<br />
20101902628 08/06/2009 BRAHMS Kryptor Compact<br />
AKL<br />
30305902727 16/06/2009<br />
AKL<br />
10305902728 16/06/2009<br />
AKL<br />
10305902729 16/06/2009<br />
AKL<br />
10305902730 16/06/2009<br />
AKL<br />
10305902731 16/06/2009<br />
AMPLICOR HIV - 1 Monitor Test,<br />
version 1,5 (HIM)<br />
AKL<br />
20207902716 16/06/2009 SIEMENS Berichrom Protein C<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
P.R. CHINA<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. TIRTA MEDILAB<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. ROCHE INDONESIA<br />
Troponin T hs STAT Elecsys and<br />
Cobas e Analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
Troponin T hs Calset Elecsys and<br />
Cobas e Analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
Troponin T hs Elecsys and Cobas e<br />
Analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
Troponin T hs STAT Calset and<br />
Cobas e Analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20208902717 16/06/2009 SIEMENS D-Dimer Control Plasma I<br />
AKL<br />
10204902718 16/06/2009<br />
AKL<br />
10208902719 16/06/2009<br />
AKL<br />
10204902720 16/06/2009<br />
AKL<br />
10208902721 16/06/2009<br />
AKL<br />
10204902722 16/06/2009<br />
AKL<br />
20103902723 16/06/2009<br />
ONE LAB E-Z Cleanser For<br />
Hematology Analyzer<br />
ONE LAB Diluent For Hematology<br />
Analyzer<br />
ONE LAB Cleanser - Rinse for<br />
Hematology Analyzer<br />
ONE LAB Lyse For Hematology<br />
Analyzer<br />
ONE LAB Probe Cleanser For<br />
Hematology Analyzer<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
GmbH., GERMANY atas penunjukan SYSMEX<br />
CORPORATION., JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
GmbH., GERMANY atas penunjukan SYSMEX<br />
CORPORATION., JAPAN<br />
SHENZEN MINDRAY BIO MEDICAL<br />
ELECTROMAS CO. LTD., CHINA<br />
SHENZEN MINDRAY BIO MEDICAL<br />
ELECTROMAS CO. LTD., CHINA<br />
SHENZEN MINDRAY BIO MEDICAL<br />
ELECTROMAS CO. LTD., CHINA<br />
SHENZEN MINDRAY BIO MEDICAL<br />
ELECTROMAS CO. LTD., CHINA<br />
SHENZEN MINDRAY BIO MEDICAL<br />
ELECTROMAS CO. LTD., CHINA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
CDI Rapid Drug Screen Panel Cup<br />
EZ Key 6 in 1 (Amp, Meth, THC,<br />
Morp, Benz, COC) CDI / BIOCHEMP CO., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103902724 16/06/2009<br />
CDI Rapid Drug Screen 6 in 1 (Amp,<br />
Meth, THC, Morp, Benz, COC) CDI / BIOCHEMP CO., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103902725 16/06/2009<br />
AKL<br />
30305902726 16/06/2009<br />
CDI Rapid Drug Screen Cup 6 in 1<br />
(Amp, Meth, THC, Morp, Benz, COC) CDI / BIOCHEMP CO., USA PT. ONCOPROBE UTAMA<br />
COBAS AMPLICOR HIV - 1 Monitor<br />
Test, version 1,5 (Ca HIM)<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
AKL<br />
30305902655 10/06/2009<br />
AKL<br />
20205902659 10/06/2009<br />
VITROS Immunodiagnostic Products<br />
Anti-HIV 1+2 Reagent Pack<br />
SIEMENS Coagulation Factor VIII<br />
Deficient Plasma<br />
AKL<br />
20207902658 10/06/2009 SIEMENS Test Thrombin Reagent<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
GmbH., GERMANY atas penunjukan SYSMEX<br />
CORPORATION., JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
GmbH., GERMANY atas penunjukan SYSMEX<br />
CORPORATION., JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
AKL<br />
20208902657 10/06/2009<br />
GmbH., GERMANY atas penunjukan SYSMEX<br />
SIEMENS D-Dimer Control Plasma II CORPORATION., JAPAN<br />
AKL<br />
20207902656 10/06/2009 SIEMENS Fibrinogen Calibrator Kit<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
GmbH., GERMANY atas penunjukan SYSMEX<br />
CORPORATION., JAPAN<br />
AKL<br />
20101902660 10/06/2009 CROMATEST Creatinine LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
20101902661 10/06/2009<br />
CROMATEST Alkaline Phospatase<br />
BR<br />
LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
10101902662 10/06/2009 CROMATEST ALT / GPT BR Opt LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
10101902663 10/06/2009 CROMATEST Triglyceride MR LINEAR CHEMICALS SL., SPAIN<br />
PT. TAWADA HEALTHCARE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101902664 10/06/2009 CROMATEST Acid Phospatase LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
10101902665 10/06/2009 CROMATEST Cholesterol MR LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
20101902666 10/06/2009 CROMATEST AST / GOT BR Opt LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
20101902667 10/06/2009 CROMATEST Albumin LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
20101902668 10/06/2009 CROMATEST Glucose MR LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
10101902669 10/06/2009 CROMATEST HDL Cholesterol LINEAR CHEMICALS SL., SPAIN<br />
AKL<br />
20101300882 10/06/2009 DIASYS Bilirubin Auto Direct FS<br />
AKL<br />
20101300890 10/06/2009 DIASYS HBDH FS<br />
AKL<br />
20101303529 10/06/2009 DIASYS TRUCAL U<br />
AKL<br />
20101402868 10/06/2009 DIASYS Calcium CPC FS<br />
AKL<br />
20101303527 10/06/2009 DIASYS Amylase CC FS<br />
AKL<br />
20101300894 10/06/2009 DIASYS Glucose GOD FS<br />
AKL<br />
20101303536 10/06/2009 DIASYS Calcium AS FS<br />
AKL<br />
10101303531 10/06/2009 DIASYS TRULAB P<br />
AKL<br />
20101902672 12/06/2009<br />
MONO Hcg One Step Pregnancy<br />
Test Cassette<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH &<br />
CO. KG., GERMANY<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
P.R. CHINA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. BINTANG MONO INDONESIA<br />
AKL<br />
20305902673 12/06/2009 ABX Pentra RF Cal ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20303902674 12/06/2009 ABX Pentra ASO Cal ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305902675 12/06/2009 ABX Pentra Myoglobin Cal ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101403795 12/06/2009 ABX Pentra Micro Albumin CP ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305403794 12/06/2009 ABX Pentra Myoglobin CP ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101403797 12/06/2009 ABX Pentra u - Alb Cal ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101403796 12/06/2009 ABX Pentra u - Alb Control L/H ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101403798 12/06/2009 ABX Pentra Protein Control L/H ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10304501499 12/06/2009 HUMAN Elisys Quattro HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20103304582 12/06/2009<br />
HUMAN Humadrug<br />
Methamphetamine HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102400533 12/06/2009 HUMAN Autohumalyzer 900 S Plus HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101401608 12/06/2009 HUMAN Prolactin (PRL) HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101902715 12/06/2009<br />
AKL<br />
20101902714 12/06/2009<br />
B.BRAUN OMNITEST EZ Blood<br />
Glucose Test Strips<br />
B.BRAUN OMNITEST EZ Blood<br />
Monitoring System<br />
INFOPIA CO. LTD., KOREA untuk B.BRAUN<br />
MEDICAL INDUSTRIES SDN. BHD.,<br />
MALAYSIA<br />
INFOPIA CO. LTD., KOREA untuk B.BRAUN<br />
MEDICAL INDUSTRIES SDN. BHD.,<br />
MALAYSIA<br />
AKL<br />
20102902416 01/06/2009 LIDA Clinical Chemistry Analyzer LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
10101902417 01/06/2009 CROMATEST Magnesium MR LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902418 01/06/2009 CROMATEST LDH BR LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902419 01/06/2009 CROMATEST Total Protein LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
10101902420 01/06/2009<br />
AKL<br />
10303902427 01/06/2009<br />
CROMATEST LDL - Cholesterol<br />
Direct<br />
INTEC Advanced Quality One Step<br />
Anti-TB Test Card<br />
LINEAR CHEMICALS S.L., SPAIN<br />
INTEC PRODUCTS INC., XIAMEN., P.R.<br />
CHINA<br />
PT. B.BRAUN MEDICAL<br />
INDONESIA<br />
PT. B.BRAUN MEDICAL<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. MITRASAMAYA SEJATI<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303902428 01/06/2009<br />
AKL<br />
20207902429 01/06/2009<br />
INTEC Advanced Quality Rapid Anti-<br />
TB Test Strip<br />
SIEMENS LA2 Confirmation<br />
Reagent<br />
AKL<br />
20207902430 01/06/2009 SIEMENS LA1 Screening Reagent<br />
AKL<br />
20207902431 01/06/2009 SIEMENS Calcium Chloride Solution<br />
AKL<br />
20207902432 01/06/2009 SIEMENS Berichrom Heparin<br />
AKL<br />
20208902433 01/06/2009 SIEMENS Control Plasma N<br />
AKL<br />
20208902434 01/06/2009 SIEMENS Control Plasma P<br />
AKL<br />
20102902435 01/06/2009<br />
INTEC PRODUCTS INC., XIAMEN., P.R.<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX CORPORATION.,<br />
JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX CORPORATION.,<br />
JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX CORPORATION.,<br />
JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX CORPORATION.,<br />
JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX CORPORATION.,<br />
JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX CORPORATION.,<br />
JAPAN<br />
PT. MITRASAMAYA SEJATI<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
SYSMEX UF-100 Fully Automated<br />
Urine Cell Analyzers SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20305902436 01/06/2009 ADVANTAGE Mal Card J. MITRA & CO. PVT. LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20305902437 01/06/2009 ADVANTAGE Pan Malaria Card J. MITRA & CO. PVT. LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20209902438 01/06/2009<br />
AKL<br />
10103902439 01/06/2009<br />
AKL<br />
20101902440 01/06/2009<br />
AKL<br />
20101902441 01/06/2009<br />
Amicus MNC Apheresis Kit, Double<br />
Needle FENWAL INC., USA PT. MEDQUEST JAYA GLOBAL<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge<br />
PCHE<br />
MULTIPLY Integrated Multisensor<br />
IMT Standard A<br />
SIEMENS QuikLYTE Multisensor<br />
Standard A<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305902442 01/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge CRP<br />
AKL<br />
20303902443 01/06/2009 RAPITEX ASL<br />
AKL<br />
20303902444 01/06/2009 SIEMENS Cellognost Amoebiasis<br />
AKL<br />
20303902445 01/06/2009 SIEMENS Enzygnost Syphilis SYPH<br />
AKL<br />
20305902446 01/06/2009<br />
AKL<br />
20305902447 01/06/2009<br />
SIEMENS N Rheumatology Standard<br />
SL<br />
SIEMENS N/T Rheumatology<br />
Control SL/1<br />
AKL<br />
20305902448 01/06/2009 SIEMENS Cellognost RF<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
AKL<br />
10101902415 01/06/2009 CROMATEST Phosphorus UV LINEAR CHEMICALS S.L., SPAIN<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
20305902609 08/06/2009 IMTEC-MPO-ANCA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305902610 08/06/2009 IMTEC-TPO-Antibodies HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101902611 08/06/2009<br />
AKL<br />
20208902613 08/06/2009<br />
SIEMENS DIMENSION ALDL -<br />
Automated LDL Cholesterol<br />
SIEMENS DIMENSION HA1c<br />
Calibrator<br />
AKL<br />
20305902614 08/06/2009 SIEMENS N Latex RF<br />
AKL<br />
20305902615 08/06/2009<br />
AKL<br />
20305902616 08/06/2009<br />
SIEMENS N Antiserum To Human<br />
IgG<br />
SIEMENS N Antiserum To Human<br />
IgA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305902617 08/06/2009 RAPITEX CRP/PCR<br />
AKL<br />
20101902618 08/06/2009 SIEMENS N Latex Cystatin C<br />
AKL<br />
20305902619 08/06/2009 SIEMENS N Protein Standard SL<br />
AKL<br />
20305902620 08/06/2009<br />
SIEMENS N Antiserum To Human<br />
C3c<br />
AKL<br />
20305902621 08/06/2009 CARDIOPHASE hsCRP<br />
AKL<br />
20305902622 08/06/2009<br />
AKL<br />
20305902623 08/06/2009<br />
AKL<br />
10304803197 09/07/2009<br />
SIEMENS N Antiserum To Human<br />
C4<br />
SIEMENS N Antiserum To Human<br />
IgM<br />
TRITURUS - ELISA Analizer and<br />
Accessories<br />
AKL<br />
20101902414 01/06/2009 SINNOWA Electrolyte Analyzer<br />
AKL<br />
10102902413 01/06/2009 SINNOWA Spectrophotometer<br />
AKL<br />
20205902412 01/06/2009<br />
AKL<br />
20101902423 01/06/2009<br />
AKL<br />
10101902424 01/06/2009<br />
AKL<br />
20303902426 01/06/2009<br />
AKL<br />
10101902421 01/06/2009<br />
AKL<br />
20209902515 04/06/2009<br />
SINNOWA Automatic Hematology<br />
Analyzer<br />
ROCHE B2 - Microglobulin Tina -<br />
Quant [a] Roche / Hitachi<br />
ROCHE B2 - Microglobulin Control<br />
Set Serum Roche / Hitachi<br />
ROCHE Preci Control CMV IgG<br />
Elecsys and Cobas e Analyzer<br />
ROCHE Preci Control CMV IgM<br />
Elecsys and Cobas e Analyzer<br />
Amicus Apheresis Kit, Single Needle<br />
with Platelet Additive Solution<br />
Connector<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
GRIFOLS ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE untuk DIAGNOSTICS GRIFOLS<br />
SA., SPAIN<br />
SINNOWA MEDICAL SCIENCE &<br />
TECHNOLOGY CO. LTD., P.R. CHINA<br />
SINNOWA MEDICAL SCIENCE &<br />
TECHNOLOGY CO. LTD., P.R. CHINA<br />
SINNOWA MEDICAL SCIENCE &<br />
TECHNOLOGY CO. LTD., P.R. CHINA<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
FENWAL INC., REP. DOMINICA untuk<br />
FENWAL INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20208902516 04/06/2009 HUMAN HbA1c Liquidirect Controls HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10209902517 04/06/2009 HUMAX 14 K HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20206902518 04/06/2009<br />
dBest One Step Occult Blood Card<br />
Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101901928 26/05/2009<br />
AKL<br />
20103902519 04/06/2009<br />
AKL<br />
20103902520 04/06/2009<br />
AKL<br />
20303902521 04/06/2009<br />
AKL<br />
20305902522 04/06/2009<br />
AKL<br />
20303902523 04/06/2009<br />
AKL<br />
20103902524 04/06/2009<br />
AKL<br />
20103902525 04/06/2009<br />
AKL<br />
20305902940 25/06/2009<br />
AKL<br />
10204902941 25/06/2009<br />
GONGDONG Vacuum Tube Sterile<br />
Plain (No Additive) Red Cap<br />
MONO One Step Marijuana (THC)<br />
Cassette<br />
MONO One Step Cocaine (COC)<br />
Test Cassette<br />
MONO One Step Anti-TP Test<br />
Cassette<br />
MONO One Step HCV Test<br />
Cassette<br />
MONO One Step Malaria (Pf/Pv)<br />
Test Cassette<br />
MONO One Step Amphetamine Test<br />
Cassette<br />
MONO One Step Multi Drug 3<br />
(AMP/THC/OPI) Test Cassette<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge<br />
RCRP<br />
SIEMENS QUIKLYTE Integrated<br />
Multisensor Flush Solution<br />
AKL<br />
20101902942 25/06/2009 SIEMENS QUIKLYTE Standard B<br />
AKL<br />
20101902913 25/06/2009<br />
AKL<br />
20303902914 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System CHEM I Calibrator<br />
SIEMENS Cellognost Toxoplasmosis<br />
H<br />
ZHEJIANG GONGDONG MEDICAL PLASTIC<br />
FACTORY., CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101902910 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System TP/ALB Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305902911 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge IGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101902912 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge<br />
AMON<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204902908 25/06/2009<br />
SIEMENS QUIKLYTE Integrated<br />
Multisensor Sample Diluent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305902909 25/06/2009<br />
AKL<br />
20101902951 25/06/2009<br />
AKL<br />
20201902952 25/06/2009<br />
AKL<br />
20101902953 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge IGM<br />
SIEMENS DIMENSION Clinical<br />
Chemistry System Flex Reagent<br />
Cartridge CA<br />
SIEMENS Supplementary Reagent<br />
for Enzygnost/TMB<br />
DIMENSION Clinical Chemistry<br />
System UCFP Calibrator<br />
AKL<br />
20303902954 25/06/2009 SIEMENS Cellognost Syphilis<br />
AKL<br />
10101902955 25/06/2009<br />
AKL<br />
10101902977 25/06/2009<br />
SIEMENS DIMENSION Clinical<br />
Chemistry System Flex Reagent<br />
Cartridge IBCT<br />
ROCHE sfIT-Elecsys and Cobas e<br />
Analyzer<br />
AKL<br />
10303902901 25/06/2009 ROCHE Light Cycler EBV Quant Kit<br />
AKL<br />
20305902902 25/06/2009<br />
ROCHE PIGF Elecsys and Cobas e<br />
Analyzer<br />
AKL<br />
10101902903 25/06/2009 ROCHE Preci Control sFit-l<br />
AKL<br />
20101902904 25/06/2009<br />
AKL<br />
20305902905 25/06/2009<br />
ROCHE sFit-Elecsys and Cobas e<br />
Analyzer<br />
ROCHE IL-6 Elecsys and Cobas e<br />
Analyzer<br />
AKL<br />
20305902906 25/06/2009 ROCHE Preci Control IL-6<br />
AKL<br />
10305902907 25/06/2009<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PreciControl Troponin Elecsys and<br />
Cobas e Analyzer ROCHE DIAGNOSTICS GmbG., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
10101902889 25/06/2009 CROMATEST GPT Colour LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902890 25/06/2009 CROMATEST Urea/BUN BR LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902891 25/06/2009 CROMATEST Amylase MR LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
10101902892 25/06/2009 CROMATEST GGT BR LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902893 25/06/2009 CROMATEST Creatinine Kinase BR LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20103902894 25/06/2009 CROMATEST Cholinesterase LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902895 25/06/2009 CROMATEST Chloride LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902896 25/06/2009 CROMATEST Calcium Arsenazo lll LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902897 25/06/2009 CROMATEST Urea Berthelot LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101902898 25/06/2009 CROMATEST GOT Colour LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20305902899 25/06/2009<br />
ROCHE PIGF Calset Elecsys and<br />
Cobas e Analyzer<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305902900 25/06/2009<br />
ROCHE CRPL 3 (C-Reactive<br />
Protyein) Gen.3 Cobas c system<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
AKL<br />
20101403528 06/07/2009 YD URICSON Glucose 1 Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101403525 26/06/2009 YD URICSON 3 GPH Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
20101403526 26/06/2009 YD URICSON Gen 11 Strip YD DIAGNOSTICS., KOREA<br />
AKL<br />
20103903011 30/06/2009<br />
AKL<br />
20301502760 07/08/2009<br />
PT. ROCHE INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
LOVIBOND Cholinesterase AF267<br />
Test Kit THE TINTOMETER LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Trimethoprim Susceptibility<br />
Discs OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305503007 30/06/2009 HEPANOSTIKA HbsAg Ultra BIO MERIEUX BV., NETHERLAND PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
30305401933 30/06/2009 VIRONOSTIKA HIV 1 Antigen BIO MERIEUX BV., NETHERLAND PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303403418 30/06/2009 TREPANOSTIKA TP Recombinant BIO MERIEUX BV., NETHERLAND PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305400061 30/06/2009<br />
AKL<br />
30305802008 30/06/2009<br />
BIO MERIEUX Hepanostika Anti<br />
HBc Uni-Form BIO MERIEUX BV., NETHERLAND PT. ENSEVAL MEDIKA PRIMA<br />
VIRONOSTIKA HIV Uni-Form ll<br />
Ag/Ab BIO MERIEUX BV., NETHERLAND PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103805362 26/06/2009<br />
INTEC Advanced Quality One Step<br />
Multy Drug Screen Test DOA-5<br />
INTEC PRODUCT INC (XIAMEN)., P.R.<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20103805363 26/06/2009<br />
INTEC Advanced Quality One Step<br />
Multy Drug Screen Test DOA-3<br />
INTEC PRODUCT INC (XIAMEN)., P.R.<br />
CHINA<br />
AKL<br />
20305301989 26/06/2009 QUANTA LITE B2 GPI IgG ELISA INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20305301990 26/06/2009 QUANTA LITE ACA IgG lll INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
10302902992 26/06/2009<br />
AKL<br />
10302902993 26/06/2009<br />
AKL<br />
10302902994 26/06/2009<br />
PT. MITRASAMAYA SEJATI<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
HARLEQUIN E.Coli / Coliform<br />
Medium LAB M LIMITED., UK PT. PYRIDAM FARMA<br />
LAB 13 A Bismuth Sulphite Agar<br />
Base A LAB M LIMITED., UK PT. PYRIDAM FARMA<br />
LAB 13 A Bismuth Sulphite Agar<br />
Base B LAB M LIMITED., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302902995 26/06/2009 LAB M Nutrient Agar LAB M LIMITED., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302902996 26/06/2009 LAB M Mac Conkey Broth LAB M LIMITED., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302902997 26/06/2009 LAB M Brilliant Green Agar LAB M LIMITED., UK PT. PYRIDAM FARMA<br />
AKL<br />
20305902998 26/06/2009 ROCHE Preci Control PIGF<br />
AKL<br />
20305902999 26/06/2009<br />
AKL<br />
20305803000 26/06/2009<br />
ROCHE IL-6 Calset Elecsys and<br />
Cobas e Analyzer<br />
ROCHE CRPL3 (C-Reactive<br />
Protein) Gen 3 Cobas C System<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH., GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101902915 25/06/2009<br />
AKL<br />
20103902919 25/06/2009<br />
AKL<br />
20103902920 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge TBIL<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge<br />
DGNA-Digoxin<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge<br />
CRBM<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101902921 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge T4<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103902922 25/06/2009<br />
AKL<br />
20103902923 25/06/2009<br />
AKL<br />
20101902924 25/06/2009<br />
PDF Creator - PDF4Free v3.0<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge COC<br />
SIEMENS DIMENSION THC Flex<br />
Reagent Cartridge<br />
SIEMENS DIMENSION CKMB Flex<br />
Reagent Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
10101902925 25/06/2009<br />
SIEMENS DIMENSION GGT Flex<br />
Reagent Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101902926 25/06/2009<br />
AKL<br />
20101902927 25/06/2009<br />
AKL<br />
20101902928 25/06/2009<br />
AKL<br />
20103902929 25/06/2009<br />
AKL<br />
10101902930 25/06/2009<br />
AKL<br />
20101902931 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge TU<br />
SIEMENS DIMENSION CK Flex<br />
Reagent Cartridge<br />
SIEMENS DIMENSION GLU Flex<br />
Reagent Cartridge<br />
SIEMENS DIMENSION BENZ Flex<br />
Reagent Cartridge<br />
SIEMENS DIMENSION URCA Flex<br />
Reagent Cartridge<br />
SIEMENS DIMENSION ALB Flex<br />
Reagent Cartridge<br />
AKL<br />
20101902932 25/06/2009 DIMENSION CSA Calibrator<br />
AKL<br />
10103902933 25/06/2009 DIMENSION PCHE Verifier<br />
AKL<br />
20101902934 25/06/2009 DIMENSION IBCT<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101902935 25/06/2009<br />
AKL<br />
20101902936 25/06/2009<br />
AKL<br />
20101902937 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge CSA<br />
SIEMENS DIMENSION BUN Flex<br />
Reagent Cartridge<br />
SIEMENS DIMENSION AMY Flex<br />
Reagent Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305902938 25/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge AGG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101902939 25/06/2009<br />
AKL<br />
20305903009 30/06/2009<br />
AKL<br />
20101903010 30/06/2009<br />
DIMENSION Clinical Chemistry<br />
System Flex Reagent Cartridge LDH<br />
VITROS Immunodiagnostic Products<br />
Myoglobin Calibrators<br />
VITROS Immunodiagnostic Products<br />
Red Cell Folate Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
PT. SIEMENS INDONESIA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20101903102 03/07/2009 ABX PENTRA Apo Cal HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305903103 03/07/2009 ABX PENTRA Accelerator I CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101903104 03/07/2009 ABX PENTRA Apo B HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101903105 03/07/2009 ABX PENTRA Apo A1 HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101903106 03/07/2009 ABX PENTRA Apo Control L/H HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305903107 03/07/2009 ABX PENTRA Accelerator II CP HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10102903108 03/07/2009<br />
AKL<br />
10102903109 03/07/2009<br />
AKL<br />
10102903110 03/07/2009<br />
AKL<br />
10102903111 03/07/2009<br />
FINE CARE Accumax Autopippettes<br />
FAP<br />
FINE CARE Accumax Autopippettes<br />
MAP<br />
FINE CARE Accumax Autopippettes<br />
VAP<br />
FINE CARE Accumax Autopippettes<br />
AJ<br />
FINE CARE BIOSYSTEMS., INDIA<br />
FINE CARE BIOSYSTEMS., INDIA<br />
FINE CARE BIOSYSTEMS., INDIA<br />
FINE CARE BIOSYSTEMS., INDIA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
10302402433 03/07/2009 Bact / ALERT 3D with Reagent BIO MERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302301783 03/07/2009 Bact / ALERT MP BIO MERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302301825 03/07/2009 Bact / ALERT FA BIO MERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302301823 03/07/2009 Bact / ALERT FN BIO MERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302301824 03/07/2009 Bact / ALERT PF BIO MERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305504952 03/07/2009<br />
AKL<br />
VITROS Chemistry Products hsCRP<br />
Reagent<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY CO.,<br />
USA<br />
PT. TAWADA HEALTHCARE<br />
20101903112 03/07/2009 ONCOPROBE Blood Glucose Meter HMD BIOMEDICAL INC., TAIWAN R.O.C PT. ONCOPROBE UTAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103903113 03/07/2009<br />
MONO One Step Morphine Test<br />
Cassette<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
PT. BINTANG MONO INDONESIA<br />
AKL<br />
10101903114 03/07/2009 FLUITEST Phos BIOCON DIAGNOSTIC GmbH., GERMANY PT. BAVARIA COMBININDO<br />
AKL<br />
20305501975 13/07/2009<br />
ABBOTT ARCHITECT Anti-Hbc IgM<br />
Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20205903208 13/07/2009 CLINIMACS CD25 Reagent MILTENYL BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20205903209 13/07/2009 CLINIMACS CD3 Reagent MILTENYL BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20101903210 13/07/2009<br />
ONCOPROBE Blood Glucose Test<br />
Strip<br />
HMD BIOMEDICAL INC., REPUBLIC OF<br />
CHINA<br />
PT. ONCOPROBE UTAMA<br />
AKL<br />
20205903211 13/07/2009 CLINIMACS CD56 Reagent MILTENYL BIOTEC GmbH., GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20101901025 30/03/2009 SIEMENS ADVIA Chemistry C4<br />
AKL<br />
20101901026 30/03/2009<br />
VITROS Vitamin B12 Reagent Pack<br />
1/2<br />
AKL<br />
20101901027 30/03/2009 VITROS PROT Slides<br />
AKL<br />
10101901028 30/03/2009 VITROS Oncology Control<br />
AKL<br />
20101901029 30/03/2009 AMP Urea UV<br />
AKL<br />
20101901030 30/03/2009 AMP Glucose PAP<br />
RANDOX LABORATORIES LTD., UK untuk<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO, UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTICS<br />
A J<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO, UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTICS<br />
A J<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON CO, UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTICS<br />
A J<br />
AMP MEDIZINTECHNIK HANDLES GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDLES GmbH.,<br />
AUSTRIA<br />
PT. SIEMENS INDONESIA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
AKL<br />
10302901048 30/03/2009 LAB M Triple Sugar Iron Agar LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901049 30/03/2009 LAB M X.L.D Agar LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901050 30/03/2009 LAB M Harlequin Salmonella ABC LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901051 30/03/2009 LAB M T.C.B.S Cholera Medium LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901052 30/03/2009 LAB M Columbia Agar Base LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901053 30/03/2009 LAB M Harlequin SMAC - BIG LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901054 30/03/2009<br />
LAB M Limited Harlequin Listeria<br />
Medium LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10101901055 30/03/2009<br />
CHOLESTECH LDX Multi-Analyte<br />
Controls<br />
AKL<br />
20305901056 30/03/2009 WAMPOLE Cardiolipin IgM ELISA II<br />
AKL<br />
20303901057 30/03/2009 WAMPOLE EBNA-1 IgG ELISA II<br />
AKL<br />
20303901058 30/03/2009<br />
AKL<br />
20303901059 30/03/2009<br />
WAMPOLE Varicella Zoster Virus<br />
IgM (VZV) ELISA II<br />
WAMPOLE Varicella Zoster Virus<br />
IgG (VZV) ELISA II<br />
AKL<br />
20305901060 30/03/2009 WAMPOLE Cardiolipin IgG ELISA II<br />
CHOLESTECH CORPORATION, USA atas<br />
penunjukan INVERNESS MEDICAL<br />
INNOVASIONS NORTH AMERICA INC, USA<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305901061 30/03/2009 WAMPOLE ENA Profile Elisa II<br />
AKL<br />
20303901062 30/03/2009 WAMPOLE Rubella IgM ELISA II<br />
AKL<br />
20305901063 30/03/2009<br />
AKL<br />
10102901064 30/03/2009<br />
AKL<br />
10102901065 30/03/2009<br />
AKL<br />
10102901039 30/03/2009<br />
AKL<br />
20101901040 30/03/2009<br />
AKL<br />
20101901043 30/03/2009<br />
AKL<br />
20101901044 30/03/2009<br />
AKL<br />
10302901045 30/03/2009<br />
AKL<br />
10302901046 30/03/2009<br />
WAMPOLE ENA Combined Screen<br />
ELISA II<br />
STARLET 200 + Accessories &<br />
Spare Part<br />
STARTEC VT, Accessories dan<br />
Spare Part<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
ZEUS SCIENTIFIC Inc., USA untuk<br />
WAMPOLE LABORATORIES (member of<br />
INVERNESS MEDICAL PROFESSIONAL<br />
DIAGN<br />
SUNOSTIK MEDICAL TECHNOLOGY CO.,<br />
LTD., CHINA<br />
SUNOSTIK MEDICAL TECHNOLOGY CO.,<br />
LTD., CHINA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
SEROPETTE Variable Volume<br />
Pipettors STANBIO LABORATORY., USA PT. DEMKA SAKTI<br />
IRMA TRUPOINT Blood Analysis<br />
System H3 Cartridge<br />
AVOXIMETER 4000 Whole Blood<br />
Oximeter Disposable Cuvettes<br />
AVOXIMETER 1000E Whole Blood<br />
Oximeter Disposable Cuvettes<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
LAB M D.C.A (Desoxycholate Citrate<br />
Agar) LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
LAB M S.S Agar (Salmonella<br />
Shigella Agar) LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
10302901047 30/03/2009 LAB M Sabouraud Dextrose Agar LAB M LIMITED, UK PT. PYRIDAM FARMA<br />
AKL<br />
20101901036 30/03/2009<br />
AKL<br />
20101600039 30/03/2009<br />
AKL<br />
20305600042 30/03/2009<br />
AKL<br />
20101901038 30/03/2009<br />
INTEC SimpDO Blood Glucose<br />
Monitoring System<br />
INTEC SimpDO Blood Glucose Test<br />
Strip<br />
INTEC Advanced Quality HCV<br />
ELISA Test Kit<br />
IRMA TRUPOINT Blood Analysis<br />
System H4 Cartridge<br />
AKL<br />
10101901031 30/03/2009 AMP Cholesterol Standard<br />
AKL<br />
10101901032 30/03/2009 AMP Uric Acid<br />
AKL<br />
10101901033 30/03/2009 AMP Triglycerides Standard<br />
AKL<br />
20101901034 30/03/2009 AMP Glucose Standard<br />
AKL<br />
20103901463 01/04/2009<br />
AKL<br />
20103600041 30/03/2009<br />
AKL<br />
30305603281 30/03/2009<br />
AKL<br />
10302901162 30/03/2009<br />
AKL<br />
10302901161 30/03/2009<br />
AKL<br />
10302901160 30/03/2009<br />
AKL<br />
10302901159 30/03/2009<br />
AKL<br />
10302901158 30/03/2009<br />
AKL<br />
20207901157 30/03/2009<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
AMP MEDIZINTECHNIK HANDLES GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDLES GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDLES GmbH.,<br />
AUSTRIA<br />
AMP MEDIZINTECHNIK HANDLES GmbH.,<br />
AUSTRIA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. DEMKA SAKTI<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
PT. SETIA GUNA MEDIKA<br />
Synthron Saliva Screen 6 (AMP,<br />
THC, MOR, MET, COC, BZD) SYNTHRON BIORESEARCH INC., USA PT. MEDIHOP<br />
INTEC One Step Barbiturate Test<br />
Card<br />
INTEC Advance Quality HIV 1&2<br />
ELISA Test Kit 3rd Generation<br />
AKL<br />
20207901156 30/03/2009 Variant II Dual Kit Reorder Pack<br />
AKL<br />
20207901155 30/03/2009<br />
AKL<br />
30305402163 31/03/2009<br />
PDF Creator - PDF4Free v3.0<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
MERCKOPLATE Mac CONKEY-<br />
Agar MERCK KGaA, GERMANY PT. MERCK Tbk<br />
MERCKOPLATE Brolacin Agar<br />
(Bromothymolblue Lactose Cystine<br />
Agar) MERCK KGaA, GERMANY PT. MERCK Tbk<br />
MERCKOPLATE Mueller - Hinton<br />
Agar MERCK KGaA, GERMANY PT. MERCK Tbk<br />
MERCKOPLATE Candida Elective<br />
Agar acc to Nickerson MERCK KGaA, GERMANY PT. MERCK Tbk<br />
FLUOROPLATE Candida Agar with<br />
Cloromphenicol and Gentamycin MERCK KGaA, GERMANY PT. MERCK Tbk<br />
Variant II Hemoglobin Testing<br />
System BIORAD LABORATORIES INC, USA PT. DIASTIKA BIOTEKINDO<br />
BIORAD LABORATORIES GmbH,<br />
GERMANY<br />
PT. DIASTIKA BIOTEKINDO<br />
Variant B Thallasemia Short Program<br />
Reorder Pack BIORAD LABORATORIES INC, USA PT. DIASTIKA BIOTEKINDO<br />
ABBOTT AXSYM HIV 1/2 gO<br />
Reagent Pack ABBOTT GmbH DIAGNOSTIKA, GERMANY PT. ABBOTT INDONESIA<br />
http://www.pdf4free.com
AKL<br />
20205901467 01/04/2009<br />
CELL-DYN Emerald Analyzer +<br />
Accessories and Spare Part ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20207901469 01/04/2009 CELL-DYN Emerald CN-Free Lyse ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10204901470 01/04/2009 CELL-DYN Emerald Cleaner ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20303903322 22/07/2009 SD Dengue IgG Capture ELISA STANDARD DIAGNOSTIC Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303903321 22/07/2009 SD BIOLINE Rota/Adeno Rapid STANDARD DIAGNOSTIC Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101903320 22/07/2009 SD BIOLINE Troponin I STANDARD DIAGNOSTIC Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303903464 28/07/2009<br />
REMEL Salmonella Hc Agglunating<br />
Sera REMEL INC., UK untuk REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101903268 17/07/2009 DIALAB G6PDH UV Kinetic DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101903269 17/07/2009 DIALAB G6PDH Lyse Reagents DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101903267 17/07/2009 DIALAB G6PDH Control Set DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101502020 17/07/2009 SD UroColor 1K STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20207903271 17/07/2009 ABX PENTRA HbA1c WB HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10302903513 03/08/2009 Mini API BIO MERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101902211 29/05/2009 INDEC Total Protein<br />
AKL<br />
20305402655 03/08/2009 BIOCON CRPslide<br />
AKL<br />
20305402652 03/08/2009 BIOCON RFslide<br />
AKL<br />
20101402922 03/08/2009 FLUITEST P-Amyl<br />
AKL<br />
20101402926 03/08/2009 FLUITEST AMYL-IFCC<br />
AKL<br />
20101402927 03/08/2009 FLUITEST AMYL-EPS<br />
AKL<br />
10303903517 03/08/2009<br />
ENZYGNOST Anti-Measles Virus /<br />
igM<br />
AKL<br />
10101402925 03/08/2009 FLUITEST Iron B<br />
AKL<br />
10103903516 03/08/2009 DIMENSION DOA-Positive Control<br />
AKL<br />
10303903514 03/08/2009<br />
AKL<br />
20103903515 03/08/2009<br />
AKL<br />
20101903518 03/08/2009 BIOSUB LA<br />
SENSATEST One-Step TB Test<br />
Cassette<br />
SENSATEST One-Step<br />
Cannabinoids (THC) Test Cassette<br />
AUDIT DIAGNOSTICS, IRELAND dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO,<br />
LTD., P.R CHINA untuk NEOPHARMA<br />
BIOTECH ASIA SDN BHD., MALAYSIA<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO,<br />
LTD., P.R CHINA untuk NEOPHARMA<br />
BIOTECH ASIA SDN BHD., MALAYSIA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. INDEC DIAGNOSTICS<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. SIEMENS INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. SIEMENS INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. BAVARIA COMBININDOA<br />
AKL<br />
20305903673 07/08/2009<br />
WAMPOLE B.burgdorferi IgM ELISA<br />
II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305903674 07/08/2009 WAMPOLE MPO IgG ELISA II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305903675 07/08/2009<br />
WAMPOLE ANA Screen IgG ELISA<br />
II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305903676 07/08/2009 WAMPOLE Proteinase-3 ELISA II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10102903860 21/08/2009 NucliSens Easy MAG Disposable BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305903677 07/08/2009<br />
WAMPOLE Legionella IgG/IgA/IgM<br />
ELISA II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305903678 07/08/2009<br />
WAMPOLE B.burgdorferi IgG/IgM<br />
ELISA II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305903679 07/08/2009<br />
WAMPOLE B.burgdorferi IgG ELISA<br />
II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305903681 07/08/2009 WAMPOLE ANCA Screen ELISA II<br />
AKL<br />
20303903680 07/08/2009 WAMPOLE HSV-1&2 IgM ELISA II<br />
AKL<br />
20305903682 07/08/2009 WAMPOLE Cardiolipin IgA ELISA II<br />
ZEUS SCIENTIFIC INC., USA melalui<br />
WAMPOLE LABORATORIES INC., USA untuk<br />
INVERNESS MEDICAL INNOVATIONS<br />
ZEUS SCIENTIFIC, Inc., USA melalui<br />
WAMPOLE Inc., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERI<br />
ZEUS SCIENTIFIC, Inc., USA melalui<br />
WAMPOLE Inc., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERI<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303801991 07/08/2009 VIDAS H.pylori IgG/HPY BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303801988 07/08/2009 VIDAS Toxo IgG Avidity/TXGA BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801990 07/08/2009 VIDAS T3 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801989 07/08/2009 VIDAS Testosterone/Tes BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102903658 07/08/2009<br />
AKL<br />
20101903659 07/08/2009 IRMA TRUPOINT GL Cartridge<br />
AKL<br />
20305903661 07/08/2009 SIEMENS NOR-Partigen C4<br />
AKL<br />
10204903662 07/08/2009 SIEMENS Cleaner SCS<br />
AKL<br />
20303903663 07/08/2009<br />
KEYLAB Discrete Random Access<br />
Analyzer and Accessories BPC BIOSED., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
ENZYGNOST Anti-Rubella-Virus /<br />
IgM<br />
AKL<br />
20103903664 07/08/2009 IMMULITE Digoxin<br />
AKL<br />
20101903665 07/08/2009 IMMULITE LBP<br />
AKL<br />
20101903666 07/08/2009 DIMENSION ALDL Calibrator<br />
AKL<br />
20305903667 07/08/2009 DIMENSION RCRP Calibrator<br />
AKL<br />
20101903668 07/08/2009<br />
DIMENSION Special Protein<br />
Calibrator<br />
AKL<br />
20101903669 07/08/2009 QUIKLYTE Integrated Multisensor<br />
AKL<br />
10303903670 07/08/2009<br />
AKL<br />
10303903672 07/08/2009<br />
WAMPOLE Mycoplasma IgG ELISA<br />
II<br />
WAMPOLE Mycoplasma IgM ELISA<br />
II<br />
AKL<br />
10303903671 07/08/2009 WAMPOLE H. Pylori IgG ELISA II<br />
INTERNATIONAL TECHNIDYNE<br />
CORPORATION., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
ZEUS SCIENTIFIC, Inc., USA melalui<br />
WAMPOLE Inc., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERI<br />
ZEUS SCIENTIFIC, Inc., USA melalui<br />
WAMPOLE Inc., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERI<br />
ZEUS SCIENTIFIC, Inc., USA melalui<br />
WAMPOLE Inc., USA untuk INVERNESS<br />
MEDICAL INNOVATIONS NORTH AMERI<br />
PT. DEMKA SAKTI<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20205903697 12/08/2009 ABX Micros ES 60 HORIBA ABX DIAGNOSTIC., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101801984 14/08/2009 VIDAS FT3 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101501056 14/08/2009 QCA CK-MB QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101501054 14/08/2009 QCA Urea UV QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101903717 14/08/2009 DIMENSION MG Magnesium<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101903718 14/08/2009 DIMENSION CKMB Verifier<br />
AKL<br />
20305903719 14/08/2009 DIMENSION CRP Calibrator<br />
AKL<br />
20101903720 14/08/2009 DIMENSION CHOL Calibrator<br />
AKL<br />
20101903721 14/08/2009 DIMENSION CK Verifier<br />
AKL<br />
20101903722 14/08/2009 DIMENSION DBIL Direct Bilirubin<br />
AKL<br />
20101903723 14/08/2009 DIMENSION CHEM II Calibrator<br />
AKL<br />
20305903724 14/08/2009 SIEMENS Turbiquant Ig M<br />
AKL<br />
20101903725 14/08/2009 SIEMENS N-Protein Standard UY<br />
AKL<br />
20305903726 14/08/2009 SIEMENS Turbiquant Ig A<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101801982 14/08/2009 VIDAS TSH BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305801983 14/08/2009 VIDAS HBe/Anti HBe BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101801987 14/08/2009 VIDAS FT4 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305801986 14/08/2009 VIDAS Ferritin/FER BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103801985 14/08/2009 VIDAS Digoxin/DIG BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101903727 14/08/2009 DIMENSION IRN Calibrator<br />
AKL<br />
20101903728 14/08/2009 DIMENSION TBIL/DBIL Calibrator<br />
AKL<br />
20101903729 14/08/2009 DIMENSION Enzyme Verifier<br />
AKL<br />
20101903730 14/08/2009 DIMENSION LIP Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305903731 14/08/2009 TURBOX Immunoglobulin A ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903732 14/08/2009 TURBOX Complement C4 ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903733 14/08/2009 TURBOX APO A - 1 ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903734 14/08/2009 TURBOX CPR ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903735 14/08/2009 TURBOX Immunoglobulin M ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
10303903736 14/08/2009 TURBOX ASO - PAIA ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903737 14/08/2009 TURBOX Immunoglobulin G ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
10102903738 14/08/2009 TURBOX Plus Protein Analyzer ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903739 14/08/2009 TURBOX APO B ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305903740 14/08/2009 TURBOX Complement 3 ORION DIAGNOSTICA OY., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20101501664 21/08/2009<br />
AKL<br />
20101403456 21/08/2009<br />
CAROLINA Total Bilirubin Reagent<br />
(TBIL) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
RANDOX - Direct HDL/LDL<br />
Cholesterol Calibrator RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20101402039 21/08/2009 RANDOX - Glucose GOD PAP RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20101403462 21/08/2009 RANDOX - CK MB Calibrator RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
10101403459 21/08/2009 RANDOX - CK MB Tri Level Control RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
10101403460 21/08/2009<br />
AKL<br />
10101403461 21/08/2009<br />
RANDOX - Assayed Urine Control<br />
Abnormal RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
RANDOX - Assayed Urine Control<br />
Normal RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20101403463 21/08/2009 RANDOX - Bilirubin Elevated Serum RANDOX LABORATORIES LTD., UK PT. SURYA DELTA MAJU<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305403080 21/08/2009<br />
AKL<br />
20305403078 21/08/2009<br />
AKL<br />
20305403083 21/08/2009<br />
AKL<br />
20305403082 21/08/2009<br />
AKL<br />
20305403347 21/08/2009<br />
AKL<br />
20305403348 21/08/2009<br />
AKL<br />
20305403079 21/08/2009<br />
AKL<br />
20305403346 21/08/2009<br />
AKL<br />
20208501650 21/08/2009<br />
AKL<br />
10101501666 24/08/2009<br />
AKL<br />
10103504154 24/08/2009<br />
THE BINDING SITE MININEPH<br />
Human C4 Kit THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE MININEPH<br />
Human IgG Kit THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE BINDARID<br />
Human IgM NL Kit THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE MININEPH<br />
Human IgM Kit THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE MININEPH<br />
Human B2 Microglobulin Kit THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE Hep-2 Cells-<br />
10x5 Well Side THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE BINDARID<br />
Human Complement C4 NL THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
THE BINDING SITE ANCA Combi<br />
Kit THE BINDING SITE LTD., UK PT. SURYA DELTA MAJU<br />
RADIOMETER Calibrating Solution<br />
2 RADIOMETER MEDICAL ApS, DENMARK<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
CAROLINA Gamma Glutamyl<br />
Transferase Reagent (GGT) CAROLINA LIQUID CHEMISTRIES CO., USA PT. RIOCA MEDICA<br />
ABBOTT Axsym Phenobarbital<br />
Control ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20306502868 24/08/2009 ARCHITECT CA 125 II Reagent Kit<br />
AKL<br />
20306502867 24/08/2009 ARCHITECT CA 125 II Calibrator<br />
AKL<br />
20306502869 24/08/2009 ARCHITECT CA 125 II Control<br />
AKL<br />
20303903909 25/08/2009<br />
AKL<br />
20103903910 25/08/2009<br />
AKL<br />
20103903912 25/08/2009<br />
AKL<br />
20103903913 25/08/2009<br />
AKL<br />
20103903914 25/08/2009<br />
AKL<br />
20303903915 25/08/2009<br />
AKL<br />
20303903916 25/08/2009<br />
INTEC Advanced Quality Rapid<br />
Malaria (P.F) Test Card<br />
INTEC ONE STEP Cocaine (COC)<br />
Test Card<br />
INTEC ONE STEP Opiates<br />
(OPI/Morphine) Test Card<br />
INTEC ONE STEP Ectasy (MDMA)<br />
Test Card<br />
INTEC ONE STEP Amphetamine<br />
(AMP) Test Card<br />
INTEC Advanced Quality Rapid<br />
Malaria (p.f/p.v) Test Card<br />
INTEC Advanced Quality Rapid<br />
Malaria (p.f/p.v) Test Strip<br />
FUJIREBIO DIAGNOSTICS, USA untuk<br />
ABBOTT LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTICS, USA untuk<br />
ABBOTT LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTICS, USA untuk<br />
ABBOTT LABORATORIES, USA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20103903917 25/08/2009<br />
AKL<br />
20103903918 25/08/2009<br />
INTEC ONE STEP Benzodiazepines<br />
(BZO) Test Card<br />
INTEC ONE STEP Cannabinoid<br />
(THC) Test Card<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
AKL<br />
21106903857 21/08/2009 RAPIDWARM Cleave VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106903858 21/08/2009 RAPIDVIT Cleave VITROLIFE SWEDEN AB., SWEDEN<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20303903887 21/08/2009 SD BIOLINE JEV IgG / IgM STANDARD DIAGNOSTICS, INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303903888 21/08/2009 SD Malaria Antigen P.F ELISA STANDARD DIAGNOSTICS, INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303903889 21/08/2009 SD Dengue NS1 Ag ELISA STANDARD DIAGNOSTICS, INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303903890 21/08/2009 SD Dengue IgM Capture ELISA STANDARD DIAGNOSTICS, INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10302903886 21/08/2009<br />
AKL<br />
10304903873 21/08/2009 SIEMENS N-Reaction Buffer<br />
AKL<br />
10303903874 21/08/2009 SIEMENS N Latex ASL<br />
AKL<br />
10304903875 21/08/2009<br />
HUMATEMP (Microplate Incubator)<br />
+ Accessories & Spareparts HUMAN GmbH, GERMANY PT. SALI POLAPA BERSAMA<br />
SIEMENS N-Supplementary<br />
Reagent L<br />
AKL<br />
20305903876 21/08/2009 SIEMENS NOR-Partigen IgM<br />
AKL<br />
10101903877 21/08/2009 SIEMENS Turbiquant Apo A-I<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103903878 21/08/2009<br />
SIEMENS Syva Emit II Plus<br />
Cannabinoid Assay<br />
AKL<br />
20305903879 21/08/2009 SIEMENS NOR-Partigen C3c<br />
AKL<br />
20305903880 21/08/2009 SIEMENS RapiTex RF<br />
AKL<br />
10101903881 21/08/2009 SIEMENS Turbiquant Apo B<br />
AKL<br />
20305903882 21/08/2009 SIEMENS NOR-Partigen IgA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
AKL<br />
20101903863 21/08/2009 CARESENS N Test Strip I - SENS Inc., KOREA<br />
AKL<br />
21106903864 21/08/2009 RAPIDVIT Blast VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106903865 21/08/2009 RAPIDVIT Blast VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
20305903867 21/08/2009 SIEMENS Complement Reagents<br />
AKL<br />
20207903868 21/08/2009 SIEMENS BC Thrombin Reagent<br />
AKL<br />
20305903869 21/08/2009 SIEMENS Berichrom F XIII<br />
AKL<br />
20208903870 21/08/2009<br />
SIEMENS BC Data-Fi Abnormal<br />
Fibrinogen Control Plasma<br />
AKL<br />
20208903871 21/08/2009 SIEMENS Batroxobin Reagent<br />
AKL<br />
20208903872 21/08/2009 SIEMENS PT. Multi Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Ltd.,<br />
SINGAP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Ltd.,<br />
SINGAP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Ltd.,<br />
SINGAP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Ltd.,<br />
SINGAP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Ltd.,<br />
SINGAP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Ltd.,<br />
SINGAP<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SUNRISE INTERMEDICA<br />
INDO<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10101303209 26/08/2009 RANDOX Iron RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
AKL<br />
20101403457 26/08/2009 RANDOX Potassium RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
AKL<br />
10101303887 26/08/2009 RANDOX HDL-Cholesterol Standard RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
AKL<br />
10101903954 26/08/2009<br />
AKL<br />
20208903955 26/08/2009<br />
AKL<br />
20103903950 26/08/2009<br />
AKL<br />
20103903951 26/08/2009<br />
RANDOX RIQAS General Clinical<br />
Chemistry RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
RANDOX Haemoglobin Human<br />
Methaemoglobin Standard RANDOX LABORATORIES Ltd., UK PT. SURYA DELTA MAJU<br />
SPEEDYTEST - DOA 3 Grug Panel<br />
Test (MOP/THC/MDMA) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST - DOA 3 Grug Panel<br />
Test (MOP/THC/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
10302904213 01/09/2009 OXOID AnaeroGen OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302904215 01/09/2009 OXOID reagent Set D OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302904412 14/09/2009 MICROSCREEN C. Difficile MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302904413 14/09/2009 MICROGEN GN - ID B Panel MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20101904129 28/08/2009 DIMENSION HCG Calibrator<br />
AKL<br />
20101904130 28/08/2009 DIMENSION HCG<br />
AKL<br />
20101904131 28/08/2009 DIMENSION T3<br />
AKL<br />
20305904132 28/08/2009 DIMENSION FERR (Ferr)<br />
AKL<br />
20305904133 28/08/2009 DIMENSION Thyroid Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20306904134 28/08/2009 DIMENSION T/F PSA Calibrator<br />
AKL<br />
20306904135 28/08/2009<br />
DIMENSION FPSA Flex Reagent<br />
Cartridge<br />
AKL<br />
20306904136 28/08/2009 DIMENSION TPSA<br />
AKL<br />
20305904137 28/08/2009 DIMENSION MYO Calibrator<br />
AKL<br />
20101904138 28/08/2009 DIMENSION T3 Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10303904139 31/08/2009 CORIS Pylori-Strip CORIS BIO CONCEPT, BELGIUM PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20208904140 31/08/2009<br />
J. Mitra Anti D IgG IgM Monoclonal<br />
Antibodies J. MITRA & CO., Ltd., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20303904141 31/08/2009 DIAGNOS Dengue Card BIOMED INDUSTRIES, INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
10303904142 31/08/2009 API CAMPY BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305904143 31/08/2009 ARCHITECT C-Peptide Reagent<br />
AKL<br />
20101904144 31/08/2009 ARCHITECT C-Peptide Calibrator<br />
AKL<br />
10101904145 31/08/2009 ARCHITECT C-Peptide Control<br />
BIOKIT S.A, FRANCE for ABBOT<br />
LABORATORIES., USA<br />
BIOKIT S.A, SPAIN for ABBOT<br />
LABORATORIES., USA<br />
BIOKIT S.A, SPAIN for ABBOT<br />
LABORATORIES., USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305904146 31/08/2009 ARCHITECT Anti-HBc II Control ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305904147 31/08/2009 ARCHITECT Anti-HBc II Reagent ABBOTT GmbH & Co KG, GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305904148 31/08/2009 ARCHITECT HCV Ag Control<br />
AKL<br />
20305904149 31/08/2009 ARCHITECT HCV Ag Reagent Kit<br />
AKL<br />
20305904150 31/08/2009 ARCHITECT HCV Ag Calibrator<br />
DENKA SEIKEN Co.Ltd., JAPAN for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
DENKA SEIKEN Co.Ltd., JAPAN for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
DENKA SEIKEN Co.Ltd., JAPAN for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20306904151 31/08/2009 SENSATEST One-Step PSA Test<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.,<br />
LTD., CHINA atas penunjukan NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., M<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305904152 31/08/2009<br />
SENSATEST One-Step HB Multi 5<br />
(HbsAg / HBc / HbeAg / Hbe /<br />
HbcAb)<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.,<br />
LTD., CHINA atas penunjukan NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., M<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20206904153 31/08/2009<br />
SENSATEST One-Step Fecal<br />
Occult Blood Test<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.,<br />
LTD., CHINA atas penunjukan NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., M<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20103904154 31/08/2009<br />
AKL<br />
20209904155 31/08/2009<br />
SENSATEST One-Step Multi Drugs<br />
Panel Test Dip 5 (MOP / THC / AMP<br />
/ MET / COC)<br />
MEDION DIAGNOSTICS Anti-H<br />
Lectin<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.,<br />
LTD., CHINA atas penunjukan NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., M<br />
MEDION DIAGNOSTICS AG.,<br />
SWITZERLAND<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10303904156 31/08/2009<br />
CLEARVIEW TB Elisa LAM Specific<br />
Direct Urinary Antigen Test<br />
AKL<br />
20303904157 31/08/2009 BINAX NOW Filariasis Test<br />
BINAX INC USA, untuk INVERNESS<br />
MEDICAL., USA<br />
BINAX INC USA, untuk INVERNESS<br />
MEDICAL., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10302802109 01/09/2009 API CORYNE BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802110 01/09/2009 API 20 C AUX BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802101 01/09/2009 API Stap BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303802103 01/09/2009 API 20 Strep BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802104 01/09/2009 API Candida BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303802105 01/09/2009 API Listeria BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802106 01/09/2009 API Suspension Medium BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20302802107 01/09/2009 API NH BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101300553 01/09/2009 QCA Glucose Liquid QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101300589 01/09/2009 QCA Uric Acid QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101300551 01/09/2009 QCA Cholesterol Liquid QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10302904214 01/09/2009 OXOID MICROBACT 12B Half Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302904216 01/09/2009 OXOID Membrane Lauryl Sulphate OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302904217 01/09/2009<br />
OXOID SIGNAL Blood Culture<br />
System OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305904296 03/09/2009 ENZYGNOST Anti-HBs II<br />
AKL<br />
20303904297 03/09/2009 ENZYGNOST Anti-HSV / IgM<br />
AKL<br />
20305904298 03/09/2009 ENZYGNOST HBe Monoclonal<br />
AKL<br />
20303904299 03/09/2009 ENZYGNOST Anti-VZV / IgG<br />
AKL<br />
20303904300 03/09/2009 ENZYGNOST Anti-CMV / IgG+IgM<br />
AKL<br />
20207904301 03/09/2009 ENZYGNOST F 1+2 (monoclonal)<br />
AKL<br />
20305904302 03/09/2009 ENZYGNOST Anti-HBc Monoclonal<br />
AKL<br />
20303904303 03/09/2009 ENZYGNOST Anti-HSV / IgG<br />
AKL<br />
20207904304 03/09/2009 ENZYGNOST TAT micro<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTCS GmbH., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20208904305 03/09/2009 SIEMENS LA Control High and low<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20208904306 03/09/2009 Dade Ci-Trol 1, 2, 3<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207904307 03/09/2009 SIEMENS Von Willebrand Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207904308 03/09/2009<br />
SIEMENS Coagulation Factor II<br />
Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20208904309 03/09/2009<br />
Dade Ci-Trol Heparin Control Low<br />
and High<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207904310 03/09/2009<br />
SIEMENS Coagulation Factor VII<br />
Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207904311 03/09/2009<br />
SIEMENS Coagulation Factor X<br />
Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC Pte, Ltd.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20303904280 03/09/2009 SD BIOLINE Chikungunya IgM STANDARD DIAGNOSTICS Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303904281 03/09/2009 SD BIOLINE Dengue NS1 Ag STANDARD DIAGNOSTICS Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303904282 03/09/2009 SD BIOLINE Malaria Antigen ELISA STANDARD DIAGNOSTICS Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303904283 03/09/2009 SD BIOLINE Malaria Antigen P.f STANDARD DIAGNOSTICS Inc., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20301904284 03/09/2009<br />
BIODISCS Trimethoprim<br />
Sulfamethoxazole - 1,25 + 23,75 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904285 03/09/2009 BIODISCS Piperacillin - 100 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904286 03/09/2009 BIODISCS Minocycline - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301904287 03/09/2009 BIODISCS Virginiamycin - 15 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904288 03/09/2009 BIODISCS Mezlocillin - 75 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904289 03/09/2009 BIODISCS Lincomycin - 2 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904290 03/09/2009 BIODISCS Kanamycin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904291 03/09/2009 BIODISCS Cefalexin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904292 03/09/2009 BIODISCS Sulfonamides - 200 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904293 03/09/2009 BIODISCS Streptomycin - 10 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
30305904294 03/09/2009 VIDAS HIV P24 II BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801838 04/09/2009 BIOMERIEUX Cholesterol RTU BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801840 04/09/2009 BIOMERIEUX Unitrol BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101801837 04/09/2009 BIOMERIEUX Creatinine Cinetique BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305600752 04/09/2009 VIDAS Myoglobin (MYO) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801842 04/09/2009 BIOMERIEUX Zymotrol BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101801841 04/09/2009<br />
AKL<br />
20101801848 04/09/2009<br />
AKL<br />
20101801847 04/09/2009<br />
BIOMERIEUX Uree Cinetique UV<br />
250 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX Enzyline Phosphatase<br />
Acide Optimise BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX Enzyline PAL<br />
Optimise BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204805803 04/09/2009 Mini VIDAS Configuration BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801845 04/09/2009 BIOMERIEUX Bilitrol BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801844 04/09/2009 BIOMERIEUX Ca-Kit BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801843 04/09/2009 BIOMERIEUX TIBC-Additif BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
30305600643 04/09/2009 VIDAS HIV DUO Ultra (HIV5) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103600750 04/09/2009 VIDAS Theophylline (THEO) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101903907 25/08/2009 BIOSINO Total Bilirubin (T.Bili) Kit<br />
AKL<br />
20101903908 25/08/2009 BIOSINO Direct Bilirubin (D.Bili) Kit<br />
AKL<br />
20101903894 25/08/2009 BIOSINO Creatinine Test System<br />
AKL<br />
10101903895 25/08/2009<br />
BIOSINO Triglyceride (TG) Assay<br />
Kit<br />
AKL<br />
10101903896 25/08/2009 BIOSINO Cholesterol Kit (CHO)<br />
AKL<br />
10101903897 25/08/2009<br />
AKL<br />
20101903898 25/08/2009<br />
AKL<br />
20305904371 14/09/2009<br />
AKL<br />
20103904372 14/09/2009<br />
AKL<br />
20101904373 14/09/2009<br />
AKL<br />
20305904374 14/09/2009<br />
AKL<br />
20103904375 14/09/2009<br />
AKL<br />
20103904376 14/09/2009<br />
AKL<br />
20305904377 14/09/2009<br />
PDF Creator - PDF4Free v3.0<br />
BIOSINO Direct HDL Cholesterol<br />
Assay Kit (D-HDL-C)<br />
DUOCARE Light Blood Glucose<br />
Monitor<br />
EXCEL CV HBsAb One Step<br />
Hepatitis B Surface Antibody<br />
EXCEL AMP One Step<br />
Amphetamine Test Device<br />
EXCEL hCG One Step Pregnancy<br />
Test Strip<br />
EXCEL HBsAg One Step Hepatitis B<br />
Surface Antigen<br />
EXCEL MOP One Step Morphine<br />
Test Device<br />
EXCEL THC One Step Marijuana<br />
Test Device<br />
EXCEL HBsAg One Step Hepatitis B<br />
Surface Antigen<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
GENEXEL-SEIN Inc., KOREA atas<br />
penunjukan dari L-TAC MEDICARE Pte. Ltd.,<br />
SINGAPORE<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
http://www.pdf4free.com<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. SEGARA PUTERA MANDIRI<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA
AKL<br />
20305904378 14/09/2009<br />
AKL<br />
20305904379 14/09/2009<br />
AKL<br />
20209904543 25/09/2009<br />
AKL<br />
20208904408 14/09/2009<br />
AKL<br />
20208904409 14/09/2009<br />
AKL<br />
20208904410 14/09/2009<br />
AKL<br />
20208904411 14/09/2009<br />
EXCEL HBsAb One Step Hepatitis B<br />
Surface Antibody<br />
EXCEL HCV Hepatitis C Virus One<br />
Step Test Device<br />
AFFIRMAGEN 3% Red Blood Cell<br />
Reagent (Pooled Cells)<br />
NOVA BIOMEDICAL Calibrator<br />
Cartridge A pHOx Plus C<br />
NOVA BIOMEDICAL Calibrator<br />
Cartridge B pHOx Plus<br />
NOVA BIOMEDICAL Calibrator<br />
Cartridge B pHOx Plus C<br />
NOVA BIOMEDICAL Calibrator<br />
Cartridge A pHOx Plus<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO., LTD.,<br />
CHINA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., USA<br />
NOVA BIOMEDICAL., USA<br />
NOVA BIOMEDICAL., USA<br />
NOVA BIOMEDICAL., USA<br />
NOVA BIOMEDICAL., USA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
AKL<br />
10303904414 14/09/2009 MICROGEN Strep ID MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10303904415 14/09/2009 ROTASCREEN II EIA MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10303904416 14/09/2009 ROTASCREEN Dipstick MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10303904417 14/09/2009 MICROGEN Strep MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20103904512 25/09/2009<br />
AKL<br />
20103904513 25/09/2009<br />
AKL<br />
20103904514 25/09/2009<br />
AKL<br />
20103904515 25/09/2009<br />
AKL<br />
20103904516 25/09/2009<br />
HOME DRUG Test<br />
(MOP/COC/THC/MET/MDMA/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
HOME DRUG Test<br />
(MOP/THC/AMP/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
HOME DRUG Test<br />
(MOP/THC/AMP) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
HOME DRUG Test<br />
(MOP/THC/MET/MDMA/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
HOME DRUG Test<br />
(MOP/COC/THC/AMP/BZO) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20303802063 25/09/2009 VIDAS RUB IgG II / RBG BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101802062 25/09/2009 VIDAS LH (Vidas Range) BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102904623 28/09/2009<br />
AKL<br />
20205904624 28/09/2009<br />
SYSMEX Fully Automated Urine<br />
Particle Analyzer Assessories +<br />
Spare Parts SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
SYSMEX Fully Automated<br />
Hematology Analyzer, Assessories +<br />
Spare Parts SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10304904652 30/09/2009 CHORUS TRIO and Accessories DIESSE DIAGNOSTIC SENESE SPA, ITALY<br />
AKL<br />
20303904653 30/09/2009 PANBIO Dengue Early ELISA<br />
AKL<br />
20303904654 30/09/2009 PANBIO Dengue Duo Cassette<br />
AKL<br />
30305501562 28/09/2009 ACON HIV 1/2/O Tri-Line<br />
PANBIO PTY LTD, AUSTRALIA, untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
PANBIO PTY LTD, AUSTRALIA, untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
ACON BIOTECH (HANGZHOU) CO, LTD.,<br />
CHINA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305904537 25/09/2009 NUCLISENS Mini MAG BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904536 25/09/2009 BIODISCS Cefamandole - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305904535 25/09/2009 NUCLISENS Isolation Reagents BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10202904538 25/09/2009 NUCLISENS Lysis Buffer BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303904539 25/09/2009 VIDAS CMV IgG CMVM BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204904544 25/09/2009<br />
AKL<br />
20101904649 30/09/2009<br />
VITROS Immunodiagnostic Products<br />
Maintenance Pack<br />
FIRST CHOICE Pregnancy Test<br />
Cassette<br />
AKL<br />
20101904650 30/09/2009 LAMSON hCG Pregnancy Test Strip<br />
AKL<br />
20101904651 30/09/2009 FIRST CHOICE Pregnancy Test<br />
AKL<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
DIVISION OF JOHNSON & JOHNSON<br />
COMPANY, UK atas penunjukan ORTHO<br />
CLINICA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
PT. TAWADA HEALTHCARE<br />
PT. PRIMA BUMI MANDIRI<br />
PT. PRIMA BUMI MANDIRI<br />
PT. PRIMA BUMI MANDIRI<br />
20101904832 02/10/2009 BPC Albumine UV G 2004 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101904833 02/10/2009 BPC BUN UV G 2008 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
10101904834 02/10/2009 BPC GPT / ALT G 2043 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
10101904835 02/10/2009 BPC Gamma-GT G 2032 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
10101904836 02/10/2009 BPC Uric Acid G 2002 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
10101904837 02/10/2009 BPC Cholesterol G 2018 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20101904838 02/10/2009 BPC Glucose G 2034 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20101904839 02/10/2009 BPC Total Proteins G 2050 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
10101904840 02/10/2009 BPC Triglycerides G 2054 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20101904841 02/10/2009 BPC GOT / AST G 2040 BPC BIOSED Srl., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20301904736 01/10/2009 BIODISCS Doxycyclin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904737 01/10/2009 BIODISCS Cefsulodin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904738 01/10/2009 BIODISCS Fosfomycin - 50 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904739 01/10/2009 BIODISCS Ticarcillin TIC 75 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904740 01/10/2009 BIODISCS Oleandomycin OL 15 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904741 01/10/2009 BIODISCS Pipemidic Acid PI 20 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904742 01/10/2009 BIODISCS Pefloxacin PEF 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904743 01/10/2009 BIODISCS Flumequine AR 30 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904744 01/10/2009 BIODISCS Teicoplanin TEC 30 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904745 01/10/2009 BIODISCS Pristinamycin PR 15 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904746 01/10/2009 BIODISCS Dibekacine DKB 10 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904747 01/10/2009 BIODISCS Fusidic Acid FA 10 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904748 01/10/2009 BIODISCS Amoxicillin - 25 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904749 01/10/2009 BIODISCS Amikacine - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904750 01/10/2009 BIODISCS Gentamycin - 10 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904751 01/10/2009 BIODISCS Aztreonam - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904752 01/10/2009 BIODISCS Cefoxitin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904753 01/10/2009 BIODISCS Cefotaxim - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904754 01/10/2009 INCUBATOR / SHAKER 50X BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904757 01/10/2009 MB/ABCT Enrichment Fluid BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904758 01/10/2009 BACT / ALERT PF Culture Bottles BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904759 01/10/2009 BACT / ALERT FA Culture Bottles BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904760 01/10/2009 MB/BACT Antibiotic Supplement BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904761 01/10/2009 BACT / ALERT MB BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904762 01/10/2009 BACT / ALERT MP BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302904763 01/10/2009 BACT / ALERT FN Culture Bottles BIOMERIEUX Inc., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101904768 01/10/2009 BPC Biosed Creatinine BPC BIOSED s.r.l., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101904769 01/10/2009 BPC Biosed HDL Cholesterol BPC BIOSED s.r.l., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20207904772 01/10/2009 TURBOX Antithrombin III ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
10303904773 01/10/2009 ROTA / Adenoscreen Dipstick MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20101904545 25/09/2009<br />
AKL<br />
10101904546 31/12/2009<br />
AKL<br />
20305904549 25/09/2009<br />
AKL<br />
10305904550 25/09/2009<br />
AKL<br />
10101904551 25/09/2009<br />
BIONIME Blood Glucose Monitoring<br />
System BIONIME CORPORATION., TAIWAN PT. SEKAR GUNA MEDIKA<br />
ROCHE NH3L COBAS C 111<br />
Ammonia ROCHE DIAGNOSTICS GmbH, GERMANY PT. ROCHE INDONESIA<br />
ORION Diagnostica CRP Control<br />
High Level, Low Level ORION DIAGNOSTICA OY, FINLAND PT. PYRIDAM FARMA<br />
ORION Diagnostica Apolipoprtein<br />
Control ORION DIAGNOSTICA OY, FINLAND PT. PYRIDAM FARMA<br />
ORION Diagnostica Urine Albumine<br />
Control ORION DIAGNOSTICA OY, FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
10101904552 25/09/2009 ORION Diagnostica Protein Control ORION DIAGNOSTICA OY, FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305904553 25/09/2009 ORION Diagnostica RF Control ORION DIAGNOSTICA OY, FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20207904554 25/09/2009<br />
SIEMENS Actin Activated<br />
Cephalosplatin Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
AKL<br />
20206505135 30/09/2009 COLO SCREEN LAB PACK HELENA LABORATORIES., USA<br />
PT. SYSMEX INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20305802061 30/09/2009 VIDAS HBsAg Ultra BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102904718 01/10/2009<br />
AKL<br />
20103904726 01/10/2009<br />
AKL<br />
20103904727 01/10/2009<br />
AKL<br />
20305904728 01/10/2009<br />
AKL<br />
20103904729 01/10/2009<br />
AKL<br />
20305904730 01/10/2009<br />
BERTHOLD Multi Crystal Gama<br />
Counter<br />
MONO Marijuana (THC) One Step<br />
Rapid Test Strip<br />
MONO Amphetamine One Step<br />
Rapid Test Strip<br />
MONO HBsAg One Step Rapid Test<br />
Strip<br />
MONO Cocaine One Step Rapid<br />
Test Strip<br />
MONO HCV One Step Rapid Test<br />
Strip<br />
AKL<br />
20101904816 01/10/2009 BRAHMS MR - proADM K - CAL<br />
AKL<br />
10101904817 01/10/2009 BRAHMS MR - proANP K - QC<br />
AKL<br />
20101904818 01/10/2009 BRAHMS MR - proADM K - 050<br />
AKL<br />
20101904819 01/10/2009 BRAHMS MR - proANP K - 050<br />
AKL<br />
20101904820 01/10/2009 BRAHMS MR - proANP K - CAL<br />
AKL<br />
10101904821 01/10/2009 BRAHMS MR - proADM K - QC<br />
BERTHOLD TECHNOLOGIES GmbH & CO.,<br />
KG, GERMANY<br />
SHANGHAI HUAGUAN BIOCHIP CO. Ltd.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. Ltd.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. Ltd.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. Ltd.,<br />
CHINA<br />
SHANGHAI HUAGUAN BIOCHIP CO. Ltd.,<br />
CHINA<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
BRAHMS AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305904814 01/10/2009 DIALAB Complement C3 DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101904810 01/10/2009 DIALAB Protein Calibrator DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101904811 01/10/2009 DIALAB Protein Control DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305904812 01/10/2009 DIALAB Koppa Light Chain DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305904813 01/10/2009 DIALAB Complement C4 DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305904815 01/10/2009 DIALAB Lambda Light Chain DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303904776 01/10/2009<br />
VITROS Immunodiagnostic Products<br />
Rubella IgG Calibrators<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTI<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
10304904777 01/10/2009<br />
VITROS Immunodiagnostic Products<br />
Metering Test Reagent<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTI<br />
PT. TAWADA HEALTHCARE<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
30305904778 01/10/2009<br />
VITROS Immunodiagnostic Products<br />
Anti-HIV 1+2 Calibrators<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTI<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
30305904779 01/10/2009<br />
VITROS Immunodiagnostic Products<br />
Rubella IgG Reagent Pack<br />
AKL<br />
20208904780 01/10/2009 SIEMENS V.E.Q COAG A, B<br />
AKL<br />
20207904781 01/10/2009 SIEMENS Kaolin Suspension<br />
AKL<br />
20207904782 01/10/2009<br />
AKL<br />
20206904783 01/10/2009<br />
AKL<br />
20207904784 01/10/2009<br />
SIEMENS Factor VIII Chromogenic<br />
Assay<br />
SIEMENS D-Cluster Platelet<br />
Aggregation Reagent<br />
SIEMENS Dade Dimertest Latex<br />
Assay<br />
AKL<br />
20205904785 01/10/2009 SYSMEX CA System Buffer<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTI<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
PT. TAWADA HEALTHCARE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20303904786 01/10/2009<br />
BIORAD Antiserum Salmonella<br />
Omnivalent Omni - O (A-60)<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
20303904787 01/10/2009<br />
BIORAD Antiserum Salmonella<br />
Monovalent H : i<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
20303904788 01/10/2009 PLATELIA HSV (1+2) IgG<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
10302904789 01/10/2009 FUNGITEST<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
10302904790 01/10/2009 AUXACOLOR 2<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
10303904791 01/10/2009<br />
Pathfinder Chlamydia Trachomatis<br />
Direct Spicemen<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
AKL<br />
21106904792 01/10/2009 OVOIL VITROLIFE AB., SWEDEN<br />
AKL<br />
21106904793 01/10/2009 EMBRYOGLUE VITROLIFE AB., SWEDEN<br />
AKL<br />
21106904794 01/10/2009 G - Rinse VITROLIFE AB., SWEDEN<br />
AKL<br />
21106904795 01/10/2009 ASP VITROLIFE AB., SWEDEN<br />
AKL<br />
21106904796 01/10/2009 HYASE VITROLIFE AB., SWEDEN<br />
PT. DOS NI ROHA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20301904943 05/10/2009 BIO - DISCS Cefinase BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20205904947 05/10/2009 VIDAS D- Dimer Exclusion (DD2) BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904949 05/10/2009 BIODISCS Mecillinam MEC 10 BIOMERIEUX SA., France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301904950 05/10/2009 BIODISCS Nitroxoline Ni 20 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802102 02/10/2009 API 20 A BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20207905079 09/10/2009 DADE Innovin<br />
AKL<br />
20305905082 09/10/2009 BERICHROM C1 Inhibitor<br />
AKL<br />
20205905083 09/10/2009 BERICHROM a2-Antiplasmin<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
http://www.pdf4free.com<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA
AKL<br />
20207905084 09/10/2009 SIEMENS Multifibren U<br />
AKL<br />
20207905085 09/10/2009 THROMBOREL S<br />
AKL<br />
20208905086 09/10/2009 SIEMENS Standard Human Plasma<br />
AKL<br />
20208905087 09/10/2009 SIEMENS Control Plasma N<br />
AKL<br />
20207905088 09/10/2009 SIEMENS vWF Ag<br />
AKL<br />
20208905089 09/10/2009 SIEMENS Control Plasma P<br />
AKL<br />
20103905332 14/10/2009 EMIT II Plus Benzodiazepine Assay<br />
AKL<br />
20103905333 14/10/2009 EMIT II Plus Opiate Assay<br />
AKL<br />
20207905334 14/10/2009 Turbiquant Antithrombin III<br />
AKL<br />
20207905335 14/10/2009 Turbiquant D - Dimer<br />
AKL<br />
20101905336 14/10/2009<br />
SIEMENS Multyply Integrated<br />
Multisensor<br />
AKL<br />
20305905337 14/10/2009 Turbiquant C3c<br />
AKL<br />
20305905338 14/10/2009 Turbiquant CRP / PCR<br />
AKL<br />
10101905339 14/10/2009<br />
AKL<br />
20305905340 14/10/2009<br />
SIEMENS N / T Protein Control SL /<br />
M<br />
SIEMENS N Antiserum to Human a1-<br />
Antitrypsin<br />
AKL<br />
10204905341 14/10/2009 DIMENSION Chemistry Wash<br />
AKL<br />
20101905342 14/10/2009<br />
AKL<br />
20101905343 14/10/2009<br />
AKL<br />
20206905344 14/10/2009<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
ONCOPROBE Urine Strip 1<br />
(Glucose) CDI / BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
ONCOPROBE Urine Strip 3 (PRO,<br />
PH, GLU) CDI / BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
ONCOPROBE FOBT (Fetal occult<br />
Blood + Transferrin) Rapid Test CDI / BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20305905345 14/10/2009 DIALAB Haptoglobin DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10204905311 13/10/2009 SIEMENS Conditioner<br />
AKL<br />
20205905312 13/10/2009<br />
RAPID POINT 400 Series Analyzer<br />
Type 405<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305905313 13/10/2009 IMTEC - RF Screen HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305905314 13/10/2009<br />
AKL<br />
20305905315 13/10/2009<br />
AKL<br />
20305905316 13/10/2009<br />
AKL<br />
20305905317 13/10/2009<br />
AKL<br />
20305905318 13/10/2009<br />
INTEC Advanced Quality One Step<br />
HBcAb Test Strip<br />
INTEC Advanced Quality One Step<br />
HBeAb Test Card<br />
INTEC Advanced Quality One Step<br />
HBeAg Test Card<br />
INTEC Advanced Quality One Step<br />
HBeAg Test Strip<br />
INTEC Advanced Quality One Step<br />
HBcAb Test Card<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20101905319 13/10/2009 ONCOPROBE Urine Strip 10 CDI / BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20303905040 08/10/2009 PAN - R Malaria Cassette<br />
AKL<br />
20103905045 08/10/2009 DIMENSION AMPH<br />
AKL<br />
20103905046 08/10/2009 DIMENSION BARB (Barbiturate)<br />
AKL<br />
20101905047 08/10/2009 DIMENSION TSH<br />
PDF Creator - PDF4Free v3.0<br />
PANBIO PTY, LTD, AUSTRALIA untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
http://www.pdf4free.com<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
20101905048 08/10/2009 DIMENSION TU Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301905049 08/10/2009 MUELLER Hinton Blood BIORAD LABORATORIES, SINGAPORE PT. DOS NI ROHA<br />
AKL<br />
20303905050 08/10/2009<br />
BIORAD Antiserum Salmonella<br />
Monovalent H, d BIORAD LABORATORIES, SINGAPORE PT. DOS NI ROHA<br />
AKL<br />
20101905051 08/10/2009 DIMENSION MMB<br />
AKL<br />
20101905052 08/10/2009 DIMENSION FT4 (Free Thyroxine)<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20303905053 08/10/2009<br />
BIORAD Antiserum Salmonella<br />
Monovalent H : a<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
10302905054 08/10/2009 Candi Select 4<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE., LTD., SINGAPO<br />
PT. DOS NI ROHA<br />
AKL<br />
10303905055 08/10/2009 PASTOREX Strep A, B, C, D, F, G BIORAD LABORATORIES, SINGAPORE PT. DOS NI ROHA<br />
AKL<br />
10303905056 08/10/2009 PLATELIA Chlamydia IgG TMB BIORAD LABORATORIES, SINGAPORE PT. DOS NI ROHA<br />
AKL<br />
20101905057 08/10/2009<br />
AKL<br />
20101905058 08/10/2009<br />
Stat Profile pHOx Plus L Calibrator<br />
Cartridge C<br />
Stat Profile pHOx Basic Calibrator<br />
Cartridge<br />
NOVA BIOMEDICAL CORPORATION., USA<br />
NOVA BIOMEDICAL CORPORATION., USA<br />
AKL<br />
20101905059 08/10/2009 Stat Profile Ultra D Calibrator Pack NOVA BIOMEDICAL CORPORATION., USA<br />
AKL<br />
20101905060 08/10/2009<br />
AKL<br />
20101905061 08/10/2009<br />
AKL<br />
20101905062 08/10/2009<br />
AKL<br />
20101905063 08/10/2009<br />
AKL<br />
20103905064 08/10/2009<br />
AKL<br />
20303905065 08/10/2009<br />
Stat Profile pHOx Plus C Calibrator<br />
Cartridge C<br />
Stat Profile pHOx Plus Calibrator<br />
Cartridge C<br />
Stat Profile pHOx Plus L Calibrator<br />
Cartridge A<br />
Stat Profile pHOx Plus L Calibrator<br />
Cartridge B<br />
MONO Multi-Drug 6<br />
(AMP,THC,MOP,MET,COC,B20)<br />
Rapid Test.<br />
MONO Dengue One Step Rapid<br />
Test<br />
NOVA BIOMEDICAL CORPORATION., USA<br />
NOVA BIOMEDICAL CORPORATION., USA<br />
NOVA BIOMEDICAL CORPORATION., USA<br />
NOVA BIOMEDICAL CORPORATION., USA<br />
SHANGHAI HUAGUAN Biochip Co, Ltd,<br />
CHINA<br />
SHANGHAI HUAGUAN Biochip Co, Ltd,<br />
CHINA<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. BINTANG MONO INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
AKL<br />
20207905066 08/10/2009<br />
SIEMENS Coagulation Factor XII<br />
Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte, Ltd<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905067 08/10/2009<br />
AKL<br />
20103904975 08/10/2009<br />
SIEMENS Coagulation Factor XI<br />
Deficient Plasma<br />
FOKUS Metamphetamine Drug<br />
Cassette<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte, Ltd<br />
PULSE SCIENTIFIC Inc. CANADA<br />
PT. SYSMEX INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
20306904979 08/10/2009 ABBOTT CEA Master Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20306904980 08/10/2009 ABBOTT CEA Control ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20306904981 08/10/2009 ABBOTT CEA Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101904982 08/10/2009<br />
AXSYM Progesteron Specimen<br />
Diluent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101904983 08/10/2009 AXSYM Progesteron Control ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101904984 08/10/2009 AXSYM Progesteron Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101904986 08/10/2009<br />
AKL<br />
20101904990 08/10/2009<br />
AXSYM Progesteron Standard<br />
Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
DIMENSION CTNI (Cardiac<br />
Troponin-I)<br />
AKL<br />
20103904991 08/10/2009 DIMENSION Drug Calibrator<br />
AKL<br />
20305904992 08/10/2009 DIMENSION MYO (Myoglobin)<br />
AKL<br />
20103904993 08/10/2009 DIMENSION OPI (Opiates)<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
10302605015 22/10/2009<br />
DAVINCI Automated Immunoassay<br />
Analyzer + Accessories BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20208905001 08/10/2009<br />
DADE Ci - Trol Coagulation Control<br />
Level 3<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte, Ltd<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20103905218 12/10/2009<br />
ACON COT One Step Cotinine Test<br />
Device (Urine)<br />
ACON BIOTECH (HANZHOU) CO., LTD.,<br />
CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20208905002 08/10/2009<br />
DADE Ci- Trol Coagulation Control<br />
Level 2<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905003 08/10/2009 DADE Hepzyme<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20202905004 08/10/2009 SIEMENS Proc Control Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20208905005 08/10/2009<br />
DADE Ci- Trol Coagulation Control<br />
Level 1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905006 08/10/2009<br />
SIEMENS Coagulation Factor V<br />
Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905007 08/10/2009 BERICHROM Heparin<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10204905008 08/10/2009<br />
SIEMENS Washing Solution for<br />
Coagulation Analyzers<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905009 08/10/2009 SIEMENS Proc Global<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905010 08/10/2009 BERICHROM Plasminogen<br />
AKL<br />
20103905023 08/10/2009<br />
SIEMENS EMIT Calibrator / Control<br />
Level 1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, atas<br />
penunjukan SYSMEX ASIA PASIFIC Pte., Lt<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SYSMEX INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10102905215 12/10/2009 RADIM Slim SEAC RADIM., ITALY PT. ES TU INDONESIA<br />
AKL<br />
20101905216 12/10/2009 RADIM Profiler SEAC RADIM., ITALY PT. ES TU INDONESIA<br />
AKL<br />
10302905217 12/10/2009 RADIM Brio SEAC RADIM., ITALY PT. ES TU INDONESIA<br />
AKL<br />
10102904969 08/10/2009<br />
AKL<br />
20101900356 26/08/2009 THYROLISA TSH<br />
AKL<br />
20303900363 26/08/2009 HEPALISA Anti HAV<br />
AKL<br />
20101905416 15/10/2009 GEA Strip Uji Kehamilan Pribadi<br />
HOSPITEX Semi Automatic<br />
Nephelometer HOSPITEX DIAGNOSTICS S.R.L., ITALY PT. GRAHA ISMAYA LTD<br />
BIOCHECK INC., U.S.A., dikemas ulang oleh<br />
PT. INDEC DIAGNOSTICS<br />
GENERAL BIOLOGICAL CORPORATION,<br />
R.O.C., dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS<br />
NEWSCEN COAST BIO-PHARMACEUTICAL<br />
CO, LTD., PR CHINA<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. MEGA PRATAMA<br />
MEDICALINDO<br />
AKL<br />
20101905036 08/10/2009 Elitech Bilirubin Total & Direct 4+1 SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905037 08/10/2009 ELITECH Bilirubin Direct 4+1 SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905038 08/10/2009 Elitech Bilirubin Total 4 + 1 SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101905039 08/10/2009 Elitech Iron TIBC SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905020 08/10/2009 ELITECH CK-MB SL SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101905021 08/10/2009 Elitech Bilirubin Total 4 + 1 SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905022 08/10/2009 ELITECH Amylase SL SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905019 08/10/2009 ELITECH Albumin SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905018 08/10/2009 ELITECH Bilirubin Direct SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20208905017 08/10/2009 ELITECH HbA1c Calibrator Set SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20208905016 08/10/2009 ELITECH HbA1c Control L+H SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20207905015 08/10/2009 ELITECH HbA1c SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101905014 08/10/2009<br />
AKL<br />
20101905013 08/10/2009<br />
ELITECH Cholesterol HDL 2G<br />
Calibrator SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
ELITECH Cholesterol LDL 2G<br />
Calibrator SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101905012 08/10/2009 ELITECH Cholesterol LDL SL 2G SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101905011 08/10/2009 ELITECH Cholesterol HDL 2G SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20103905024 08/10/2009<br />
AKL<br />
20103905025 08/10/2009<br />
AKL<br />
20103905026 08/10/2009<br />
AKL<br />
20101905027 08/10/2009<br />
SIEMENS EMIT Calibrator / Control<br />
Level 5<br />
SIEMENS EMIT Calibrator / Control<br />
Level 2<br />
SIEMENS EMIT Calibrator / Control<br />
Level 3<br />
SIEMENS EMIT Calibrator / Control<br />
Level 0<br />
AKL<br />
20305905028 08/10/2009 SIEMENS Nor Partigen IgG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERAMNY<br />
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
20305905029 08/10/2009 SIEMENS ENZYGNOST HBsAg 5.0 PRODUCTS GmbH., GERAMNY<br />
AKL<br />
20305905030 08/10/2009 SIEMENS N Latex IgE Mono<br />
AKL<br />
20103905031 08/10/2009<br />
AKL<br />
20207904971 08/10/2009<br />
SIEMENS EMIT Plus II Cocaine<br />
Metabolite Assay<br />
PRIMUS PDQ (Glycated<br />
Hemoglobin Analyzer)<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERAMNY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PRIMUS CORPORATION., USA melalui<br />
ACUTEST SYSTEM (M) SDN., BHD.,<br />
MALAYSIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. WACANA INDO MITRA<br />
AKL<br />
20301905353 14/10/2009 OXOID Nalidixic Acid NA 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305905495 19/10/2009<br />
INTEC Advanced Quality One Step<br />
HBeAb Test Strip<br />
AKL<br />
10204905496 19/10/2009 SIEMENS Deproteinizer<br />
AKL<br />
20103805497 19/10/2009 IMMULITE Digitoxin<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. MITRASAMAYA SEJATI<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20209905474 16/10/2009 ELDONCARD 2511 ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
20209905468 16/10/2009 ELDONCARD 2521 + 2522 ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
20209905469 16/10/2009 ELDONCARD Rh D ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL 2020990<br />
5471 16/10/2009 ELDONCARD 2551 ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
20209905472 16/10/2009 ELDON ID CARD ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
20209905473 16/10/2009 ELDONCARD 2532 + 2531 ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
20303905500 19/10/2009<br />
AKL<br />
20301905354 14/10/2009<br />
SD BIOLINE Salmonella Thypi<br />
IgG/IgM STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
OXOID Triethoprim<br />
Sulphamethoxazole SXT 25 OXOID Limited, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10204905041 08/10/2009 SYSMEX Urinopack SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20303902710 08/10/2009 FOKUS ASO Latex Check Test PULSE SCIENTIFIC INC., CANADA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
20301905476 16/10/2009 BIODISCS Clindamycin - 2 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905477 16/10/2009 BIODISCS Ampicillin - 10 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301905479 16/10/2009 BIODISCS Cefixim - 10 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905480 16/10/2009 BIODISCS Carbenicillin - 100 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905481 16/10/2009 BIODISCS Imipenem - 2 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905482 16/10/2009 BIODISCS Rifampicin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905483 16/10/2009 BIODISCS Nitrofurantoin - 300 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905484 16/10/2009 BIODISCS Neomycin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905485 16/10/2009 BIODISCS Nalidixin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303905486 16/10/2009 RPR - Nosticon II BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305905487 16/10/2009 HEPANOSTIKA HCV Ultra<br />
AKL<br />
10201403627 14/10/2009<br />
BEIJING UNITED BIOMEDICAL CO. LTD.,<br />
P.R. CHINA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
MERCK Wright's Eosin Methylene<br />
Blue MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10206400260 14/10/2009 MERCK Hayem's Reagent MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20305904967 08/10/2009 QUANTIA ASP-RF Control I<br />
AKL<br />
20305905490 19/10/2009<br />
BIOKIT S.A., SPAIN for ABBOT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
HEPANOSTIKA HbsAg UNI-FORM<br />
II BIOMERIEUX BV., NETHERLANDS PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905491 19/10/2009 BIODISCS Chloramphenicol - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905492 19/10/2009 BIODISCS Cephalothin - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905493 19/10/2009 BIODISCS Ceftazidim - 30 ug BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301905494 19/10/2009 BIODISCS Erythromycin BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10206904988 08/10/2009 MERCK Turk's Solution MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302904996 08/10/2009 OXOID Campylobacter agar Base OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905000 08/10/2009 OXOID Blood Agar Base No. 2 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905033 08/10/2009 OXOID Cycloheximide OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905034 08/10/2009 OXOID Novobiocin Supplement OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905035 08/10/2009 OXOID Laked Horse Blood OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21501505009 12/10/2009 Gene Flash SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103502711 14/10/2009 FOKUS Cocain Check Test Device PULSE SCIENTIFIC INC., CANADA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
20301905176 12/10/2009 ATB Enteroc 5 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
11501905174 12/10/2009 SYPNOTICS G Box HR SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102905173 12/10/2009<br />
SYNOPTICS Gel Documentation<br />
and Analysis SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
11501905171 12/10/2009 SYNOPTICS G Box Chemi HR 16 SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
11501905172 12/10/2009 SYNOPTICS G Box XT SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
11501905169 12/10/2009 SYNOPTICS G Box Chemi XT 16 SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
11501905170 12/10/2009 SYNOPTICS G Box Chemi HR SYNOPTICS LTD., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802100 12/10/2009 API 20 E Reagent BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802099 12/10/2009 API 50 CH BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802098 12/10/2009 API 50 CHB / E Medium BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802097 12/10/2009 API 50 CHL Medium BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302802096 12/10/2009 API 20 NE BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA`<br />
AKL<br />
10302801846 12/10/2009 RAPID ID23 Strep BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204802095 12/10/2009 BIOMERIEUX Mineral Oil BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20306801979 15/10/2009 VIDAS FPSA BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303801839 15/10/2009 VIDAS Toxo IgM TXM BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801978 15/10/2009 VIDAS Estradiol II E211 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303801599 15/10/2009 VIDAS CA 19-9 / 199 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801980 15/10/2009 VIDAS Prolactin BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101801981 15/10/2009 VIDAS FSH BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802111 15/10/2009 ID 32 E BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802112 15/10/2009 ID Color Catalase BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305904962 08/10/2009<br />
AKL<br />
20305904960 08/10/2009<br />
AKL<br />
20103904955 08/10/2009<br />
AKL<br />
10303904956 08/10/2009<br />
AKL<br />
20303904958 08/10/2009<br />
AKL<br />
10303904959 08/10/2009<br />
AKL<br />
20103904961 08/10/2009<br />
AKL<br />
20303904963 08/10/2009<br />
INST. ANSWER HBsAB One Step<br />
Test Strip<br />
INST. ANSWER HBsAB One Step<br />
Test Device<br />
INST. ANSWER COC COCAINE<br />
Test Strip.<br />
INST. ANSWER TB Tuberculosis<br />
One Step Strip<br />
INST. ANSWER Dengue IgG // IgM<br />
Test Device<br />
INST. ANSWER TB Tuberculosis<br />
One Step Device<br />
INST. ANSWER COC COCAINE<br />
Test Device<br />
INST. ANSWER SYPHILIS ULTRA<br />
One Step Device<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
PT. TIRTA MEDILAB<br />
AKL<br />
20301504922 16/10/2009 OXOID Cefotiam CTM 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504920 16/10/2009 OXOID Cefpirome OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504921 16/10/2009 OXOID Meropenem MEM 10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504909 16/10/2009 OXOID Linezolid OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301504927 16/10/2009 OXOID Methicillin Met 5 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101905603 20/10/2009<br />
AKL<br />
10101905604 20/10/2009<br />
AKL<br />
20305905605 20/10/2009<br />
AKL<br />
10303905606 20/10/2009<br />
ABL 80 FLEX CO - OX and<br />
Accessories<br />
INTEC Advanced Quality One Step<br />
Pregnancy Midstream Test<br />
INTEC Advanced Quality One Step<br />
Myoglobin/CK-MB/Troponin I Test<br />
Card<br />
INTEC Advanced Quality Rapid Anti-<br />
H Pylori Test Card<br />
RADIOMETER MEDICAL ApS., DENMARK<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20306905607 20/10/2009<br />
AKL<br />
20305905608 20/10/2009<br />
INTEC Advanced Quality One Step a-<br />
Fetoprotein (AFP) Test Card<br />
INTEC Advanced Quality One Step<br />
Myoglobin/CK-MB/Troponin I Test<br />
Strip<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
10101905609 20/10/2009<br />
INTEC Advanced Quality One Step<br />
Luteinizing Hormone (LH) Test Strip<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20306905610 20/10/2009<br />
INTEC Advanced Quality One Step a-<br />
Fetoprotein (AFP) Test Strip<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
10101905611 20/10/2009<br />
PDF Creator - PDF4Free v3.0<br />
INTEC Advanced Quality One Step<br />
Luteinizing Hormone (LH) Test Card<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
http://www.pdf4free.com<br />
PT. MITRASAMAYA SEJATI
AKL<br />
20209905470 16/10/2009 DOCTOR'S KIT DKS Rh D ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
20209905471 16/10/2009 ELDONCARD 2551 ELDON BIOLOGICAL A/S, DENMARK PT. ZOE PELITA NUSANTARA<br />
AKL<br />
10303905612 20/10/2009<br />
INTEC Advanced Quality Rapid Anti-<br />
H Pylori Test Strip<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20301905613 20/10/2009 OXOID Streptomycin S 10 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905614 20/10/2009 OXOID Neomycin N 10 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905615 20/10/2009 OXOID MUG Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905623 20/10/2009 OXOID Amikacin AK 30 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905624 20/10/2009 MERCKOPLATE Rambach Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302905625 20/10/2009<br />
AKL<br />
20305905626 20/10/2009<br />
AKL<br />
20303905627 20/10/2009<br />
AKL<br />
20207905628 20/10/2009<br />
MERCK Tb - Color Modified Staining<br />
Kit MERCK KGaA., GERMANY PT. MERCK Tbk<br />
INTEC Advanced Quality One Step<br />
Multi - HBV Test<br />
INTEC Advanced Treponema<br />
Pallidum (TP) ELISA Test<br />
SIEMENS Berichrom Antithrombin III<br />
(A)<br />
AKL<br />
20207905629 20/10/2009 SIEMENS Innovance D-Dimer<br />
AKL<br />
20207905630 20/10/2009 SIEMENS Protein C Reagent<br />
AKL<br />
20207905631 20/10/2009 SIEMENS D-Dimer Plus<br />
AKL<br />
20208905632 20/10/2009<br />
AKL<br />
20207905633 20/10/2009<br />
AKL<br />
20207905634 20/10/2009<br />
SIEMENS Innovance D-Dimer<br />
Controls (1 & 2)<br />
SIEMENS Dade Actin FSL Activated<br />
PTT Reagent<br />
SIEMENS Dade Actin FS Activated<br />
PTT Reagent<br />
AKL<br />
20207905635 20/10/2009 SIEMENS Berichrom PAI Test Kit<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20101801854 22/10/2009 BIOMERIEUX BOLI - T/D BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101905666 20/10/2009 CORMAY HDL PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905667 20/10/2009<br />
AKL<br />
10101905668 20/10/2009<br />
LIQUICK COR-Total Protein<br />
Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
LIQUICK COR-UA Uric Acid<br />
Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905669 20/10/2009 LIQUICK COR-BIL Direct PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905670 20/10/2009 LIQUICK COR-BIL Total PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905671 20/10/2009<br />
LIQUICK COR-CK Creatinine Test<br />
System PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905672 20/10/2009 CRMAY HDL Direct PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905673 20/10/2009<br />
AKL<br />
10101905674 20/10/2009<br />
AKL<br />
10101905676 20/10/2009<br />
LIQUICK COR-ASAT Aspartate<br />
Amino Transferase Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
LIQUICK COR-ALAT Alanine Amino<br />
Transferase Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
LIQUICK COR-CHOL Cholesterol<br />
Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905677 20/10/2009 LIQUICK COR-Creatinine Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905678 20/10/2009 LIQUICK COR-Glucose Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905679 20/10/2009 LIQUICK COR-Albumin PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905680 20/10/2009 LIQUICK COR-CK-MB PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20102905681 20/10/2009 SPHERA EDIF INSTRUMENTS S.r.l., ITALY<br />
AKL<br />
20205905685 20/10/2009 SINNOWA Coagulometer CL-2000<br />
SINNOWA MEDICAL SCIENCE &<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. BAVARIA COMBININDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20102905686 20/10/2009<br />
SINNOWA Fully Automatic<br />
Biochemistry Analyzer D-320<br />
AKL<br />
10304905687 20/10/2009 SINNOWA ELISA Reader ER-500<br />
AKL<br />
10101801852 22/10/2009<br />
AKL<br />
10101801853 22/10/2009<br />
AKL<br />
10101801851 22/10/2009<br />
SINNOWA MEDICAL SCIENCE &<br />
TECHNOLOGY CO. LTD., CHINA<br />
SINNOWA MEDICAL SCIENCE &<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
BIOMERIEUX Acide Urique<br />
Enzymatique PAP 150 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
ENZYLINE ALAT / GPT 20<br />
Monoreactif BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
BIOMERIEUX Triglycerid<br />
Enzymatique PAP 150 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101801849 22/10/2009 BIOMERIEUX Glucose RTU BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101801850 22/10/2009 BIOMERIEUX Proteines Kit (PROT) BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101600751 22/10/2009 ENZYLINE PAL Standardise BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302802113 22/10/2009 ID 32 Staph BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303904994 08/10/2009 RAPID Staph Plus System REMEL ONC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20306904985 08/10/2009 AXSYM CEA Reagent Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101905651 20/10/2009<br />
INNOFAST Hcg Rapid Test<br />
Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
10101905650 20/10/2009 INNOFAST LH Rapid Test Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
20103905636 20/10/2009<br />
AKL<br />
20103905637 20/10/2009<br />
AKL<br />
20103905638 20/10/2009<br />
AKL<br />
20103905639 20/10/2009<br />
AKL<br />
20103905640 20/10/2009<br />
AKL<br />
20206905641 20/10/2009<br />
AKL<br />
20103905642 20/10/2009<br />
INNOFAST Cocaine Rapid Test<br />
Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST Cocaine Rapid Test<br />
Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST MDMA Rapid Test<br />
cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST Amphetamine Rapid<br />
Test Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST Morphine Rapid Test<br />
Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST FOB Rapid Test<br />
Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST Amphetamine Rapid<br />
Test Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
20103905643 20/10/2009 INNOFAST MDMA Rapid Test Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
20103905644 20/10/2009<br />
AKL<br />
20103905645 20/10/2009<br />
AKL<br />
20103905646 20/10/2009<br />
INNOFAST Marijuana (THC) Rapid<br />
Test Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST Marijuana (THC) Rapid<br />
Test Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
INNOFAST Morphine Rapid Test<br />
Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
10101905647 20/10/2009 INNOFAST FSH Rapid Test Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
10101905648 20/10/2009<br />
INNOFAST FSH Rapid Test<br />
Cassette IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
10101905652 20/10/2009 INNOFAST LH Rapid Test Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
20101905649 20/10/2009 INNOFAST hCG Rapid Test Strip IND DIAGNOSTIC Inc., CANADA PT. COMBIPHAR<br />
AKL<br />
10101905682 20/10/2009 BIOSINO Uric Acid (UA) Assay Kit<br />
AKL<br />
20101905683 20/10/2009 BIOSINO AST / GOT Kit<br />
AKL<br />
20101905684 20/10/2009 BIOSINO Glucose (GLU) Assay Kit<br />
AKL<br />
20101905688 20/10/2009 BIOSINO Urea (BUN) Assay Kit<br />
AKL<br />
10101905689 20/10/2009 BIOSINO ALT / GPT Kit<br />
AKL<br />
10101905882 26/10/2009<br />
AKL<br />
10302905808 23/10/2009<br />
ABBOTT Immunoassay - MCC<br />
Liquid Level 1,2,3<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIOSINO BIOTECHNOLOGY & SCIENCE<br />
INC., CHINA<br />
BIORAD LABORATORIES, USA untuk<br />
ABBOTT LABORATORIES, USA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. LESTARI MANDIRI SENTOSA<br />
PT. ABBOTT INDONESIA<br />
OXOID Sorbitol Macconkey Agar No.<br />
3 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101905866 26/10/2009<br />
PDF Creator - PDF4Free v3.0<br />
DIMENSION Xpand Clinical<br />
Chemistry System and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA
AKL<br />
20207905867 26/10/2009<br />
Dade Fibrinogen Determination<br />
Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10302905869 26/10/2009 REMEL Coagulase Plasma REMEL INC., PUSPA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101905870 26/10/2009 FOKUS 1P Urine Reagent Strip PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20103905871 26/10/2009<br />
MONO Morphine One Step Rapid<br />
Test Strip<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. BINTANG MONO INDONESIA<br />
AKL<br />
20101905872 26/10/2009 BPC Direct Bilirubin BPC BIOSED s.r.l., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20101905873 26/10/2009 BPC Total Bilirubin BPC BIOSED s.r.l., ITALY PT. INTIMEDIKA PUSPA INDAH<br />
AKL<br />
20305905874 26/10/2009<br />
AKL<br />
20101905875 26/10/2009<br />
AKL<br />
10101905876 26/10/2009<br />
AKL<br />
20101905877 26/10/2009<br />
VITROS Immunodiagnostic Product<br />
HBsAg ES Reagent Pack<br />
VITROS Chemistry Products<br />
250/350 Reference Fluid<br />
VITROS Chemistry Products DT<br />
Isoenzymes Control I<br />
VITROS Chemistry Products DT<br />
Isoenzyme Calibrator Kit<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY, UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY, USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY, USA<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY, USA<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
30305905878 26/10/2009<br />
VITROS Immunodiagnostic Products<br />
Anti-HIV 1+2 Controls<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK atas<br />
penunjukan ORTHO CLINICAL DIAGNOSTI<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20305905885 26/10/2009 ARCHITECT Toxo IgM Control ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305905886 26/10/2009 ARCHITECT Anti- HBc II Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305905888 26/10/2009<br />
AKL<br />
20305905889 26/10/2009<br />
AKL<br />
20305905890 26/10/2009<br />
AKL<br />
20303905891 26/10/2009<br />
AKL<br />
20103905892 26/10/2009<br />
AKL<br />
20103905893 26/10/2009<br />
AKL<br />
20103905894 26/10/2009<br />
AKL<br />
20103905895 26/10/2009<br />
AKL<br />
20103905896 26/10/2009<br />
AKL<br />
20103905897 26/10/2009<br />
AKL<br />
20103905898 26/10/2009<br />
AKL<br />
20103905899 26/10/2009<br />
ABBOTT Clinical Chemistry C-<br />
Reactive Protein Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Immunoglobulin G ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry C-<br />
Reactive Protein Reagent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
INTEC Advanced Quality Rapid<br />
Malaria (P.f) Test Strip<br />
INTEC One Step Ketamine Test<br />
Strip<br />
INTEC One Step Ketamine Test<br />
Card<br />
INTEC One Step Ecstasy (MDMA)<br />
Test Strip<br />
INTEC One Step Ecstasy (MDMA)<br />
Test Card<br />
INTEC One Step Phencyclidine Test<br />
Strip<br />
INTEC One Step Phencyclidine Test<br />
Card<br />
INTEC One Step Methadone Test<br />
Strip<br />
INTEC One Step Methadone Test<br />
Card<br />
AKL<br />
20207905868 26/10/2009 THROMBOREL S<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
INTEC PRODUCTS, INC. (XIAMEN), P.R.<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD.<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10303905816 23/10/2009<br />
PATHFINDER Chlamydia Culture<br />
Confirmation System<br />
BIORAD LABORATORIES LTD, FRANCE,<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
PT. DOS NI ROHA<br />
AKL<br />
20303905817 23/10/2009 PLATELIA HSV (1+2) IgM<br />
BIORAD LABORATORIES LTD, FRANCE,<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE, LTD, SINGAPORE<br />
PT. DOS NI ROHA<br />
AKL<br />
20101905818 23/10/2009 TURBOX Microalbumin ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
10305905819 23/10/2009<br />
TURBOX a1 - Acid Glycoprotein<br />
(Orosomucoid) ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
10305905820 23/10/2009 TURBOX Prealbumin ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303905821 23/10/2009 TURBOX ASO PAIA Control ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305905822 23/10/2009 TURBOX Transferrin ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20207905823 23/10/2009 TURBOX Fibrinogen ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305905824 23/10/2009 TURBOX a1 - Antitrypsin ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305905825 23/10/2009 TURBOX Haptoglobin ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305905826 23/10/2009 TURBOX RF - PAIA ORION DIAGNOSTICA., FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20305905827 23/10/2009<br />
AKL<br />
20101905828 23/10/2009<br />
AKL<br />
20101905829 23/10/2009<br />
AKL<br />
20101905830 23/10/2009<br />
VITROS Immunodiagnostic Product<br />
HBsAg ES Confirmatory Kit<br />
VITROS Immunodiagnostic Product<br />
HBsAg ES Calibrator<br />
VITROS Immunodiagnostic Product<br />
Total T3 Calibrator<br />
VITROS Immunodiagnostic Product<br />
Total T4 Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY, UK<br />
ORTHO-CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY, UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
ORTHO CLINICAL DIAGNOSTICS A<br />
JOHNSON & JOHNSON COMPANY., UK<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
PT. TAWADA HEALTHCARE<br />
AKL<br />
20303905835 23/10/2009 ARCHITECT Toxo IgM Reagent Kit ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20301905836 23/10/2009 REMEL NIACIN Reagent Strip REMEL INC., PUSPA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10208905837 23/10/2009 SYSMEX IP Reagent A SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20205905838 23/10/2009 DADE Owners Veronal Buffer<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD.<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905839 23/10/2009 SIEMENS Protein S Ac<br />
AKL<br />
20207905841 23/10/2009<br />
SIEMENS BC von Willebrand<br />
Reagent<br />
AKL<br />
20206905842 23/10/2009 SIEMENS Pathromtin SL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX SINGAPORE Pte, Ltd., S<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD.<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD.<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10302905810 23/10/2009 OXOID Anaerobe Basal Broth OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905811 23/10/2009 OXOID Gas Generating Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905813 23/10/2009 OXOID Oxytetracycline OT 30 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302905814 23/10/2009 OXOID MRVP Medium OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20102905866 26/10/2009<br />
DIMENSION Xpand Clinical<br />
Chemistry System and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
AKL<br />
PLASMATEC LABORATORY PRODUCTS<br />
10303502755 05/11/2009 PRO Diagnostics ASO Latex Test Kit LTD., UK<br />
AKL<br />
20102905905 30/10/2009<br />
PT. SIEMENS INDONESIA<br />
PT. PILAR REJEKI OPTIMA<br />
ARCHITECT C4000 System &<br />
Accessories ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101905939 02/11/2009 QuickVue One-Step hCG Urine Test<br />
AKL<br />
20101905940 02/11/2009<br />
AKL<br />
20101905941 02/11/2009<br />
AKL<br />
10101905942 02/11/2009<br />
AKL<br />
10102905943 02/11/2009<br />
AKL<br />
20101905944 02/11/2009<br />
QuickVue One-Step hCG Combo<br />
Test<br />
QuickVue+ One -Step hCG Combo<br />
Test<br />
UriMate C010Liquid Human Urine<br />
Controls<br />
STRIPMAX 1210 Urine Chemistry<br />
Analyzer<br />
UriMate 10 Reagent Strips For<br />
Urinalysis<br />
QUIDEL CORPORATION, USA atas<br />
penunjukan BIOMERIEUX SA, FRANCE<br />
QUIDEL CORPORATION, USA atas<br />
penunjukan BIOMERIEUX SA, FRANCE<br />
QUIDEL CORPORATION, USA atas<br />
penunjukan BIOMERIEUX SA, FRANCE<br />
BIOCARE CORPORATION, TAIWAN<br />
BIOCARE CORPORATION, TAIWAN<br />
BIOCARE CORPORATION, TAIWAN<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20207905843 23/10/2009<br />
DADE ACTIN FSL Activated PTT<br />
Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20208905844 23/10/2009 SIEMENS Standard Human Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905845 23/10/2009 DADE Thrombin Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905846 23/10/2009<br />
SIEMENS Coagulation Factor IX<br />
Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20207905847 23/10/2009<br />
DADE ACTIN FS Activated PTT<br />
Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20205905848 23/10/2009 SIEMENS Imidozale Buffer Solution<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20208905849 23/10/2009<br />
AKL<br />
20101905850 23/10/2009<br />
SIEMENS D - Dimer PLUS Standard<br />
Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY, atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE LTD,<br />
PT. SYSMEX INDONESIA<br />
OPTI CCA / OPTI - TS / OPTI R<br />
Calibration Gas OPTI MEDICAL SYSTEMS INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
10101905851 23/10/2009 OPTI R Fluid Pack OPTI MEDICAL SYSTEMS INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
20101905852 23/10/2009<br />
CORE Beta Clear HCG Pregnancy<br />
Test Strip<br />
AKL<br />
20303905853 23/10/2009 DENGUE DX IgG/IgM<br />
AKL<br />
20303905854 23/10/2009 DENGUE DX NS1 Antigen<br />
AKL<br />
20305905784 23/10/2009 SIEMENS Turbiquant IgG<br />
AKL<br />
20305905785 23/10/2009 Turbiquant C4<br />
AKL<br />
10304905786 23/10/2009 Siemens N Diluent for Sample<br />
AKL<br />
10305905787 23/10/2009<br />
DIMENSION PALB - Prealbumin<br />
Flex Reagent Catridge<br />
AKL<br />
20101905788 23/10/2009 DIMENSION MMB Calibrator<br />
AKL<br />
20101905789 23/10/2009 DIMENSION CTNI Calibrator<br />
AKL<br />
10101905790 23/10/2009 EMIT Drug Buffer Concentrate<br />
AKL<br />
20103905791 23/10/2009 EMIT Calibrator Level 0 (Negative)<br />
AKL<br />
10101905792 23/10/2009<br />
DIMENSION LA - Lactic Acid Elex<br />
Reagent Cartridge<br />
AKL<br />
20101905793 23/10/2009 DIMENSION PALB Calibrator<br />
AKL<br />
20101905794 23/10/2009 DIMENSION AMON Calibrator<br />
AKL<br />
20101905795 23/10/2009 DIMENSION T4 Calibrator<br />
AKL<br />
20306905796 23/10/2009 IMMULITE PSA<br />
AKL<br />
20101905797 23/10/2009 IMMULITE Thyroid Uptake<br />
AKL<br />
20101905798 23/10/2009 IMMULITE Cortisol<br />
AKL<br />
20101905799 23/10/2009 IMMULITE 2000 Cortisol<br />
CORE DIAGNOSTIC, UK<br />
STANDARD DIAGNOSTIC, INC., KOREA<br />
atas penunjukan FOCUS DIAGNOSTIC INC.,<br />
USA<br />
STANDARD DIAGNOSTIC, INC., KOREA<br />
atas penunjukan FOCUS DIAGNOSTIC INC.,<br />
USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTIC Inc.,<br />
USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS Ltd., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS Ltd., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS Ltd., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS Ltd., UK<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. NELTA MULTI GRACIA<br />
PT. NELTA MULTI GRACIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303905800 23/10/2009 IMMULITE Rubella Quantitative IgG<br />
AKL<br />
10207905803 23/10/2009<br />
COPACK Haemoglobin Colour Scale<br />
Strip<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS Ltd., UK<br />
COPACK FRITZ HEINEMAN & CO. GmbH.,<br />
GERMANY<br />
AKL<br />
COPACK FRITZ HEINEMAN & CO. GmbH.,<br />
20207905806 23/10/2009 COPACK Haemoglobin Colour Scale GERMANY<br />
AKL<br />
10101905801 23/10/2009<br />
PT. SIEMENS INDONESIA<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ABHIMATA MANUNGGAL<br />
LIQUICK COR-TG Triglycerides<br />
Reagent PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20101905802 23/10/2009 LIQUICK COR-Urea PZ CORMAY S.A., POLAND PT. TRIGUNA MEDIKA<br />
AKL<br />
20102906062 06/11/2009<br />
AKL<br />
30505501964 09/11/2009<br />
NOVA 1 Electrolyte Analyzer and<br />
Accessories<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
HIV Combi Elecsys and Cobas e<br />
analyzer ROCHE DIAGNOSTIC GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
10303301645 09/11/2009 PLASMATEC ASO Latex Test Kit<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCTS, UK<br />
PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20208802072 09/11/2009 ABX Minotrol 16 Twin Pack 2L HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10102802005 11/11/2009 Biolyzer 100 - Chemistry Analyzer<br />
AKL<br />
10101403527 11/11/2009 DSI Lp(a) FS<br />
AKL<br />
10102905347 14/10/2009<br />
MULTISKAN FC With Incubator and<br />
Accessories<br />
AKL<br />
10102905346 14/10/2009 MULTISKAN FC and Accessories<br />
AKL<br />
20303904957 08/10/2009<br />
INST. ANSWER SYPHILIS ULTRA<br />
One Step Strip<br />
ANALYTICON BIOTECHNOLOGIES AG,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GmbH,<br />
GERMANY<br />
THERMO FISHER SCIENTIFIC, FINLAND<br />
atas penunjukan THERMO FISHER<br />
SCIENTIFIC, SINGAPORE<br />
THERMO FISHER SCIENTIFIC, FINLAND<br />
atas penunjukan THERMO FISHER<br />
SCIENTIFIC, SINGAPORE<br />
ACON BIOTECH (HANGZHOU) CO, LTD<br />
P.R., CHINA<br />
PT. BAVARIA COMBININDO<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIPA PUSPA LABSAINS<br />
PT. DIPA PUSPA LABSAINS<br />
PT. TIRTA MEDILAB<br />
AKL<br />
20102905938 02/11/2009<br />
CORELAB Automatic Electrolyte<br />
Analyzer EA 206 S and Accessories<br />
AKL<br />
10102906067 06/11/2009 MINDRAY Microplate Washer<br />
SANHE MEDICAL EQUIPMENT CO, LTD.,<br />
CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO., LTD, CHINA<br />
PT. PERMATA<br />
CAKRAWALABARU<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20301501084 13/11/2009 OXOID Teicoplanin-TEC 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305504542 12/11/2009<br />
AEROSET/c8000 Quantia b2-<br />
Microglobulin<br />
AKL<br />
20305301646 12/11/2009 ANTEC CRP Latex Test Kit<br />
AKL<br />
20305301648 12/11/2009 ANTEC RA Latex Test Kit<br />
AKL<br />
20303301651 12/11/2009 ANTEC VDRL Carbon Antigen<br />
AKL<br />
20303301650 12/11/2009 ANTEC TPHA Test Kit<br />
AKL<br />
20303301649 12/11/2009 ANTEC RPR Test Kit<br />
AKL<br />
10102906068 06/11/2009 MINDRAY Microplate Reader<br />
AKL<br />
20102906069 06/11/2009<br />
AKL<br />
20102906070 06/11/2009<br />
NOVA 8 Electrolyte Analyzer and<br />
Accessories<br />
NOVA 12 Electrolyte Analyzer and<br />
Accessories<br />
BIOKIT S.A., SPAIN for ABBOTT GmbH &<br />
CO. KG., GERMANY<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCT, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCT, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCT, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCT, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK untuk ANTEC DIAGNOSTIC<br />
PRODUCT, UK<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO., LTD, CHINA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
PT. ABBOTT INDONESIA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
AKL<br />
20301906071 06/11/2009 OXOID Levofloxacin M.I.C Evaluator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906072 06/11/2009 OXOID Doripenem DOR 10 ug OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906073 06/11/2009 OXOID Amoxycillin M.I.C Evaluator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906074 06/11/2009 OXOID Mupirocin 20ug MUP20 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10203906075 06/11/2009<br />
WESCOR 7150 Aerospray<br />
Hematology Slide Stainer /<br />
Cytocentrifuge WESCOR INC, USA PT. MRK DIAGNOSTICS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10203906076 06/11/2009<br />
AKL<br />
10203906077 06/11/2009<br />
AKL<br />
10203906078 06/11/2009<br />
AKL<br />
20101906047 06/11/2009<br />
AKL<br />
20101906048 06/11/2009<br />
AKL<br />
20102906049 06/11/2009<br />
WESCOR 7620 Cytopro<br />
Cytocentrifuge WESCOR INC, USA PT. MRK DIAGNOSTICS<br />
WESCOR 7320 Aerospray Gram<br />
Slide Stainer / Cytocentrifuge WESCOR INC, USA PT. MRK DIAGNOSTICS<br />
WESCOR Aerospray Acid Fast<br />
Bacteria WESCOR INC, USA PT. MRK DIAGNOSTICS<br />
Stat Profile pHOX Plus L and<br />
Accessories<br />
Stat Profile pHOX Plus C and<br />
Accessories<br />
NOVA 11 Electrolyte Analyzer and<br />
Accessories<br />
NOVA BIOMEDICAL, USA<br />
NOVA BIOMEDICAL, USA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906050 06/11/2009 NOVA 1 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906051 06/11/2009 NOVA 4 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906052 06/11/2009 NOVA 5 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906053 06/11/2009 NOVA 8 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906054 06/11/2009 NOVA 10 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906055 06/11/2009 NOVA 11 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906056 06/11/2009 NOVA 12 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906057 06/11/2009 NOVA 13 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906058 06/11/2009 NOVA 14 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20101906059 06/11/2009 NOVA 16 Calibrator Pack NOVA BIOMEDICAL CORPORATION, USA<br />
AKL<br />
20102906060 06/11/2009<br />
AKL<br />
20102906061 06/11/2009<br />
AKL<br />
20102906063 06/11/2009<br />
AKL<br />
20102906064 06/11/2009<br />
AKL<br />
20102906065 06/11/2009<br />
AKL<br />
20205906066 06/11/2009<br />
NOVA 10 Electrolyte Analyzer and<br />
Accessories<br />
NOVA 16 Electrolyte Analyzer and<br />
Accessories<br />
NOVA 4 Elctrolyte Analyzer and<br />
Accessories<br />
NOVA 13 Electrolyte Analyzer and<br />
Accessories<br />
NOVA 14 Electrolyte Analyzer and<br />
Accessories<br />
Stat Profile pHOX CO-Oximeter and<br />
Accessories<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
NOVA BIOMEDICAL, USA<br />
AKL<br />
10102905973 03/11/2009 NuAncer 10 2 BIOCARE CORPORATION, TAIWAN<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
AKL<br />
20301905974 03/11/2009 OXOID Cefotaxime M.I.C Evaluator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301905975 03/11/2009 OXOID Linezolid M.I.C Evaluator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20103905976 03/11/2009<br />
ONEMED Drug Abuse Multipanel<br />
Test (Morphine / Amphetamine /<br />
Methamphetamine / Cocaine/THC)<br />
AKL<br />
20103905977 03/11/2009 ONEMED Amphetamine Test<br />
AKL<br />
20103905978 03/11/2009 ONEMED Metamphetamine Test<br />
AKL<br />
20103905979 03/11/2009 ONEMED Morphine Test<br />
AKL<br />
20103905980 03/11/2009 ONEMED Cocaine Test<br />
AKL<br />
20103905981 03/11/2009 ONEMED THC Test<br />
AKL<br />
20103905982 03/11/2009 ONEMED Alcohol Test<br />
AKL<br />
20103905983 03/11/2009 ONEMED Combi 10 Test<br />
AKL<br />
10102906117 20/11/2009<br />
BOECO Micropipettes Adjustable<br />
Volume<br />
AKL<br />
20103906258 16/12/2009 MONO Metamphetamine Strip<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO., LTD., CHINA<br />
BIOHIT OYJ, FINLAND untuk BOECKEL +<br />
Co. KG., GERMANY<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. BAVARIA COMBININDO<br />
PT. BINTANG MONO INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103906259 16/12/2009 MONO Metamphetamine Device<br />
AKL<br />
20305703118 30/07/2009<br />
BIOLINE Dengue IgG/IgM Rapid<br />
Test Device<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) Co,Ltd.,<br />
CHINA<br />
PT. BINTANG MONO INDONESIA<br />
PT. SAPTA LARONA MUDA<br />
AKL<br />
20303906264 16/12/2009 SD BIOLINE HAV IgG/IgM STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10302906267 16/12/2009<br />
C.L.E.D Medium MacConkey Agar<br />
No. 3 Dipslides OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906268 16/12/2009 OXOID Dextrose Tryptone Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101906271 16/12/2009 ABX Pentra Urinary Proteins CP HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
21106906563 23/12/2009 The Stripper Tips-135um MID-ATLANTIC DIAGNOSTICS INC., USA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20207604912 31/07/2009 CYPRESS Haemoglobin Test CYPRESS DIAGNOSTICS., BELGIUM PT. SAPTA LARONA MUDA<br />
AKL<br />
20207300967 23/12/2009 BIO - CK BIOLABO SA, FRANCE<br />
AKL<br />
20101906653 28/12/2009<br />
BD Microtainer PST Tubes with LH<br />
(Lithium Heparin)<br />
BECTON DICKINSON CARIBE LTD.,<br />
PUERTO RICO untuk BECTON DICKINSON<br />
& COMPANY., USA melalui BECTON DICK<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
10302906666 28/12/2009 OXOID Anaerob Basal Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906663 28/12/2009<br />
OXOID Clostridium Difficile Agar<br />
Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906664 28/12/2009 OXOID Biggy Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301505350 14/10/2009 OXOID Cartridge Blank OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906670 28/12/2009 OXOID Azide Blood Agar Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906665 28/12/2009 OXOID Alkaline Peptone Water OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906667 28/12/2009<br />
OXOID Plate Count Agar / O.G.Y.E<br />
Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906668 28/12/2009 OXOID Cary-Blair Medium OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906669 28/12/2009 OXOID Blood Agar Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303906545 23/12/2009 DENGUE IgG Indirect ELISA<br />
AKL<br />
20303906546 23/12/2009<br />
DENGUE DUO IgM & IgG Capture<br />
ELISA<br />
AKL<br />
20303906547 23/12/2009 DENGUE IgM Capture ELISA<br />
AKL<br />
20303906548 23/12/2009 DENGUE IgG Capture ELISA<br />
AKL<br />
20303906549 23/12/2009 LEPTOSPIRA IgM ELISA<br />
AKL<br />
20206906555 23/12/2009 BeneCheck Hb Hemoglobin Strips<br />
AKL<br />
20301906556 23/12/2009<br />
PANBIO PTY LTD, AUSTRALIA untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
PANBIO PTY LTD, AUSTRALIA untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
PANBIO PTY LTD, AUSTRALIA untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
PANBIO PTY LTD, AUSTRALIA untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
PANBIO PTY LTD, AUSTRALIA untuk<br />
INVERNESS MEDICAL, AUSTRALIA<br />
GENERAL LIFE BIOTECHNOLOGY CO,<br />
LTD, TAIWAN<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. INDOCORE PERKASA<br />
OXOID Oxacillin M.I.C Evaluator<br />
Strips OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303906425 23/12/2009<br />
AKL<br />
20305906426 23/12/2009<br />
AKL<br />
10203906427 23/12/2009<br />
FOKUS DIAGNOSTIC Hellicobacter<br />
Pylori Antigen Cassette<br />
FOKUS DIAGNOSTIC Fecal Occult<br />
Blood Cassette<br />
PULSE SCIENTIFIC INC, CANADA<br />
PULSE SCIENTIFIC INC, CANADA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
ThinPrep 2000 Processor and<br />
Accessories HOLOGIC CYTIC CORPORATION, USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20301906421 23/12/2009 OXOID Ampicillin AMP 25 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906422 23/12/2009 OXOID Sulphafurazole SF300 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906423 23/12/2009 OXOID Ertapenem ETP 10 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906424 23/12/2009 OXOID Erythromycin E10 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101906433 23/12/2009<br />
B.R.A.H.M.S Free BhCG Kryptor-<br />
075 BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20306906434 23/12/2009 B.R.A.H.M.S AFP Kryptor-075 BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303906412 23/12/2009 VIKIA Strepto A BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303906413 23/12/2009 QuickVue H. Pylori gll Test<br />
AKL<br />
10303906414 23/12/2009 QuickVue Chlamydia Test<br />
AKL<br />
10303906415 23/12/2009 QuickVue In-Line Strep A<br />
AKL<br />
10303906416 23/12/2009 QuickVue Dipstick Strep A Test<br />
AKL<br />
10303906417 23/12/2009 QuickVue+ Strep A Test<br />
QUIDEL CORPORATION, USA untuk<br />
BIOMERIEUX SA, FRANCE<br />
QUIDEL CORPORATION, USA untuk<br />
BIOMERIEUX SA, FRANCE<br />
QUIDEL CORPORATION, USA untuk<br />
BIOMERIEUX SA, FRANCE<br />
QUIDEL CORPORATION, USA untuk<br />
BIOMERIEUX SA, FRANCE<br />
QUIDEL CORPORATION, USA untuk<br />
BIOMERIEUX SA, FRANCE<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303906418 23/12/2009 Q-Test MTB Test (WB) AMGENIX DIAGNOSTICS PVT, LTD., INDIA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303906419 23/12/2009 Q-Test S. Typhi IgM Test (WB) AMGENIX DIAGNOSTICS PVT, LTD., INDIA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303906420 23/12/2009<br />
Q-Test Dengue Combo IgG + IgM<br />
Test (WB) AMGENIX DIAGNOSTICS PVT, LTD., INDIA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305906432 23/12/2009 B.R.A.H.M.S Ferritin Kryptor QC BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101906430 23/12/2009<br />
AKL<br />
10303906431 23/12/2009<br />
ROCHE ALBT2 Tina-quant [a] cobas<br />
c 111<br />
FOKUS DIAGNOSTIC Hellicobacter<br />
Pylori Urease Cassette<br />
AKL<br />
10101906429 23/12/2009 ROCHE IRON2 cobas c 111<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH, GERMANY<br />
PULSE SCIENTIFIC INC, CANADA<br />
ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND untuk ROCHE<br />
DIAGNOSTICS GmbH, GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20306906439 23/12/2009 B.R.A.H.M.S AFP Kryptor Diluent BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101906438 23/12/2009<br />
AKL<br />
20305906437 23/12/2009<br />
B.R.A.H.M.S Free BhCG Kryptor<br />
Calibrator BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
B.R.A.H.M.S Ferritin Kryptor<br />
Calibrator BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20306906436 23/12/2009 B.R.A.H.M.S AFP Kryptor Calibrator BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305906435 23/12/2009 B.R.A.H.M.S Ferritin Kryptor - 050 BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101906440 23/12/2009<br />
B.R.A.H.M.S PAPP-A Kryptor<br />
Calibrator BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
10203906452 23/12/2009 PreservCyt Solution HOLOGIC CYTIC CORPORATION, USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20305906451 23/12/2009 B.R.A.H.M.S PAPP-A Kryptor - 075 BRAHMS AG., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101906455 23/12/2009 VIDAS NT-Pro BPN (PBNP) BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20205906454 23/12/2009<br />
AKL<br />
20101906331 22/12/2009<br />
AKL<br />
20101906332 22/12/2009<br />
AKL<br />
20301906557 23/12/2009<br />
Beckman Coulter Hematology<br />
System BECKMAN COULTER INC, USA PT. MENDJANGAN<br />
Test Pack + Plus hCG Combo With<br />
OBC<br />
Test Pack + Plus hCG Urine with<br />
OBC<br />
UNIPATH LTD., UK melalui INVERNESS<br />
MEDICAL, AUSTRALIA<br />
UNIPATH LTD., UK melalui INVERNESS<br />
MEDICAL, AUSTRALIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
OXOID Cefotaxime M.I.C Evaluator<br />
Strips OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906329 22/12/2009 OXOID Dextrose Tryptone Broth OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906330 22/12/2009 OXOID Corn Meal Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906328 22/12/2009 OXOID Hektoen Enteric Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906327 22/12/2009 OXOID Listeria Selective Agar Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906326 22/12/2009 OXOID TTC Solution 5 % OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906325 22/12/2009<br />
AKL<br />
20205906324 22/12/2009<br />
AKL<br />
20205906323 22/12/2009<br />
AKL<br />
20205906322 22/12/2009<br />
PDF Creator - PDF4Free v3.0<br />
OXOID Dermasel Selective<br />
Supplement OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
Lyphocheck Coagulation Control<br />
Level I BIO-RAD LABORATORIES, USA PT. DIASTIKA BIOTEKINDO<br />
Lyphocheck Coagulation Control<br />
Level 3 BIO-RAD LABORATORIES, USA PT. DIASTIKA BIOTEKINDO<br />
Lyphocheck Coagulation Control<br />
Level 2 BIO-RAD LABORATORIES, USA PT. DIASTIKA BIOTEKINDO<br />
http://www.pdf4free.com
AKL<br />
20303906444 23/12/2009<br />
AKL<br />
20303906443 23/12/2009<br />
S. Paratyphi C-H Phase 1 Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
S. Paratyphi B-H Phase 1 Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20101906442 23/12/2009 BIOLABO Alkaline Phosphatase BIOLABO SA., FRANCE<br />
AKL<br />
20101906441 23/12/2009 IL TEST Direct Bilirubin<br />
INSTRUMENTATION LABORATORY, SPA.,<br />
ITALY<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. MENDJANGAN<br />
AKL<br />
10204906453 23/12/2009 ABX PENTRA Deprotinizer CP HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20303906450 23/12/2009<br />
AKL<br />
20303906449 23/12/2009<br />
AKL<br />
20303906448 23/12/2009<br />
AKL<br />
20303906447 23/12/2009<br />
AKL<br />
20303906446 23/12/2009<br />
S. Paratyphi C-O Group C Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
S. Paratyphi B-O Group Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
S. Paratyphi A-O Group A Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
S. Paratyphi A-H Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
S. Typhi O Group D Stained<br />
Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20303906445 23/12/2009 S. Typhi d-H Stained Suspension REMEL EUROPE LTD., UK PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20303906608 28/12/2009 Parascreen ZEPHYR BIOMEDICALS, INDIA PT. ADIWARA WORLDWIDE<br />
AKL<br />
20101906642 28/12/2009<br />
AKL<br />
10101906643 28/12/2009<br />
AKL<br />
20101906644 28/12/2009<br />
CENTURY Healthcare Pregnancy<br />
Test Strip<br />
CENTURY Healthcare Ovulation<br />
Test Strip<br />
DUOCARE Blood Glucose and<br />
Pressure Monitor<br />
W.H.P.M BIORESEARCH & TECHNOLOGY<br />
CO., LTD., CHINA<br />
W.H.P.M BIORESEARCH & TECHNOLOGY<br />
CO., LTD., CHINA<br />
GENEXEL-SEIN INC., KOREA melalui dari L-<br />
TAC MEDICARE PTE. LTD., SINGAPORE<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
PT. SEGARA PUTERA MANDIRI<br />
AKL<br />
10204906645 28/12/2009 CLINIMACS PBS / EDTA Buffer MILTENYI BIOTEC GMBH, GERMANY PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20303906646 28/12/2009 FOKUS DENGUE Antigen PULSE SCIENTIFIC INC, CANADA<br />
AKL<br />
10209906647 28/12/2009 DIAMED DiaCent - 12 DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20101906648 28/12/2009 AKURAT Compact<br />
NANTONG EGENS BIOTECHNOLOGY CO,<br />
LTD., P.R CHINA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. DANPAC PHARMA<br />
AKL<br />
10303906656 28/12/2009 VIDAS EBV EBNA IgG BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303906657 28/12/2009 VIDAS EBV VCA/EA IgG BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906658 28/12/2009 Etest LE Levofloxacin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906659 28/12/2009 Etest MYC Micafungin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906660 28/12/2009 Etest AZ Azithromycin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10303906661 28/12/2009 VIDAS EBV VCA IgM BIOMERIEUX SA, FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20207906650 28/12/2009 BeneCheck Hb Hemoglobin Meter<br />
AKL<br />
20306906671 28/12/2009<br />
AKL<br />
20306906672 28/12/2009<br />
AKL<br />
20306906673 28/12/2009<br />
AKL<br />
20306906674 28/12/2009<br />
AKL<br />
20306906675 28/12/2009<br />
ABBOTT Axsym System CA 19-9<br />
Calibrators<br />
ABBOTT Axsym System CA 19-9<br />
Specimen Diluent<br />
ABBOTT Axsym System CA 19-9<br />
Reagent Pack<br />
ABBOTT Axsym System CA 19-9<br />
Controls<br />
ABBOTT Axsym System CA 19-9<br />
Master Calibrators<br />
GENERAL LIFE BIOTECHNOLOGY CO,<br />
LTD, TAIWAN<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. INDOCORE PERKASA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101906676 28/12/2009<br />
ABBOTT AXSYM Insulin Standard<br />
Calibrators<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101906677 28/12/2009<br />
ABBOTT AXSYM Insulin Master<br />
Calibrator<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101906678 28/12/2009<br />
ABBOTT AXSYM Insulin Reagent<br />
Pack<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305906679 28/12/2009<br />
ABBOTT AXSYM System B2-<br />
Microglobulin Reagent Pack<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305906680 28/12/2009<br />
ABBOTT AXSYM System B2-<br />
Microglobulin Master Calibrators<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305906681 28/12/2009<br />
ABBOTT AXSYM System B2-<br />
Microglobulin Controls<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305906682 28/12/2009<br />
ABBOTT AXSYM System B2-<br />
Microglobulin Standard Calibrator<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305906683 28/12/2009<br />
AKL<br />
20101902943 25/06/2009<br />
ABBOTT AXSYM System B2-<br />
Microglobulin Specimen Diluent<br />
SIEMENS DIMENSION Clinical<br />
Chemistry System Flex Reagent<br />
Cartridge UCFP<br />
AXIS - SHIELD DIAGNOSTICS LTD, UK<br />
untuk ABBOTT GmbH & CO.KG, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. ABBOTT INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301601194 30/12/2009 OXOID Ceftizoxime - ZOX 30 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906270 16/12/2009 OXOID Cefpodoxime CPD10 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301907008 30/12/2009 BIORAD Voriconazole 1 ug<br />
AKL<br />
10101907009 30/12/2009 ONE MED Menopouse Test<br />
AKL<br />
20101907010 30/12/2009 ONE MED Siklus HCG Test<br />
AKL<br />
20209907011 30/12/2009 HAIER Deep Freezer<br />
AKL<br />
20209907012 30/12/2009 HAIER Blood Bank Refrigerator<br />
AKL<br />
20209907013 30/12/2009 HAIER ULT Freezer<br />
AKL<br />
20101907014 30/12/2009<br />
Urinalysis Reagent Strips 2AG<br />
(Protein, Glucose)<br />
AKL<br />
20101907015 30/12/2009 Urinalysis Reagent Strips 10A<br />
AKL<br />
20101907016 30/12/2009<br />
AKL<br />
20101906983 30/12/2009<br />
Urinalysis Reagent Strips 3AG<br />
(Protein, Glucose, PH)<br />
PRODIGY Autocode Blood Glucose<br />
Test Strip<br />
AKL<br />
20101906984 30/12/2009 PRODIGY Autocode Talking Meter<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
IND DIAGNOSTIC INC., CANADA melalui AI<br />
DE DIAGNOSTIC CO. LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui AI<br />
DE DIAGNOSTIC CO. LTD., CHINA<br />
HAIER MEDICAL AND LABORATORY<br />
PRODUCTS CO. LTD., CHINA<br />
HAIER MEDICAL AND LABORATORY<br />
PRODUCTS CO. LTD., CHINA<br />
HAIER MEDICAL AND LABORATORY<br />
PRODUCTS CO. LTD., CHINA<br />
SUZHOU HONGYI BIOTECH CO. LTD., P.R.<br />
CHINA<br />
SUZHOU HONGYI BIOTECH CO. LTD., P.R.<br />
CHINA<br />
SUZHOU HONGYI BIOTECH CO. LTD., P.R.<br />
CHINA<br />
PRODIGY DIABETES CARE LTD, TAIWAN<br />
untuk DIAGNOSTIC DEVICE INC, USA<br />
PRODIGY DIABETES CARE LTD, TAIWAN<br />
untuk DIAGNOSTIC DEVICE INC, USA<br />
PT. DOS NI ROHA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ABHIMATA MANUNGGAL<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20101906982 30/12/2009<br />
PRODIGY Autocode Pocket Blood<br />
Glucose Monitoring System<br />
AKL<br />
20301907002 30/12/2009 BIORAD Fluconazole 25 ug<br />
AKL<br />
20301907003 30/12/2009 BIORAD Ampicillin 10 ug<br />
AKL<br />
20303907004 30/12/2009 PLATELIA Rubella IgG<br />
AKL<br />
20303907005 30/12/2009 PLATELIA Rubella IgM<br />
AKL<br />
20301907006 30/12/2009 BIORAD Amoxycillin 25 ug<br />
AKL<br />
20301907007 30/12/2009 BIORAD Penicillin 6 ug<br />
AKL<br />
20103906989 30/12/2009 NAPSA TEST 8 (THC) Card Test<br />
AKL<br />
20103906990 30/12/2009 NAPSA TEST 5 (MAMP) Card Test<br />
AKL<br />
20103906991 30/12/2009 NAPSA TEST 4 (MDMA) Card Test<br />
PRODIGY DIABETES CARE LTD, TAIWAN<br />
untuk DIAGNOSTIC DEVICE INC, USA<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
BOSON BIOTECHNOLOGY CO, LTD.,<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO, LTD.,<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO, LTD.,<br />
CHINA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103906992 30/12/2009 NAPSA TEST 2 (BZO) Card Test<br />
AKL<br />
20103906987 30/12/2009 NAPSA TEST 1 (AMP) Card Test<br />
AKL<br />
20103906988 30/12/2009 NAPSA TEST 6 (OPI) Card Test<br />
BOSON BIOTECHNOLOGY CO, LTD.,<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO, LTD.,<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO, LTD.,<br />
CHINA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
AKL<br />
20301906981 30/12/2009 ETEST NA Nalidixic Acid AB BIOMERIREUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10302906662 28/12/2009 OXOID Polycarbonat Jar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601193 31/12/2009<br />
OXOID Piperacillin / Tazobact - TZP<br />
85 ug OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601198 31/12/2009 OXOID Levofloxacin - LEV 5 ug OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301600499 30/12/2009 OXOID Oxacillin (OX1) OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301600532 30/12/2009 OXOID Cephalothin - KF 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301600497 30/12/2009 OXOID Nitrofuration - F50 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601190 30/12/2009 OXOID Chloramfenicol - C10 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301600498 30/12/2009 OXOID Norfloxacin - NOR 5 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601199 30/12/2009 OXOID Cefprozil - CPR 30 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601191 30/12/2009 OXOID Doxycycline - DO 30 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302907069 30/12/2009 OXOID Disc Dispenser MK III OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301906975 30/12/2009 ETEST AM Ampicillin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906976 30/12/2009 ETEST OX Oxacillin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906977 30/12/2009 ETEST PG Benzylpenicillin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906978 30/12/2009<br />
ETEST AB Ampicillin / Sulbactam<br />
(2/1) AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906979 30/12/2009 ETEST TXL Ceftriaxone AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906980 30/12/2009 ETEST CI Ciprofloxacin AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906973 30/12/2009 ETEST CL Chloramphenicol AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301906974 30/12/2009 ETEST SX Sulfamethoxazole AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20102907017 30/12/2009 BEP III System and Accessories<br />
AKL<br />
20102907018 30/12/2009<br />
AKL<br />
20305907083 30/12/2009<br />
DIMENSION RxL Clinical Chemistry<br />
Analyzer and Accessories<br />
SIEMENS N-Antiserum to Human<br />
Ceruloplasmin<br />
AKL<br />
20102907084 30/12/2009 VIVA-E System and Accessories<br />
AKL<br />
20102907085 30/12/2009 BEP 2000 System and Accessories<br />
AKL<br />
20303907079 30/12/2009<br />
AKL<br />
20305907080 30/12/2009<br />
AKL<br />
20305907081 30/12/2009<br />
AKL<br />
20305907082 30/12/2009<br />
ENZYGNOST Anti-Rubella Virus /<br />
IgG<br />
SIEMENS Cellognost -<br />
Mononucleosis<br />
SIEMENS N-Antiserum to Human<br />
Albumin<br />
SIEMENS N-Antiserum to Human<br />
Fibronectin<br />
AKL<br />
20101907078 30/12/2009 SIEMENS Multiply IMT Standard B<br />
AKL<br />
20101907076 30/12/2009 DIMENSION Salt Bridge Solution<br />
AKL<br />
20101907077 30/12/2009 DIMENSION AHDL Calibrator<br />
AKL<br />
20101907074 30/12/2009 SIEMENS Multiply IMT Standard C<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
20101907075 30/12/2009<br />
DIMENSION QUIKLYTE Diluent<br />
Check<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301907073 30/12/2009 BRILLIANCE MRSA Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102907068 30/12/2009 ComboStik R-700 and Accessories DFI CO, LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
20102907066 30/12/2009<br />
ALFA WASSERMANN ACE Alera<br />
Clinical Chemistry Analyzer System<br />
ALFA WASSERMANN INC, USA melalui<br />
ALFA WASSERMANN BV, NETHERLANDS<br />
PT. MARADA PHARMA MEDICA<br />
AKL<br />
10102907067 30/12/2009 ComboStik R-300 and Accessories DFI CO, LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
20206907065 30/12/2009 INNOVANCE Antithrombin<br />
AKL<br />
20306907063 30/12/2009<br />
AKL<br />
10302906986 30/12/2009<br />
LIQUICHECK Cardiac Markers Plus<br />
Control Trilevel<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH, GERMANY<br />
BIORAD LABORATORIES INC, USA melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE, LTD., SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
OXOID Desoxycholate Citrate Agar<br />
(Hynes) OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906993 30/12/2009 OXOID Tryptone Water OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906994 30/12/2009 OXOID Mycoplasma Broth Base OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302906996 30/12/2009 OXOID Vancomycin Supplement OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302907069 30/12/2009 OXOID Disc Dispenser MK III OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302907071 30/12/2009 OXOID Penase OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20102906999 30/12/2009 SPOTCHEM EZ SP-4430 ARKRAY FACTORY INC., JAPAN<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
10204907001 30/12/2009 ABX Pentra Etching CP HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101907064 30/12/2009 IL TEST ALT/GPT<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
PT. MENDJANGAN<br />
AKL<br />
10101907137 31/12/2009 DIALAB UIBC DIALAB GMBH, AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101504781 31/12/2009 IL Test Creatinine<br />
AKL<br />
20103600350 31/12/2009 DIMA One Step Cocaine Test<br />
INSTRUMENTATION LABORATORY SPA,<br />
ITALY<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbH., GERMANY<br />
AKL<br />
10302907155 31/12/2009 CHROMagar Salmonella CHROMAGAR MICROBIOLOGY, FRANCE<br />
AKL<br />
20209907140 31/12/2009 CLAS Anti - B CLAS TECHNOLOGY LIMITED, UK<br />
AKL<br />
20209907141 31/12/2009 CLAS Anti - A CLAS TECHNOLOGY LIMITED, UK<br />
AKL<br />
20209907142 31/12/2009 CLAS Anti - D (IgG / IgM) Blend CLAS TECHNOLOGY LIMITED, UK<br />
PT. MENDJANGAN<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
AKL<br />
20208907143 31/12/2009 ABX Minotrol 16 Twin-Pack 2N HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20208907144 31/12/2009 ABX Minotrol 16 Twin-Pack 2H HORIBA ABX DIAGNOSTICS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10303907145 31/12/2009 BIORAD SIR Mycoplasma<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
PT. DOS NI ROHA<br />
AKL<br />
10302907146 31/12/2009 BIORAD Sabouraud Agar<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
PT. DOS NI ROHA<br />
AKL<br />
10302907147 31/12/2009<br />
BIORAD Sabouraud<br />
Chloramphenicol Agar<br />
AKL<br />
20303907148 31/12/2009 CHORUS Varicella Zoster IgG<br />
AKL<br />
20303907149 31/12/2009 CHORUS Varicella Zoster Virus IgM<br />
AKL<br />
10303907150 31/12/2009 CHORUS Helicobacter Pylori IgG<br />
AKL<br />
10303907151 31/12/2009 CHORUS Helicobacter Pylori IgA<br />
BIORAD LABORATORIES, FRANCE melalui<br />
BIORAD LABORATORIES, SINGAPORE<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
DIESSE DIAGNOSTICA SENESE SPA,<br />
ITALY<br />
AKL<br />
10302907152 31/12/2009 CHROMagar Staph Aureus CHROMAGAR MICROBIOLOGY, FRANCE<br />
AKL<br />
10302907153 31/12/2009 CHROMagar Salmonella Plus CHROMAGAR MICROBIOLOGY, FRANCE<br />
PDF Creator - PDF4Free v3.0<br />
PT. DOS NI ROHA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
http://www.pdf4free.com
AKL<br />
10302907154 31/12/2009 CHROMagar Candida CHROMAGAR MICROBIOLOGY, FRANCE<br />
AKL<br />
10302907156 31/12/2009 CHROMagar Orientation CHROMAGAR MICROBIOLOGY, FRANCE<br />
AKL<br />
10302907157 31/12/2009 CHROMagar VRE Base CHROMAGAR MICROBIOLOGY, FRANCE<br />
AKL<br />
20101907158 31/12/2009<br />
AKL<br />
20101907159 31/12/2009<br />
AKL<br />
20101907160 31/12/2009<br />
AKL<br />
20103907161 31/12/2009<br />
AKL<br />
20103907162 31/12/2009<br />
GlucoSure STAR Blood Glucose<br />
Monitoring System<br />
GlucoSure STAR Blood Glucose<br />
Test Strips<br />
MultiSure Blood Glucose & Uric Acid<br />
Monitoring System<br />
APEX BIOTECHNOLOGY CORP, TAIWAN,<br />
R.O.C<br />
APEX BIOTECHNOLOGY CORP, TAIWAN,<br />
R.O.C<br />
APEX BIOTECHNOLOGY CORP, TAIWAN,<br />
R.O.C<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
NAPSA CHECK 4 (MDMA) Card<br />
Test LUMIQUICK DIAGNOSTICS INC, USA PT. INDOMED BERKAT JAYA<br />
NAPSA CHECK 5 (MAMP) Card<br />
Test LUMIQUICK DIAGNOSTICS INC, USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103907163 31/12/2009 NAPSA CHECK 8 (THC) Card Test LUMIQUICK DIAGNOSTICS INC, USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103907164 31/12/2009 NAPSA CHECK 6 (OPI) Card Test LUMIQUICK DIAGNOSTICS INC, USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103907165 31/12/2009 NAPSA CHECK 1 (AMP) Card Test LUMIQUICK DIAGNOSTICS INC, USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103907166 31/12/2009 NAPSA CHECK 2 (BZO) Card Test LUMIQUICK DIAGNOSTICS INC, USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103600342 31/12/2009<br />
DIMA One Step<br />
Tetrahydrocannabinol Test<br />
AKL<br />
21106906559 23/12/2009 TPC Holding Pipettes (LHS)<br />
AKL<br />
20305600348 31/12/2009 DIMA One Step HBsAg Test<br />
AKL<br />
20103600344 31/12/2009 DIMA One Step Morphine Test<br />
AKL<br />
20103600345 31/12/2009<br />
DIMA One Step Methamphetamine<br />
Test<br />
AKL<br />
20305600347 31/12/2009 DIMA HBsAb Test<br />
AKL<br />
20103600346 31/12/2009 DIMA One Step Barbiturate Test<br />
AKL<br />
10103902624 08/06/2009<br />
SIEMENS DIMENSION DOA -<br />
Negative Control<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
THE PIPETTE COMPANY (TPC),<br />
AUSTRALIA<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
DIMA GESELLSCHAFT FUR DIAGNOSTIKA<br />
mbh., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101900366 10/03/2009 ABBOTT AXSYM - Uptake Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20805904115 28/08/2009<br />
FRESENIUS Bibag Sodium<br />
Bicarbonate<br />
AKL<br />
20101804949 05/02/2010 ANALYTICON LDH-P Mono<br />
AKL<br />
20101804668 05/02/2010 Fluitest TP<br />
AKL<br />
20306603524 25/10/2010 ADVIA CENTAUR CA 15-3<br />
AKL<br />
20303504849 30/06/2010<br />
FRESENIUS MEDICAL CARE AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. FRESENIUS MEDICAL CARE<br />
INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. SIEMENS INDONESIA<br />
SPAN Stained Salmonella S.<br />
Paratyphi C (H) Antigen SPAN DIAGNOSTICS Ltd, India PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
21109013748 28/12/2010 VITROLIFE Thaw-Kit VITROLIFE AB., SWEDEN<br />
AKL<br />
21108013749 28/12/2010 VITROLIFE ICSI VITROLIFE AB., SWEDEN<br />
AKL<br />
20301011147 21/05/2010 BIORAD NYSTATIN<br />
BIORAD LABORATORIES LTD., FRANCE<br />
atas penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE. LTD., SINGAPOR<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DOS NI ROHA<br />
AKL<br />
20301800398 26/04/2010 VITEK 2 NH BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305600967 30/04/2010 ARCHITECT Ferritin Controls<br />
AKL<br />
20305600963 30/04/2010 ARCHITECT Ferritin Calibrators<br />
AKL<br />
20305600682 30/04/2010 ARCHITECT Ferritin Reagent Kit<br />
AKL<br />
20101600228 06/05/2010 ARCHITECT Total T3 Calibrators<br />
AKL<br />
ARCHITECT Total T3<br />
20101600264 06/05/2010 (Triiodothyronine) Reagent Test<br />
PDF Creator - PDF4Free v3.0<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
http://www.pdf4free.com<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA
AKL<br />
20101600993 06/05/2010 ARCHITECT Free T3 Calibrator<br />
AKL<br />
10101600994 30/04/2010 ARCHITECT Free T3 Controls<br />
AKL<br />
10101600986 30/04/2010 ARCHITECT TSH Control<br />
AKL<br />
20101600227 06/05/2010 ARCHITECT TSH Calibrator<br />
AKL<br />
10302704281 28/12/2010<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
OXOID Plate Count Agar and<br />
MacConkey Agar No. 3 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20208703373 28/12/2010 DIAMED ID-Diluent 2 DIAMED GmbH., SWITZERLAND<br />
AKL<br />
10102604597 20/08/2010<br />
AKL<br />
20101600988 06/05/2010<br />
Stat Fax 2100 Microplate Reader and<br />
Accessories<br />
ARCHITECT Total B- hCG<br />
Calibrator<br />
AKL<br />
10101010631 30/04/2010 ARCHITECT Total B-hCG Control<br />
AKL<br />
20101600991 06/05/2010 ARCHITECT Total T4 Calibrator<br />
AWARENESS TECHNOLOGY INC., USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
AKL<br />
ABBOTT IRELAND DIAGNOSTICS<br />
20101504473 30/04/2010 ARCHITECT Total T4 Reagent Pack DIVISION., IRELAND<br />
AKL<br />
10101601636 06/05/2010 ABBOTT Architect Testosterone<br />
AKL<br />
20205010623 29/04/2010<br />
PROCLEIX TIGRIS System and<br />
Accessories<br />
AKL<br />
10102011081 12/05/2010 PROCLEIX Optiva RAS<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTIC<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20205600499 30/04/2010 BOULE MEDONIC M16M BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20102011266 11/06/2010 SELECTRA JUNIOR VITAL SCIENTIFIC B.V., NETHERLANDS PT. MRK DIAGNOSTICS<br />
AKL<br />
20101402400 05/11/2010 MEDI-TEST Glucose<br />
MACHEREY NAGEL GmbH & Co KG,<br />
Germany<br />
PT. MRK DIAGNOSTICS<br />
AKL<br />
10208602453 06/08/2010 MEDONICT M - Series Diluent BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
10101601007 06/05/2010<br />
ABBOTT Architect Prolactin<br />
Controls<br />
AKL<br />
20101601008 30/04/2010 ARCHITECT Prolactin Calibrator<br />
AKL<br />
20208600232 06/05/2010<br />
ARCHITECT Concentrated Wash<br />
Buffer<br />
AKL<br />
20205600957 06/05/2010 ARCHITECT Pre-Trigger Solution<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10302700034 28/06/2010 OXOID X+V factor Disc OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101702695 06/05/2010<br />
ProDia Triglyserides LS GPO-POP<br />
Method<br />
PRODIA INTERNATIONAL, UNITED ARAB<br />
EMIRATES<br />
AKL<br />
10302701489 05/11/2010 DIAMED ID-Incubator DIAMED GMBH., SWITZERLAND<br />
AKL<br />
10102701408 15/11/2010 DIAMED ID-Dispenser DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20101601029 03/05/2010<br />
AKL<br />
20305501461 06/05/2010 ARCHITECT HBsAg Calibrators<br />
AKL<br />
20303700531 29/12/2010<br />
AKL<br />
10303700538 29/12/2010<br />
AKL<br />
10303700537 29/12/2010<br />
AKL<br />
10303700539 29/12/2010<br />
PT. SUMIFIN CITRA ABADI<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
ABBOTT Clinical Chemistry Alkaline<br />
Phosphatase ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
Shingella Flexneri Polyvalent (1 to 6,<br />
X & Y)<br />
REMEL E. COLI Polyvalent 3<br />
Agglutinating Sera<br />
REMEL E.Coli Polyvalent 4<br />
Agglutinating Sera<br />
REMEL E.COLI Polyvalent 2<br />
Agglutinating Sera<br />
AKL<br />
10303700532 29/12/2010 REMEL E.Coli Angglutinating Sera<br />
AKL<br />
10101700259 28/12/2010 UF II Sheath<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
REMEL EUROPE LTD, UK untuk REMEL<br />
INC., USA<br />
REMEL EUROPE LTD, UK untuk REMEL<br />
INC., USA<br />
REMEL EUROPE LTD, UK untuk REMEL<br />
INC., USA<br />
REMEL EUROPE LTD, UK untuk REMEL<br />
INC., USA<br />
REMEL EUROPE LTD., UK untuk REMEL<br />
INC., USA<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. ABBOTT INDONESIA<br />
PT. DIPA PUSPA LABSAINS<br />
PT. DIPA PUSPA LABSAINS<br />
PT. DIPA PUSPA LABSAINS<br />
PT. DIPA PUSPA LABSAINS<br />
PT. DIPA PUSPA LABSAINS<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101700260 28/12/2010 UF II Search - Bac<br />
AKL<br />
10101700257 28/12/2010 UF II Search - Sed<br />
AKL<br />
10102700253 30/12/2010<br />
SYSMEX UF-1000i Urine<br />
Fluorescene Flow Cytometry +<br />
Accessories<br />
AKL<br />
10101700258 28/12/2010 UF II Pack - Bac<br />
AKL<br />
20101401907 06/05/2010<br />
AKL<br />
10302700047 28/12/2010<br />
AKL<br />
10302700045 28/12/2010<br />
AKL<br />
10302700046 28/12/2010<br />
ABBOTT Axsym CK-MB Reagent<br />
Pack<br />
SYSMEX CORPORATION, JAPAN atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD., JAPAN<br />
SYSMEX CORPORATION, JAPAN atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD., JAPAN<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD., JAPAN<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ABBOTT INDONESIA<br />
OXOID Legionella Pneumaphila 1x60<br />
test OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Legion. Pneumaphila 2-14<br />
Latex Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Legionella Pneumaphila 1<br />
Reag. Kit 1x50 TST OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700043 22/12/2010 OXOID Charcoal Agar 500 g Oxoid Limited, England PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700041 28/12/2010 OXOID Microbat Reagent VP II OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700035 22/12/2010 OXOID Karmali Agar Base 500 g OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700037 22/12/2010 OXOID Lab Lemco Agar 500 g OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700032 28/12/2010<br />
AKL<br />
10302700031 28/12/2010<br />
AKL<br />
10302700029 22/12/2010<br />
OXOI Ddryspot Legionella Species60<br />
tests OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Dryspot Legionella Pneumop<br />
Serogr 2-14 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Yersinia sel. Agar Base 500<br />
g OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305604993 29/12/2010 DIALAB RF (Rheumatoid Factor) DIALAB GESM.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10305604994 28/12/2010 DIALAB Apolipoprotein A2 DIALAB GESM.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303013143 05/11/2010<br />
REMEL Wellcogen N.Meningitidis<br />
B/E Coli K1 REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302604819 03/08/2010 OXOID Mycoplasma Agar Base OXOID LIMITED,UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301605032 03/08/2010 OXOID Cinoxacin CIN100 OXOID LIMITED,England PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301604097 03/08/2010 OXOID Metronidazole 50 ug MTN 50 OXOID LIMITED,UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101604946 01/09/2010<br />
PTS Panels HDL Cholesterol Test<br />
Strips<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
USA<br />
PT. MITRAASA PRATAMA<br />
AKL<br />
10302605037 06/08/2010 OXOID Microbact Reagent Nitrate B OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302605043 06/08/2010<br />
OXOID C-T Supplement for E-coli<br />
0157 OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
10101604949 01/09/2010 PTS Panels Triglycerides Test Strips USA<br />
AKL<br />
10101604955 01/09/2010 PTS Panels Cholesterol Test Strips<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
USA<br />
PT. MITRAASA PRATAMA<br />
PT. MITRAASA PRATAMA<br />
AKL<br />
20301605033 03/08/2010 OXOID Cephalodirine CR5 OXOID LIMITED,England PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101012368 01/09/2010<br />
PTS Panels CHOL + HDL Panel test<br />
strips<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
USA<br />
AKL<br />
20305604793 29/12/2010 FOKUS HBsAg Cassette PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20305604791 29/12/2010 FOKUS Anti HCV Strip PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20305012013 09/08/2010<br />
PROCLEIX Ultrio Plus Assay HCV<br />
Discriminatory Probe Reagents<br />
GEN PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
AKL<br />
20205012950 28/10/2010 QUO-TEST A1C Test Cartridge QUOTIENT DIAGNOSTICS., UK<br />
AKL<br />
20303012455 03/09/2010<br />
AKL<br />
20101604664 29/12/2010<br />
PDF Creator - PDF4Free v3.0<br />
ARCHITECT Syphilis TP Reagent<br />
Kit<br />
ON CALL Plus Blood Glucose Test<br />
Strip<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
http://www.pdf4free.com<br />
PT. MITRAASA PRATAMA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. ABBOTT INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI
AKL<br />
20101604663 29/12/2010 ON CALL Plus Blood Glucose Meter ACON BIOTECH (HANGZHOU) CO., CHINA<br />
AKL<br />
20305012012 09/08/2010 PROCLEIX Ultrio Plus Assay<br />
AKL<br />
10302604942 06/08/2010<br />
AKL<br />
20301011921 06/08/2010<br />
GEN PROBE INC., USA untuk CHIRON<br />
LIMITED., HONGKONGNC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MEDQUEST JAYA GLOBAL<br />
OXOID Campylobacter Gas<br />
Generating Kit OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Oxolinic Acid OA 2<br />
Susceptibility Test Disc OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101012603 08/09/2010 VACUETTE K3 EDTA Tubes GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20209700460 28/12/2010<br />
AKL<br />
20209700459 28/12/2010<br />
KUBOTA High Capacity Refrigerated<br />
Centrifuge<br />
DIAMED Swing Twin Sampler<br />
Automated Pipettor for ID-Cards and<br />
Microplates<br />
KUBOTA MANUFACTURING CORP., JAPAN<br />
DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20209700461 28/12/2010 DIAMED SAXO ID-Reader DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20209700458 28/12/2010<br />
DIAMED LYRA MP - Reader<br />
Centrifuge with Automated Reader for<br />
Microplates<br />
AKL<br />
20101012473 03/09/2010 VACUPRO ETDA K3<br />
AKL<br />
20303013144 05/11/2010<br />
DIAMED GMBH., SWITZERLAND<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. ANTAR MITRA SEMBADA<br />
REMEL Wellcogen N. Meningtidis<br />
ACY W135 REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20306013139 05/11/2010 ELSA CA 15 - 3 CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101604352 28/12/2010<br />
AKL<br />
20101604371 25/10/2010<br />
AKL<br />
20101604364 25/10/2010<br />
AKURAT Strip Deteks iDini<br />
Kehamilan AMGENIX INTERNATIONAL INC., INDIA PT. DANPAC PHARMA<br />
ABBOTT Axsym CA 125 Standard<br />
Calibrators<br />
ABBOTT Axsym CK-MB Standard<br />
Calibrators<br />
AKL<br />
10101604362 05/11/2010 ABBOTT Axsym CK-MB Controls<br />
AKL<br />
10102604260 15/11/2010 Cobas c 111 + Accessories<br />
AKL<br />
10101604256 05/11/2010<br />
AKL<br />
10101604257 05/11/2010<br />
AKL<br />
20301604158 20/12/2010<br />
AKL<br />
20301604157 28/12/2010<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, SLIGO-IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD-IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ROCHE INSTRUMENT CENTER AG,<br />
SWITZERLAND for ROCHE DIAGNOSTIC<br />
GmbH., GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
HDLC3-HDL-Cholesterol Plus 3<br />
Generation COBAS INTEGRA-cobas<br />
c system ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
Roche/Hitachi HDL-C Plus (HDL<br />
Cholesterol 3 Gen) ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
BD BBL Sensi Disc Piperacilin TZP-<br />
110 parts BECTON DICKINSON & COMPANY, USA PT. ANUGRAH ARGON MEDICA<br />
BD BBL Sensi Disc Ticarcillin /<br />
Clavulanic 85 ug BECTON DICKINSON & COMPANY, USA PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20209013199 05/11/2010 CrossLys and Accessories DIAGAST., FRANCE PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10303012029 09/08/2010<br />
AKL<br />
20101604127 29/12/2010 RAVITEST Test Strip<br />
AKL<br />
20101012026 09/08/2010 MULTICARE In Glucose Strip<br />
AKL<br />
20207011578 05/07/2010<br />
AKL<br />
10303605035 09/08/2010<br />
OXOID Microbact Staph fast Blue<br />
Reagent OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
BLUE CROSS BIOMEDICAL (BEIJING) Co.<br />
Ltd., China<br />
BIOCHEMICAL SYSTEM INTERNATIONAL<br />
Srl., ITALY<br />
PT. OSADHA GRAHA<br />
SEJAHTERA<br />
PT. ISOTEKINDO INTERTAMA<br />
coaguChek XS System and<br />
Accessories ROCHE DIAGNOSTICS GmbH., Germany PT. ROCHE INDONESIA<br />
OXOID PBP2 (Penicillin Binding<br />
Protein) Test OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301013623 20/12/2010<br />
BD BBL Sensi Disc Fosfomycin +<br />
Glucose-Phosphate FOS - 200 ug BECTON BICKINSON & COMPANY., USA PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
10201013771 28/12/2010 MERCK Congo Red Staining Kit MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10101604063 28/12/2010 DIALAB Iron Standard DIALAB GESM.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101304587 02/07/2010<br />
COBAS C.f.a.s ( Calibrator for<br />
Automated Systems ) ROCHE DIAGNOSTICS GmbH., Germany PT. ROCHE INDONESIA<br />
AKL<br />
10101013775 28/12/2010 DIALAB Cholesterol Standard DIALAB Ges M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20205012949 28/10/2010 QUA-TEST AIC System QUOTIENT DIAGNOSTICS., UK<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101601013 05/02/2010 AXSYM LH Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20103012022 09/08/2010 ARCHITECT i Digoxin Reagent Kit<br />
AKL<br />
20305603779 07/10/2010<br />
ROCHE Linear Array Detection Kit<br />
(La DK)<br />
AKL<br />
20103012021 09/08/2010 ARCHITECT i Digoxin calibrators<br />
AKL<br />
20305603780 07/10/2010<br />
ROCHE Linear Array HCV<br />
Genotyping<br />
AKL<br />
10101012090 10/08/2010 AXSYM Free T4 Controls<br />
BIOKIT S.A, SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
BIOKIT S.A, SPAIN untuk ABBOTT<br />
LABORATORIES, USA<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD-IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101603883 07/07/2010 ABBOTT Ultra HDL ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10303011145 21/05/2010 SD Bioline H. Pylori STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305603598 11/06/2010 SD Bioline HBsAg STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305603597 11/06/2010 SD Bioline HCV STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305603595 11/06/2010 SD Bioline Anti - HBs STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10302012030 09/08/2010<br />
AKL<br />
20101011653 05/07/2010<br />
AKL<br />
10208011651 05/07/2010<br />
OXOID Biochemical Identification<br />
System (O.B.I.S)-PYR OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
ARCHITECT STAT Troponon-I<br />
Calibrators<br />
ARCHITECT Folate RBC Lysis<br />
Diluent<br />
AKL<br />
20305011655 07/07/2010 AXSYM Ferritin Standard Calibrator<br />
FISHER DIAGNOSTICS, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD - IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD - IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10302602704 12/07/2010 REMEL Endo Agar REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302604929 06/08/2010 OXOID CCDA selective Supplement OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101504868 16/08/2010 STANBIO Glucose Liquicolor STANBIO LABORATORIES., USA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
10201011944 06/08/2010 MERCK Microcopy Cytocolor MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20303012456 03/09/2010 ARCHITECT Syphilis TP calibrator<br />
AKL<br />
20101604951 09/08/2010 PTS PANELS Ketone Test Strip<br />
AKL<br />
20303012457 03/09/2010 ARCHITECT Syphilis TP Control<br />
AKL<br />
20101604954 09/08/2010 PTS PANELS Glucose Test Strip<br />
AKL<br />
20301605018 06/08/2010<br />
AKL<br />
20101012476 03/09/2010 FRIDA HCG Test Strip<br />
AKL<br />
10101012477 03/09/2010 FRIDA LH Test Strip<br />
AKL<br />
10302604095 06/08/2010<br />
AKL<br />
20101012471 03/09/2010 VACUPRO 3.2% Sodium Citrate<br />
AKL<br />
20301604668 06/08/2010<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
USA<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
USA<br />
PT. ABBOTT INDONESIA<br />
PT. MITRAASA PRATAMA<br />
PT. ABBOTT INDONESIA<br />
PT. MITRAASA PRATAMA<br />
OXOID Clindamycin DA 10<br />
Susceptibility Disc OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
NANTONG EGENS BIOTECHNOLOGY CO.<br />
LTD, P.R. CHINA<br />
NANTONG EGENS BIOTECHNOLOGY CO.<br />
LTD, P.R. CHINA<br />
PT. ALFA OMEGA FARMA<br />
PT. ALFA OMEGA FARMA<br />
OXOID Neomycin Selective<br />
Supplement OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
PT. ANTAR MITRA SEMBADA<br />
OXOID Amoxycillin Susceptibility<br />
Disc (AML 2 dan AML 10) OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20209601842 02/07/2010 DIALAB Anti B Monoclonal DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602712 06/05/2010 REMEL Thymune - T REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305602669 06/05/2010 REMEL Thymune - M REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303602713 06/05/2010<br />
REMEL Salmonella Agglutinating<br />
Serum Polyvalent G-H REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302011657 07/07/2010 REMEL RapID NF Plus System REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602691 12/07/2010 REMEL Loefflers Medium Slant REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101010960 06/05/2010 ARCHITECT Phenytoin Calibrator<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION, USA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10303011658 07/07/2010 REMEL Staphaurex REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602715 06/05/2010<br />
REMEL Tryptic Soy Agar with<br />
Lecithin & Polysorbate 80 REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602716 12/07/2010 REMEL GN Broth REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602695 12/07/2010 REMEL RapID ANA II System REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602697 06/05/2010 REMEL RapID Yeast Plus System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602693 06/05/2010 REMEL RapID One System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602694 06/05/2010 REMEL RapID Spot Indole Reagent REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602690 06/05/2010 REMEL RapID CB Plus System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602706 12/07/2010 REMEL BactiDrop OXIDASE REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700033 28/06/2010 OXOID X-Factor Disc OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602689 06/05/2010 REMEL RapID Inoculation Fluid REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301602673 06/05/2010 OXOID COLISTIN SULPHATE OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602671 06/05/2010 OXOID Microbact Reagent Nitrate A OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602670 06/05/2010<br />
OXOID O.R.S.A.B. SELECTIVE<br />
SUPPLEMENT OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301602672 24/03/2010 OXOID LOMEFLOXACIN LOM 10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301602674 06/05/2010 OXOID Furazolidone FR 100 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602676 06/05/2010 OXOID Microbat Reagent TDA OXOID LIMITED., ENGLAND<br />
AKL<br />
ABBOTT IRELAND DIAGNOSTICS<br />
20101603140 12/07/2010 AXSYM Free T4 standard Calibrators DIVISION, LONGFORD-IRELAND<br />
AKL<br />
10202011656 07/07/2010<br />
PT. DIPA PUSPA LABSAINS,<br />
Jakarta<br />
PT. ABBOTT INDONESIA<br />
REMEL Phytohaemagglutinin (PHA)<br />
Reagent REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20207011579 05/07/2010 CoaguCheck XS PT Test ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20306501734 21/05/2010<br />
AKL<br />
20306502198 21/05/2010<br />
AKL<br />
20306500916 21/05/2010<br />
ABBOTT AXSYM System CA 15-3S<br />
peciment Diluent<br />
ABBOTT AXSYM CA 15-3 Reagent<br />
Pack<br />
ABBOTT AXSYM Free and Total<br />
PSA Speciment Diluent<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10208602452 06/08/2010 MEDONIC M - Series Lyse BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20205602454 06/05/2010<br />
AGGRAM Advanced Modular<br />
System and Accessories<br />
HELENA LABORATORIES, USA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20209011126 14/05/2010 MiniCollect Tube K3EDTA GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20209011128 14/05/2010 VACUETTE Glucose Tubes GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20305011031 06/05/2010<br />
QUANTIA Ferritin / Myoglobin / IgE<br />
Control<br />
BIOKIT S.A, SPAIN untuk ABBOTT<br />
LABORATORIES., USA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101602115 06/05/2010 ELECSYS Prolactin II ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101301092 27/05/2010 ARCHITECT Folate Calibrator<br />
AKL<br />
20101301091 27/05/2010 ARCHITECT Folate Reagent Kit<br />
AKL<br />
20101301110 07/07/2010 ARCHITECT Folate Manual Diluent<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD - IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10302602098 14/05/2010 OXOID Manitol Salt Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302602100 14/05/2010<br />
AKL<br />
OXOID Eosin Methylene Blue Agar<br />
(Modified) Levine OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
20301011161 21/05/2010 OXOID Mueller Hinton Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302602094 14/05/2010 OXOID Peptone Water OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101602011 30/03/2010 SYSMEX TG.KL.R1.R2<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10305602728 02/07/2010 DIALAB APO A1 DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
10303602035 06/05/2010 OXOID Dry Spot E.Coli 0157 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303602034 16/08/2010 OXOID Salmonella Latex OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101011214 27/05/2010 ARCHITECT Folate Controls<br />
AKL<br />
10208011213 27/05/2010 ARCHITECT Folate Lysis Reagent<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20303605038 03/08/2010 OXOID VDRL Test Cards OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303601942 06/05/2010<br />
PULSE Febrile Antigen O-C<br />
Paratyphi<br />
PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20303601941 06/05/2010 PULSE Febrile Antigen H Typi PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20303601938 06/05/2010<br />
AKL<br />
20303601936 06/05/2010<br />
AKL<br />
20303601937 06/05/2010<br />
AKL<br />
20303601934 06/05/2010<br />
AKL<br />
20303601935 06/05/2010<br />
AKL<br />
20101601933 06/05/2010<br />
AKL<br />
20101601939 06/05/2010<br />
AKL<br />
20101601940 06/05/2010<br />
AKL<br />
10302601926 06/05/2010<br />
PULSE Febrile Antigen H-C<br />
Paratyphi<br />
PULSE Febrile Antigen H-A<br />
Paratyphi<br />
PULSE Febrile Antigen H-B<br />
Paratyphi<br />
PULSE Febrile Antigen O-D<br />
Paratyphi<br />
PULSE Febrile Antigen O-B<br />
Paratyphi<br />
PULSE 3P Parameter Reagent<br />
Strips<br />
PULSE 1 G Parameter Reagent<br />
Strips<br />
PULSE 10 L Parameter Reagent<br />
Strips<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
OXOID Brilliant Green Bile (2%)<br />
Broth OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601923 06/05/2010 OXOID Brain Heart Infusion OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601921 14/05/2010 OXOID Nutrient Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601919 14/05/2010<br />
AKL<br />
20101012024 09/08/2010 ARCHITECT BNP Calibrator<br />
AKL<br />
20101012025 09/08/2010 ARCHITECT BNP Reagent<br />
AKL<br />
10101603145 09/08/2010 AXSYM Ferritin Control<br />
OXOID Tryptone Soya Broth - Soy<br />
bean Casein Digest Medium OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES., USA<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES., USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD-IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20301012001 09/08/2010 OXOID Gentamycin CN 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101012023 09/08/2010 ARCHITECT BNP Control<br />
AKL<br />
20209601840 02/07/2010<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES., USA<br />
PT. ABBOTT INDONESIA<br />
DIALAB Anti D ( IgM/IgG )<br />
Monoclonal DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
20209601843 02/07/2010 DIALAB Anti AB Monoclonal DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101012472 03/09/2010 VACUPRO ETDA K2<br />
AKL<br />
10302012028 09/08/2010<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
PT. ANTAR MITRA SEMBADA<br />
MICROBACT Reagent Indole -<br />
Kovacs OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20209601841 02/07/2010 DIALAB Anti A Monoclonal DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
20102603827 25/10/2010 ARKRAY Pocketchem UA ARKRAY GLOBAL BUSSINES INC., JAPAN<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20302601844 06/05/2010 OXOID Rifampicin RD5 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601848 06/05/2010 OXOID Mecillinam MEL 25 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20605504169 06/05/2010 AXSYM Myoglobin Reagent Pack<br />
AKL<br />
20101601805 06/05/2010<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
ACTH Calset Elecsys and Cobas e<br />
Analyzer ROCHE DIAGNOSTIC GmbH, GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20101601803 06/05/2010 ACTH Elecsys ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10101601804 06/05/2010<br />
ELECSYS PreciControl and Cobas e<br />
Analyzer ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20301601774 06/05/2010 OXOID NYSTATIN NS-100 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10301601775 06/05/2010<br />
OXOID Wilkins - Chalgren Anaerobe<br />
Agar OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302011162 21/05/2010 OXOID CO2 GEN OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305601675 06/05/2010 COBAS INTEGRA Ferr T Standard ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10305601623 06/05/2010 ROCHE Cardiac Control ProBNP ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101601679 06/05/2010 ROCHE System C.f.a.s. PAC ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101601678 06/05/2010 ROCHE C.f.a.s. LP (a) ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20206601663 30/03/2010<br />
ACON FOB One Step Fecal Occult<br />
Blood Test Device<br />
ACON BIOTECH (HANGZHOU) CO., CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20306601660 30/03/2010<br />
AKL<br />
20306601664 30/03/2010<br />
ACON PSA Prostat Specific Antigen<br />
Semi Quantitative RapidTest Device<br />
ACON CEA (Carcino Embryonic<br />
Antigen) Rapid Test Device<br />
ACON BIOTECH (HANGZHOU) CO., CHINA<br />
ACON BIOTECH (HANGZHOU) CO., CHINA<br />
AKL<br />
10102601665 30/03/2010 MISSION U 120 Urine Analyzer ACON BIOTECH (HANGZHOU) CO., CHINA<br />
AKL<br />
20205601567 30/03/2010<br />
AKL<br />
20205601558 30/03/2010<br />
SYSMEX Automated Hematology<br />
AnalyzerXS + Accessories<br />
SYSMEX Automated Hematology<br />
Analyzer XS series + Accessories<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20209011127 14/05/2010 VACUETTE Heparin Tubes GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20209011129 14/05/2010 VACUETTE Serum Separator Tubes GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20303012656 21/09/2010<br />
ViroSeq HIV-1 Genotyping System<br />
v2.0 Pack 1<br />
CELERA CORPORATION., USA melalui<br />
ABBOTT MOLECULAR INC., USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
20303013083 01/11/2010 ADVANTAGE malaria Card J. MITRA CO. LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
10102013078 01/11/2010 COMBI SCAN 500<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
10101010097 05/02/2010 AXSYM LH Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20305601504 05/11/2010 CORE HBsAg CORE DIAGNOSTICS LIMITED., UK<br />
AKL<br />
20101601512 06/05/2010<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
COBAS INTEGRA Amylase EPS2<br />
(AMYL 2) ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20305601498 30/03/2010<br />
ACON Dengue IgG/IgM Rapid Test<br />
Device (WB, Serum, Plasma)<br />
ACON BIOTECH (HANGZHOU) CO., CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10302601520 06/05/2010 OXOID AFPA Base OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302605029 06/08/2010 OXOID Liver Digest Neutralised OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601517 06/05/2010 OXOID Stuart Transport Medium OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601516 06/05/2010 OXOID Desoxycholate Citrate Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301011270 11/06/2010 OXOID Sulpha Methoxazole RL 100 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20302011322 14/06/2010<br />
OXOID Ampicillin Selective<br />
Supplement OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601488 30/04/2010 OXOID Cefoperazone scf 105 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601478 30/04/2010 OXOID Cefoxitin - Fox 30 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301601480 24/03/2010 OXOID Colistin Sulphate - CT10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601487 30/04/2010 OXOID Cefepime FEP 30 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301501479 30/04/2010 OXOID Sulphamet/Trimethop SX 25 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301601457 30/04/2010 OXOID Bacitracin Single OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303601459 30/04/2010 OXOID Streptoccal Grouping Kit OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10201601455 06/05/2010<br />
AKL<br />
20101601345 30/03/2010<br />
AKL<br />
20101601343 30/03/2010<br />
AKL<br />
20101601342 30/03/2010<br />
AKL<br />
20101601340 30/03/2010<br />
OXOID Basic Fuchsin - Technical<br />
Dye OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
MISSION Urinalysis Reagent Strip<br />
(10 Parameter) ACON BIOTECH (HANGZHOU) CO., CHINA<br />
MISSION Urinalysis Reagent Strip<br />
Glukosa (1 Parameter)<br />
ACON BIOTECH (HANGZHOU) CO., CHINA<br />
MISSION Urinalysis Reagent Strip (3<br />
Parameter)<br />
ACON BIOTECH (HANGZHOU) CO., CHINA<br />
MISSION Urinalysis Reagent Strip<br />
(11 Parameter) ACON BIOTECH (HANGZHOU) CO., CHINA<br />
AKL<br />
10101600684 06/05/2010 ARCHITECT Total B-hCG<br />
AKL<br />
20101600680 06/05/2010 ARCHITECT Free T3 Reagent Kit<br />
AKL<br />
10101600683 06/05/2010<br />
AKL<br />
20306010675 03/05/2010<br />
AKL<br />
20306010676 03/05/2010<br />
AKL<br />
20306010678 03/05/2010<br />
AKL<br />
20306010679 03/05/2010<br />
AKL<br />
20306010677 03/05/2010<br />
AKL<br />
20306010674 03/05/2010<br />
AKL<br />
20305600830 24/03/2010<br />
ABBOTT Architect Prolactin Reagent<br />
Kit<br />
ABBOTT AXSYM System Free PSA<br />
Controls<br />
ABBOTT AXSYM System Total PSA<br />
Standard Calibrator<br />
ABBOTT AXSYM System AFP<br />
Controls<br />
ABBOTT AXSYM System AFP<br />
Standard Calibrator<br />
ABBOTT AXSYM System total PSA<br />
Controls<br />
ABBOTT AXSYM System CA 15-3<br />
Standard Calibrators<br />
SP-NANBASE C-96 (TMB) EIA Anti<br />
HCV<br />
AKL<br />
20305601626 24/03/2010 ANTICORASE MB-96 (TMB) EIA<br />
AKL<br />
20305600829 24/03/2010 ANTISURASE B-96 (TMB) EIA<br />
AKL<br />
20305600825 24/03/2010 SURASE B-96 (TMB) EIA<br />
AKL<br />
20305600822 24/03/2010 EASE BN-96 (TMB) EIA<br />
AKL<br />
20305600819 24/03/2010 ANTICORASE B-96 (TMB) EIA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101601704 06/05/2010 ARCHITECT LH Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101601638 06/05/2010<br />
ARCHITECT Testosterone<br />
Calibrator<br />
AKL<br />
10101601701 06/05/2010 ARCHITECT LH Reagent Kit<br />
AKL<br />
10101601700 06/05/2010 ARCHITECT LH Control<br />
AKL<br />
10101601637 06/05/2010<br />
AKL<br />
20306600651 03/05/2010<br />
AKL<br />
10302601306 06/05/2010<br />
ABBOTT ARCHITECT Testosterone<br />
Controls<br />
ABBOTT Axsym System CA 15-3<br />
Controls<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
OXOID Listeria Secondary<br />
Enrichment Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601305 06/05/2010 OXOID SPUTASOL (Liquid) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601304 30/04/2010<br />
AKL<br />
10302601303 06/05/2010<br />
AKL<br />
10302601301 05/02/2010<br />
PDF Creator - PDF4Free v3.0<br />
OXOID CHROMOGENIC CANDIDA<br />
SELECTIVE SUPPLEMENT OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID CAMPYLOBACTER<br />
SELECTIVE SUPPLEMENT OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Bordetella Selective<br />
Suplement OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
http://www.pdf4free.com
AKL<br />
10302601297 14/05/2010<br />
OXOID Helicobacter PylorySelective<br />
Supplement (IDENT) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302011325 14/06/2010 OXOID M17 Broth OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303010121 05/02/2010<br />
AKL<br />
20301010050 02/02/2010<br />
OXOID VTEC-RPLA Toxin Detection<br />
Kits OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Haemophilus Testing<br />
Medium OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601296 05/02/2010 OXOID MALT Extract Broth OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601276 06/05/2010 OXOID Mal Extract Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20306600257 08/03/2010<br />
ABBOTT ARCHITECT CEA<br />
Controls<br />
AKL<br />
20306600965 24/03/2010 ARCHITECT CA 19-9XR Control<br />
AKL<br />
20306600964 24/03/2010<br />
AKL<br />
20303600653 03/05/2010<br />
AKL<br />
20303600657 03/05/2010<br />
AKL<br />
20303600656 03/05/2010<br />
ARCHITECT CA 19-9XR Reagent<br />
Kit<br />
ABBOTT Architect Syphilis TP<br />
Reagent Kit<br />
ABBOTT Architect Syphilis TP<br />
Calibrator<br />
ABBOTT Architect Syphilis TP<br />
Control<br />
AKL<br />
20306600966 24/03/2010 ARCHITECT CA 19-9XR Calibrator<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
FUJIREBIO DIAGNOSTICS INC., USA for<br />
ABBOTT LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTICS INC., USA for<br />
ABBOTT LABORATORIES, USA<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT JAPAN CO. LTD., JAPAN<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT JAPAN CO. LTD., JAPAN<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT JAPAN CO. LTD., JAPAN<br />
FUJIREBIO DIAGNOSTICS INC., USA for<br />
ABBOTT LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101011402 25/06/2010 ABBOTT Cholesterol ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101011576 05/07/2010 Speedytest -Pregnancy Test Casset IND DIAGNOSTICS INC ., Canada PT. LANIROS DIAN PHARMA<br />
AKL<br />
30305011644 05/07/2010<br />
AKL<br />
20306600256 08/03/2010<br />
AKL<br />
20305010316 08/03/2010<br />
AKL<br />
20306010319 08/03/2010<br />
BIOMERIEUX Vironostika HIV<br />
Ag/Ab<br />
ABBOTT ARCHITECT Free PSA<br />
Reagent Kit<br />
ARTHITECT Stat Troponin-I<br />
Controls<br />
ABBOTT ARCHITECT AFP<br />
Reagent Kit<br />
AKL<br />
20305600251 05/02/2010 ARCHITEC Total PSA Controls<br />
AKL<br />
20306600253 05/02/2010 ARCHITECT Total PSA<br />
AKL<br />
20305600252 05/02/2010 architect tOTAL psa cALIBRATORS<br />
AKL<br />
20306600259 08/03/2010<br />
AKL<br />
20305501171 08/03/2010<br />
AKL<br />
20306600255 08/03/2010<br />
AKL<br />
20205010098 05/02/2010<br />
AKL<br />
20103505119 30/03/2010<br />
AKL<br />
20103505122 30/03/2010<br />
ABBOTT ARCHITECT CEA<br />
Reagent Kit<br />
ARCHITECT Stat Myoglobin<br />
Controls<br />
ABBOTT ARCHITECT Free PSA<br />
Controls<br />
SHANGHAI BIOMERIEUX BIO-<br />
ENGINEERING CO.LTD., China untuk<br />
BIOMERIEUX S.A., France<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
ABBOTT Cell-Dyn 1700 System<br />
Lytic Agent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
One Step Amphetamin Rapi<br />
CardInsta Test<br />
One Step THC (Marijuana) Rapi<br />
CardInsta Test<br />
AKL<br />
20101505120 30/03/2010 URS 10 Parameter<br />
AKL<br />
20103505118 30/03/2010<br />
One Step Opiate/Her/Mor RapiCard<br />
Insta Test<br />
AKL<br />
20101505117 30/03/2010 URS 11 parameter<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101505130 06/05/2010 TECO URS-1G TECO DIAGNOSTIC, U.S.A PT. RIOCA MEDICA<br />
AKL<br />
20208505040 30/03/2010 ABX Erytrol HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208505035 30/03/2010 ABX Minipack LMG HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101505127 06/05/2010 TECO URS-10 TECO DIAGNOSTIC, U.S.A PT. RIOCA MEDICA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20205504984 30/03/2010 ABX PENTRA DF 120 SPS HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20205504986 30/03/2010 ABX PENTRA DX 120 SPS HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101504959 05/11/2010<br />
AKL<br />
20101504960 05/11/2010<br />
AKL<br />
20303504941 30/06/2010<br />
AKL<br />
10302504935 16/08/2010<br />
AKL<br />
20301504876 29/04/2010<br />
AKL<br />
20101504771 06/05/2010<br />
MEDISMART Blood Glucose Test<br />
Strip LOBECK MEDICAL AG., SWITZERLAND PT. GRAHA ISMAYA LTD<br />
MEDISMART Blood Glucose<br />
Monitoring System LOBECK MEDICAL AG., SWITZERLAND PT. GRAHA ISMAYA LTD<br />
SPAN Stained Salmonella S.<br />
Paratyphi C (O) SPAN DIAGNOSTICS Ltd, India PT. SETIA ANUGERAH MEDIKA<br />
OXOID Food Borne Pathogens<br />
Monograph 5<br />
BD BBL Sensi Disc Levofloxacin 5<br />
ug<br />
ARCHITECT Total T3 Manual<br />
Diluent<br />
AKL<br />
20101504770 06/05/2010 ARCHITECT TSH Reagent Kit<br />
AKL<br />
20306600254 08/03/2010<br />
AKL<br />
20306010315 08/03/2010<br />
AKL<br />
20306010313 08/03/2010<br />
AKL<br />
20306010314 08/03/2010<br />
ABBOTT ARCHITECT Free PSA<br />
Calibrators<br />
ABBOTT ARTHITECT AFP Manual<br />
Diluent<br />
ABBOTT ARCHITECT AFP<br />
Controls<br />
ABBOTT ARTHITECT AFP<br />
Calibrators<br />
AKL<br />
10208010261 18/02/2010 IL TEST Referril G Diluent<br />
OXOID LIMITED., ENGLAND<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. MENDJANGAN<br />
AKL<br />
20205504644 06/05/2010 ABX Pentra DX 120 HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305504631 06/05/2010<br />
InstaTest One Step HBsAg Rapi<br />
Card<br />
DIAGNOSTIC AUTOMATION / CORTEZ<br />
DIAGNOSTIC INC., USA<br />
AKL<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
20305504632 06/05/2010 Insta Test One Step HBsAg RapiDIP DIAGNOSTIC INC., USA<br />
AKL<br />
20305504630 06/05/2010<br />
AKL<br />
20305504629 06/05/2010<br />
InstaTest One Step HBsAb<br />
RapiCard<br />
Insta Test One Step HBsAb Rapid<br />
Dip<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10101504380 06/05/2010 SEBIA Hydragel 2 IF SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20103504333 28/12/2010<br />
NARCOTEST 5 in 1 (Amphetamine,<br />
Benzodiazepine, Opiates,<br />
Methamphetamine, THC)<br />
AKL<br />
20103504332 28/12/2010 NARCOTEST 5 Cocaine<br />
AKL<br />
10101504310 28/12/2010 Ovutest<br />
AKL<br />
20103504306 28/12/2010<br />
Narcotest 2 Opiates (Morfin, Heroin,<br />
Putauw)<br />
AKL<br />
20103504308 28/12/2010 Narcotest 1 (Methamphetamine)<br />
AKL<br />
20305504169 06/05/2010 AXSYM Myoglobin Reagent Pack<br />
AKL<br />
20305504167 06/05/2010 AXSYM Myoglobin Calibrators<br />
AKL<br />
20305504166 06/05/2010 AXSYM Myoglobin Controls<br />
AKL<br />
20305504168 06/05/2010 Abbott Axsym Ferritin Reagent Pack<br />
AKL<br />
20101010099 05/02/2010<br />
AKL<br />
20303502998 29/04/2010<br />
AKL<br />
20303502997 29/04/2010<br />
AKL<br />
20303502994 29/04/2010<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGPORE<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGPORE<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGPORE<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGPORE<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGPORE<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. DANPAC PHARMA<br />
PT. DANPAC PHARMA<br />
PT. DANPAC PHARMA<br />
PT. DANPAC PHARMA<br />
PT. DANPAC PHARMA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AXSYM Cylosporine Reagent<br />
Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
CDI Febrile Antigen Check<br />
Paratyphoid C Somatic<br />
CDI Febrile Antigen Check<br />
Paratyphoid A Flagellar<br />
CDI Febrile Antigen Check<br />
Paratyphoid B Somatic<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303502995 29/04/2010<br />
AKL<br />
20303502996 29/04/2010<br />
AKL<br />
20303502993 29/04/2010<br />
AKL<br />
20303502992 29/04/2010<br />
AKL<br />
20101502931 05/02/2010 BIO-CAL E<br />
AKL<br />
20303502847 06/05/2010<br />
AKL<br />
20305501174 08/03/2010<br />
AKL<br />
20305010300 08/03/2010<br />
AKL<br />
20303502991 29/04/2010<br />
AKL<br />
20305010294 08/03/2010<br />
CDI Febrile Antigen Check Typhoid<br />
H Flagellar<br />
CDI Febrile Antigen Check<br />
Paratyphoid O Somatic<br />
CDI Febrile Antigen Check<br />
Paratyphoid O Somatic<br />
CDI Febrile Antigen Check<br />
Paratyphoid B Flagellar<br />
AMPLICOR Amplilute Liquid Media<br />
Extraction Kit (Extrn)<br />
ARCHITECT Stat Myoglobin<br />
calibrators<br />
ABBOTT MUREX Syfacard-R<br />
VD32/33<br />
CDI Febrile Antigen Check<br />
Paratyphoid C Flagellar<br />
ARCHITECT Stat Troponin-I<br />
Reagent Kit<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
MUREX BIOTECH LTD., ENGLAND untuk<br />
ABBOTT LABORATORIES., USA<br />
CAL-TECH DIAGNOSTICS INC., USA<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. BAVARIA COMBININDO<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101502342 01/02/2010 TECO Tecal N<br />
AKL<br />
20306502197 06/05/2010<br />
AKL<br />
20306502196 06/05/2010<br />
AKL<br />
20101502199 06/05/2010<br />
AKL<br />
10208010120 05/02/2010<br />
ABBOTT Axsym CA 125 Reagent<br />
Pack<br />
ABBOTT Axsym CA 125 Speciment<br />
Diluent<br />
ABBOTT Axsym Free T4 Reagent<br />
Pack<br />
AKL<br />
20102011149 21/05/2010 GEM PREMIER 3000<br />
AKL<br />
20102010525 06/04/2010 ILAB 300 Plus<br />
AKL<br />
10204501590 06/05/2010<br />
AKL<br />
10208501596 06/05/2010<br />
AKL<br />
10208501586 06/05/2010<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTS + TRADING GmbH., GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 1800 CN-Free<br />
Diff Lyse ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Solution 2 (Ion<br />
Capture)<br />
ABBOTT AXSYM Solution 3 Matrix<br />
Cell Wash<br />
ABBOTT AXSYM Solution 4 (Line<br />
Diluent)<br />
AKL<br />
10204501589 06/05/2010 ABBOTT AXSYM Solution 1 (MUP)<br />
AKL<br />
10302010562 12/04/2010 BBL TAXO Bacitracin<br />
AKL<br />
20301501205 12/04/2010 BD BBL CEFINASE<br />
AKL<br />
20306501182 21/05/2010<br />
ABBOTT AXSYM System Free PSA<br />
Reagent Pack<br />
AKL<br />
10101010295 08/03/2010 ARTHITECT Stat CK-MB Control<br />
AKL<br />
20306600258 08/03/2010<br />
AKL<br />
20101011153 21/05/2010<br />
ABBOTT ARCHITECT CEA<br />
Calibrators<br />
ABBOTT AXSYM System Troponin-<br />
1 ADV Reagent Pack<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101501101 21/05/2010 SYSMEX ALP-L.R1 & ALP-PL.R1 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20101501104 25/10/2010<br />
SYSMEX Ca. Color Solution (R2)<br />
CaColorSolution(R2)<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20101501102 21/05/2010 SYSMEX ALP-L.R2 & ALP-PL.R2 SYSMEX CORPORATION., JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101501097 06/05/2010<br />
AKL<br />
10101501098 06/05/2010<br />
AKL<br />
SYSMEX - GTP (3C) - L.R1 dan<br />
PL.R1<br />
SYSMEX - GTP (3C) - L.R2 dan<br />
PL.R2<br />
20305501462 30/04/2010 ARCHITECT HbsAg Manual Diluent<br />
PDF Creator - PDF4Free v3.0<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
http://www.pdf4free.com<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ABBOTT INDONESIA
AKL<br />
20305501460 06/05/2010 ARCHITECT HBsAg Controls<br />
AKL<br />
20305501457 06/05/2010<br />
AKL<br />
20305501459 06/05/2010<br />
AKL<br />
20305501458 06/05/2010<br />
ARCHITECT HBsAg Confirmatory<br />
V.1 Reagent Pack<br />
ARCHITECT HBsAg Confirmation<br />
V.1 Control<br />
ARCHITECT HbsAg Confirmatory<br />
V.1 Calibrator<br />
AKL<br />
20305501456 30/04/2010 ARCHITECT HBsAg Reagent Kit<br />
AKL<br />
20101501041 20/12/2010<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
CYBOW 10 reagent strip for<br />
urinalysis DFI Co, Ltd, Korea PT. RAJAWALI NUSINDO<br />
AKL<br />
10102501043 20/12/2010 CYBOW Reader 300 DFI Co, Ltd, Korea PT. RAJAWALI NUSINDO<br />
AKL<br />
20101501048 11/06/2010 QCA Potasium QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101501058 21/05/2010 QCA Seriscann Normal QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101011310 14/06/2010 QCA Creatinine QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101501049 11/06/2010 QCA Amylase BPS QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101501440 11/06/2010 QCA Urea Enzimatic QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
10101011215 27/05/2010 QCA LDL cholesterol Direct QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20207011311 14/06/2010 QCA Glycohemoglobin QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101010624 29/04/2010<br />
BD vacutainer K2 EDTA (K2E) 10.8<br />
mg Plus Blood Collection Tubes<br />
AKL<br />
20207500789 01/02/2010 TECO Teclot TT<br />
AKL<br />
20101010626 29/04/2010<br />
AKL<br />
20101010248 18/02/2010<br />
BD Vacutainer CPT Cell Preparation<br />
Tube with Sodium Cirate<br />
VACUTAINER Tabung Cirate 2.7 ml<br />
plus<br />
AKL<br />
20101500706 06/05/2010 AXSYM Free T3 Reagent Pack<br />
AKL<br />
10101500711 06/05/2010 ABBOTT AXSYM Free T3 Controls<br />
AKL<br />
20101500555 06/05/2010<br />
ABBOTT Axsym 3rd Generation<br />
TSH Reagent Pack<br />
AKL<br />
10208500504 05/02/2010 STROMATOLYZER iM (SIM-220A)<br />
AKL<br />
20101500409 25/10/2010 CRE-S-R1<br />
AKL<br />
10208010101 05/02/2010<br />
AKL<br />
20101500016 25/10/2010 AMY-L-R1 dan PL.R1<br />
AKL<br />
10101500021 06/05/2010<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTS + TRADING GmbH., GERMANY<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON CON & CO.,<br />
SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
ABBOTT CELL-DYN 1200 CN-Free<br />
Diff Lyse ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
SYSMEX HDL-C-Reagent KL (C-<br />
KL.R1 dan C-P.KL.R1)<br />
AKL<br />
20101500020 25/10/2010 AMY-L-R2 dan PL.R2<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD., JAPAN<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20303010100 05/02/2010 AXSYM Rubella IgM Index Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101402401 05/11/2010<br />
MEDI-TEST Combi 10 SGL (Blood,<br />
Urobilinogen, Bilirubin, Protein, Nitrite, MACHEREY-NAGEL GMBH & CO. KG.,<br />
Ketones, Glucose, pH, Dens GERMANY<br />
PT. MRK DIAGNOSTICS<br />
AKL<br />
10102010163 10/02/2010<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
DRAGONMED micropipet 8 Channel P.R CHINA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
TSH CalSet Elecsys and Cobas e<br />
20101401527 11/06/2010 Analyzers ROCHE DIAGNOSTIC GmbH., GERMANY PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20306010680 03/05/2010<br />
ABBOTT AXSYM System Free PSA<br />
Standard Calibrators<br />
AKL<br />
10206010161 10/02/2010 DRAGONMED ESR Analyzer<br />
AKL<br />
10102010162 10/02/2010<br />
AKL<br />
10102010164 10/02/2010<br />
DRAGONMED Midi Plus Pipetting<br />
Aid<br />
DRAGONMED Micropipet 12<br />
Channel<br />
AKL<br />
20301400676 24/03/2010 BD BBL Sensi Disc Ticarcillin 75 ug<br />
AKL<br />
20101907062 17/02/2010 BD Vacutainer Liq. K3 EDTA<br />
AKL<br />
10202010681 03/05/2010 FALCON Tissue Culture Flask<br />
AKL<br />
10209400714 06/05/2010 BD Vacutainer SST 6 ML<br />
AKL<br />
20301400666 29/04/2010<br />
BD BBL SENSI-DISC Cofataxime 30<br />
ug<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
P.R CHINA<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
P.R CHINA<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
P.R CHINA<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON AND<br />
COMPANY, SINGAPORE<br />
BECTON DICKINSON CO, USA melalui<br />
BECTON DICKINSON CO, SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON AND<br />
COMPANY, SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
AKL<br />
20301400658 24/03/2010<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
BD BBL Sensi Disc Axtreonam 30 ug LTD., SINGAPORE<br />
AKL<br />
10102010158 10/02/2010 DRAGONMED Micropipet Electronic<br />
AKL<br />
10102010159 10/02/2010<br />
AKL<br />
10102010160 10/02/2010<br />
AKL<br />
10305805616 05/11/2010<br />
DRAGON ONEMED Fixed<br />
micropipet<br />
DRAGON ONEMED Adjustable<br />
Micropipet<br />
SEKISUI Human Adiponectin ELISA<br />
Kit<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
P.R CHINA<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
P.R CHINA<br />
DRAGON MEDICAL (SHANGHAI) LIMITED,<br />
P.R CHINA<br />
SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
20209806048 28/12/2010 ID-DiaClon ABO/Rh for Newborns DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20101603980 28/12/2010 MULTISTIX 10 SG Reagent Strips<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
AKL<br />
20101012295 23/08/2010 AUTION MAX ARKRAY GLOBAL BUSSINES INC., JAPAN<br />
AKL<br />
20209700457 28/12/2010<br />
DIAMED ID-Centrifuge Micro Typing<br />
System<br />
DIAMED GMBH., SWITZERLAND<br />
PT. ABBOTT INDONESIA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGERAH PHARMINDO<br />
LESTARI<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20101600061 06/05/2010 DIRUI Urine Analyzer H - 100 DIRUI INDUSTRIAL CO. LTD., CHINA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20101600065 25/06/2010 DIRUI Uristik 1A DIRUI INDUSTRIAL CO. LTD., CHINA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
10101011405 25/06/2010<br />
AEROSET/ARCHITECT Cystatin C<br />
Control Set<br />
SENTINEL CH SpA, ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101600060 25/06/2010 DIRUI Uristik H10 DIRUI INDUSTRIAL CO. LTD., CHINA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20305012015 09/08/2010<br />
PROCLEIX Ultrio Plus HBV<br />
Discriminatory Probe Reagent<br />
AKL<br />
20101604957 03/08/2010 PTS CARDIOCHEK Analyzer<br />
AKL<br />
20101604953 03/08/2010<br />
PTS PANELS CHOL + GLU Panel<br />
Test Strip<br />
GEN PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
POLYMER TECHNOLOGY SYSTEMS<br />
INC,USA<br />
POLYMER TECHNOLOGY SYSTEMS<br />
INC,USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MITRAASA PRATAMA<br />
PT. MITRAASA PRATAMA<br />
AKL<br />
10302605030 09/08/2010 OXOID Agar Tablets OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101011939 06/08/2010<br />
AKL<br />
20101012027 09/08/2010 MULTICARE In Meter<br />
AKL<br />
20101603536 25/10/2010 ADVIA CENTAUR FrT4<br />
AKL<br />
20306500033 25/10/2010<br />
ABBOTT Aspartate<br />
Aminotransferase ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ADVIA CENTAUR AFP (Alpha<br />
Fetoprotein)<br />
BIOCHEMICAL SYSTEM INTERNATIONAL<br />
Srl., ITALY<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20205402731 11/06/2010 COBAS b 221 and Accessories ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101603879 28/12/2010 SIEMENS Calibrator O<br />
AKL<br />
20101603881 28/12/2010 SIEMENS Centaur Calibrator U<br />
AKL<br />
20101604412 28/12/2010<br />
ADVIA CENTAUR COR Ready<br />
Pack<br />
AKL<br />
20101603878 25/10/2010 SIEMENS Calibrator G<br />
AKL<br />
10207013770 28/12/2010 ZENIX Diluent<br />
AKL<br />
20101603841 25/10/2010 SIEMENS Calibrator X<br />
AKL<br />
10206600070 12/04/2010<br />
AKL<br />
10206600068 06/04/2010<br />
AKL<br />
20903600067 29/04/2010<br />
AKL<br />
10302602692 12/07/2010<br />
AKL<br />
20209011130 14/05/2010<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY, melalui<br />
SYSMEX ASIA PASIFIC Pte, Ltd., SINGA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SIEMENS INDONESIA<br />
BD vacutainer Blood Collection Set<br />
25 G BECTON DICKINSON & COMPANY, USA PT. ANUGRAH ARGON MEDICA<br />
BD Vacutainer Blood Collection Set<br />
23 G BECTON DICKINSON & CO, USA PT. ANUGRAH ARGON MEDICA<br />
BD Vacutainer Flashback Blood<br />
Collection Needle BECTON DICKINSON & CO., SINGAPORE PT. ANUGRAH ARGON MEDICA<br />
REMEL Lowenstein - Jensen<br />
Medium REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
VACUETTE Evacuated Blood<br />
Collection System GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
10206010636 30/04/2010 VACUETTE ESR Pipette GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
11603010634 30/04/2010 GREINER Mini Collect Lancet GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20101010637 30/04/2010 VACUETTE Coagulation Tubes GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20101010635 30/04/2010 VACUETTE Serum Tubes GREINER BIO-ONE GmbH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
10101700262 28/12/2010 UF II Control<br />
AKL<br />
10302603961 16/08/2010<br />
BD BACTEC MGIT 960 System and<br />
Accessories<br />
AKL<br />
10102011401 25/06/2010 BIOTECH BT-260 Plus<br />
AKL<br />
20301604666 06/08/2010<br />
AKL<br />
10101700256 28/12/2010 UF II Pack - Sed<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
NANCHANG BIOTECHA&C BIOTECHNICAL<br />
INDUSTRY CO., CHINA<br />
PT. SYSMEX INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
OXOID Penicillin G Susceptibility<br />
Disc (P1.5, P2 dan P5) OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
SYSMEX CORPORATION, JAPAN atas<br />
penunjukan SYSMEX ASIA PACIFIC PTE.<br />
LTD., JAPAN<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20301010033 02/02/2010 ETEST VA Vancomycin AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103010093 05/02/2010 NAPSA CHECK 6 OPI LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103010094 05/02/2010 NAPSA CHECK 8 THC LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103010095 05/02/2010 NAPSA CHECK 1 AMP LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103010096 05/02/2010 NAPSA CHECK 4 MDMA LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103010102 05/02/2010 NAPSA CHECK 2 BZO LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20103010103 05/02/2010 NAPSA CHECK 5 MAMP LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
AKL<br />
20101010104 05/02/2010 Combo Stik 2GP DFI CO. LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
10101010105 05/02/2010<br />
AKL<br />
20101010106 05/02/2010<br />
LIQUICHECK Immunoassay Plus<br />
Control Trilevel<br />
ACCU-CHECK Inform II System and<br />
Accessories<br />
AKL<br />
10101010107 05/02/2010 DIASYS Phosphate FS<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES, SINGAPORE<br />
ROCHE DIAGNOSTIC LTD., SWITZERLAND<br />
untuk ROCHE DIAGNOSTIC GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
GERMANY<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ROCHE INDONESIA<br />
PT. MULTIREDJEKI KITA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101010108 05/02/2010 FLUITEST PHOS<br />
AKL<br />
20101010109 05/02/2010 ONE LAB Direct Creatinine Kit<br />
AKL<br />
10101010110 05/02/2010 ONE LAB LDL Cholesterol Kit<br />
AKL<br />
10101010111 05/02/2010 ONE LAB Cholesterol Kit<br />
AKL<br />
10101010112 05/02/2010 ONE LAB Direct HDL Cholesterol kit<br />
AKL<br />
10101010113 05/02/2010 ONE LAB Uric Acid Kit<br />
AKL<br />
10101010114 05/02/2010 ONE LAB HDL Cholesterol Kit<br />
AKL<br />
20101010115 05/02/2010 ONE LAB Direct Bilirubin Kit<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE., CHINA<br />
PT. BAVARIA COMBININDO<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20101010116 05/02/2010 Combo Stik G DFI CO. LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
20101010117 05/02/2010 Combo Stik 10M DFI CO. LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
20101010118 05/02/2010 Combo Stik 3 DFI CO. LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
20101010119 05/02/2010 Combo Stik 5 DFI CO. LTD., KOREA PT. SABA INDOMEDIKA<br />
AKL<br />
30305010124 05/02/2010 TRUGENE HIV-1 genotyping Kit<br />
AKL<br />
30305010125 05/02/2010<br />
VERSANT HIV-1 RNA 3.0 Assay<br />
(bDNA)<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301010034 02/02/2010 ETEST IX Cefixime AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010035 02/02/2010 ETEST TS Trim/Sulfa 1/19 AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010036 02/02/2010 ETEST GA Gatifloxacin AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010037 02/02/2010 ETEST TGC Tigecycline AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103010038 02/02/2010 NAPSA Test 1 AMP<br />
AKL<br />
20103010039 02/02/2010 NAPSA Test 8 THC<br />
AKL<br />
20103010040 02/02/2010 NAPSA Test 2 BZO<br />
AKL<br />
20103010041 02/02/2010 NAPSA Test 6 OPI<br />
AKL<br />
20103010042 02/02/2010 NAPSA Test 5 MAMP<br />
AKL<br />
20103010043 02/02/2010 NAPSA Test 4 MDMA<br />
AKL<br />
20301010044 02/02/2010<br />
AKL<br />
20301010045 02/02/2010<br />
AKL<br />
20301010046 02/02/2010<br />
AKL<br />
20301010047 02/02/2010<br />
AKL<br />
20301010048 02/02/2010<br />
AKL<br />
20301010049 02/02/2010<br />
BOSON BIOTECHNOLOGY CO. LTD., P.R.<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO. LTD., P.R.<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO. LTD., P.R.<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO. LTD., P.R.<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO. LTD., P.R.<br />
CHINA<br />
BOSON BIOTECHNOLOGY CO. LTD., P.R.<br />
CHINA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
PT. INDOMED BERKAT JAYA<br />
OXOID Metronidazol M.I.C.<br />
Evaluator Strips OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID gentamicin M.I.C. Evaluator<br />
Strips OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Ciprofloxacin M.I.C.<br />
Evaluator Strips OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID penicillin M.I.C. Evaluator<br />
Strips OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Imipenem M.I.C. Evaluator<br />
Strips OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Tetracycline M.I.C. Evaluator<br />
Strip OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301010052 02/02/2010 ETEST TLC Ticarcillin/CLAV AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010053 02/02/2010 ETEST EM Erythromycin AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010054 02/02/2010 ETEST TP Teicoplanin AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010055 02/02/2010 ETEST TC Tetracyclin AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010056 02/02/2010 ETEST ESBL TZ/TZL AB. BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10206010057 02/02/2010<br />
SEDIMAT 15 With Reading in 15<br />
Minutes LP ITALIANA SPA, ITALY PT. ELO KARSA UTAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101010059 02/02/2010 ONE LAB Alkaline Phosphatase Kit<br />
AKL<br />
10101010060 02/02/2010 ONE MED Baby Test<br />
AKL<br />
20305010061 02/02/2010 ONE MED Detecto HBs Antibody<br />
AKL<br />
20305010062 02/02/2010 ONE MED Detecto HBsAg<br />
AKL<br />
20101010063 02/02/2010 ONE MED Combi Test<br />
AKL<br />
20101010064 02/02/2010 ONE MED Gluco Protein Test<br />
AKL<br />
10101010065 02/02/2010 ONE LAB Direct LDL Cholesterol Kit<br />
AKL<br />
20101010066 02/02/2010 ONE MED Gluco Test<br />
AKL<br />
20101010067 02/02/2010<br />
ANDALAN Strip Uji Kehamilan<br />
Instan<br />
AKL<br />
20101010068 02/02/2010 ONE MED HCG Test<br />
AKL<br />
20101010069 02/02/2010 ONE LAB Total Bilirubin Kit<br />
AKL<br />
20101010070 02/02/2010 ONE LAB Albumin Kit<br />
AKL<br />
20101010071 02/02/2010 ONE LAB Total Protein Kit<br />
AKL<br />
10101010072 02/02/2010<br />
ONE LAB Alanine Aminotransferase<br />
Kit<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO. LTD., CHINA<br />
IND. DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO. LTD., CHINA<br />
IND. DIAGNOSTIC INC., CANADA melalui<br />
AIDE DIAGNOSTIC CO. LTD., CHINA<br />
GUILIN ZHONGHUI TECHNOLOGY CO.<br />
LTD., CHINA<br />
GUILIN ZHONGHUI TECHNOLOGY CO.<br />
LTD., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
GUILIN ZHONGHUI TECHNOLOGY CO.<br />
LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui AI<br />
DE DIAGNOSTIC CO. LTD., CHINA<br />
IND DIAGNOSTIC INC., CANADA melalui AI<br />
DE DIAGNOSTIC CO. LTD., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
AKL<br />
BIOSINO BIO-TECHNOLOGY AND<br />
10101010073 02/02/2010 ONE LAB y Glutamyl Transferase Kit SCIENCE INC., CHINA<br />
AKL<br />
10101010074 02/02/2010 ONE LAB Tryglicerides Kit<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20305602485 30/06/2010<br />
AKL<br />
10102012294 23/08/2010<br />
COBAS INTEGRA CRPHS C-<br />
Reactive Protein (latex) Jigh Sensitive ROCHE DIAGNOSTICS GmbH, GERMANY PT. ROCHE INDONESIA<br />
ACURA Manual Digital Reading<br />
Micropipettes 815 Fixed<br />
AKL<br />
20205010217 16/02/2010 XT 4000i and Accessories<br />
AKL<br />
20101010218 16/02/2010<br />
AKL<br />
20101010219 16/02/2010<br />
AKL<br />
20101010220 16/02/2010<br />
AKL<br />
20101010221 16/02/2010<br />
AKL<br />
30305010260 18/02/2010<br />
AKL<br />
10302603962 20/12/2010<br />
LABONACHECK Wing Blood<br />
Glucose Monitor<br />
LABONACHECK Wing Blood<br />
Glucose Strip<br />
BETACHEK G5 Blood Glucose<br />
Meter<br />
LABONACHECK Gluppy Blood<br />
Glucose Strip<br />
DETERMINE HIV-1/2 Ag/Ab Combo<br />
Test<br />
AKL<br />
20205010283 23/02/2010 IMMULITE 2000 ECP<br />
SOCOREX ISBA S.A., SWITZERLAND<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
HUBIT INC., KOREA<br />
HUBIT INC., KOREA<br />
NATIONAL DIAGNOSTIC PRODUCTS PTY.<br />
LTD., AUSTRALIA<br />
HUBIT INC., KOREA<br />
INVERNESS MEDICAL JAPAN CO. LTD.,<br />
JAPAN melalui INVERNESS MEDICAL<br />
PROFESSIONAL DIAGNOSTICS., UK<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. SYSMEX INDONESIA<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. ARYASA TEKAD PRATAMA<br />
BD Bactec MGIT 960 Supplement<br />
Kit BECTON DICKINSON & COMPANY, USA PT. ANUGRAH ARGON MEDICA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302604818 06/08/2010 OXOID Mycoplasma Supplement-G OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303011580 05/07/2010<br />
AKL<br />
10302604930 16/08/2010<br />
OnSite Leptospire lgG/lgM Combo<br />
Rapid Test<br />
CTK BIOTECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
OXOID Campylobacter Blood - Free<br />
Agar Base OXOID LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601267 05/02/2010 OXOID Mac Conkey Agar OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601262 05/02/2010 OXOID Thioglycollate Medium U.S.P OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601202 05/02/2010 OXOID Lactose Bacteriological OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101010126 05/02/2010 FLUITEST Fe-FZ<br />
AKL<br />
10302010122 05/02/2010<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
OXOID Sealing Clips for Anaerogen<br />
Compact OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302010123 05/02/2010 OXOID Trichomonas Medium Base OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303010051 02/02/2010 SLIDEX Strepto A, B, D BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010050 02/02/2010<br />
OXOID Haemophilus Testing<br />
Medium OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101010058 02/02/2010 BD VAcutainer Precision Glide BECTON DICKINSON & COMPANY., USA PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101010262 18/02/2010 IL TEST Referril G<br />
AKL<br />
20101010263 18/02/2010 IL TEST Referril HDL Colesterol<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
AKL<br />
20205010289 08/03/2010 HAEMONETICS ACD-A Solution HAEMONETICS CORPORATION, USA PT. TRANSMEDIC INDONESIA<br />
AKL<br />
10302010250 18/02/2010 OXOID C. Difficile Test Kit OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301010251 18/02/2010<br />
AKL<br />
20301010252 18/02/2010<br />
AKL<br />
20301010253 18/02/2010<br />
AKL<br />
20301010254 18/02/2010<br />
OXOID Amoxycillin/Clavulanic M.I.C<br />
Evaluator Strip OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Ampicillin M.I.C. Evaluator<br />
Strip OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Erythromycin M.I.C.<br />
Evaluator Strip OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Vancomycin M.I.C. Evaluator<br />
Strip OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302010255 18/02/2010 RAPID Staph Plus System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302010256 18/02/2010 RAPID SS/u System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10208010257 18/02/2010 DIMENSION Sample Diluent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA meklalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305010258 18/02/2010<br />
SIEMENS N-Antiserum to Human<br />
Transferrin<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA meklalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305010259 18/02/2010 SIEMENS N-Latex RF Kit<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA meklalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
AKL<br />
20101010264 18/02/2010 NOVA Calibrator Pack CCX NOVA BIOMEDICAL CORPORATION., USA<br />
AKL<br />
20101010265 01/02/2010 ONELAB Creatine Kinase Kit<br />
AKL<br />
20101010266 18/02/2010 ONELAB Acid Phosphatase Kit<br />
AKL<br />
20101010267 18/02/2010<br />
ONELAB Aspartate<br />
Aminotransferase Kit<br />
AKL<br />
20101010268 18/02/2010 ONELAB Glucose Kit<br />
AKL<br />
20101010269 18/02/2010 ONELAB Urea (BUN) kit<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
BIOSINO BIO-TECHNOLOGY AND<br />
SCIENCE INC., CHINA<br />
PT. SIEMENS INDONESIA<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20103801450 11/03/2010<br />
BIORAD TOX/See Drug Screen Test<br />
MTD<br />
ABON BIOPHARM CO., CHINA for BIORAD<br />
LABORATORIES LTD., FRANCE atas<br />
penunjukan dari BIORAD LABORATORI<br />
PT. DOS NI ROHA<br />
AKL<br />
20103801451 11/03/2010<br />
BIORAD TOX/See Drug Screen Test<br />
AMP<br />
AKL<br />
20101502863 08/03/2010 ARCHITECT Stat CK-MB Reagent<br />
AKL<br />
20101502871 08/03/2010<br />
AKL<br />
20305501175 08/03/2010<br />
ARCHITECT Stat CK-MB<br />
Calibrators<br />
ARCHITECT Stat Myoglobin<br />
Reagent Kit<br />
AKL<br />
30305600960 08/03/2010 ABBOTT MUREX HTLV I+II<br />
AKL<br />
20101502656 08/03/2010 ANALYTICON CK-NaC<br />
ABON BIOPHARM CO., CHINA for BIORAD<br />
LABORATORIES LTD., FRANCE atas<br />
penunjukan dari BIORAD LABORATORI<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES., USA<br />
MUREX BIOTECH LTD., ENGLAND untuk<br />
ABBOTT LABORATORIES., USA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
AKL<br />
20303010291 08/03/2010 FOKUS Malaria Pan/Pv/Pf Casette CORE DIAGNOSTICS LTD., UK<br />
PT. DOS NI ROHA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303010292 08/03/2010 Influenza A Rapid Test Kit PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
10102010293 08/03/2010 PROCLEIX Target Capture System<br />
AKL<br />
10204010298 08/03/2010 Probe Rinse ILAB 300 Plus<br />
AKL<br />
10101504748 08/03/2010 SERACHEM Control Level 1 & 2<br />
AKL<br />
10101504750 08/03/2010 IL TEST Chol<br />
AKL<br />
10101504724 08/03/2010 IL TEST Phosphorus<br />
AKL<br />
10101504729 08/03/2010 IL TEST LDL Cholesterol ILAB 300<br />
AKL<br />
20301010310 08/03/2010<br />
AKL<br />
10101010296 08/03/2010 B.R.A.H.M.S TM1 Kryptor Qc<br />
AKL<br />
10101010297 08/03/2010 B.R.A.H.M.S GM Kryptor Qc<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
INSTRUMENTATION LABORATORY S.P.A.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY S.P.A.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY S.P.A.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY S.P.A.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY S.P.A.,<br />
ITALY<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
MERCKOPLATE Mueller - Hinton<br />
Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
B.R.A.H.M.S AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
B.R.A.H.M.S AKTIENGESELLSCHAFT.,<br />
GERMANY<br />
PT. MULTI SARANA MEDIKA<br />
PT. MULTI SARANA MEDIKA<br />
AKL<br />
20102010309 08/03/2010 DIALAB Autolyzer DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101503014 08/03/2010 DIALAB HDL Cholesterol Calibrator DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10305010290 08/03/2010<br />
SIEMENS N - Antiserum to Human<br />
Prealbumin<br />
AKL<br />
10204010301 08/03/2010 DIMENSION IMT Probe Cleaner<br />
AKL<br />
10204010302 08/03/2010 DIMENSION Sample Probe Cleaner<br />
AKL<br />
10204010303 08/03/2010<br />
DIMENSION IMT/ISE Cleaning<br />
Solution A<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
AKL<br />
10204010304 08/03/2010<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIMENSION Reagent Probe Cleaner DIAGNOSTICS LIMITED., HONGKONG<br />
AKL<br />
20102010305 08/03/2010<br />
AKL<br />
10101010306 08/03/2010<br />
AKL<br />
10303010307 08/03/2010<br />
AKL<br />
20305010308 08/03/2010<br />
DIMENSION AR Clinical Chemistry<br />
and Accessories<br />
AHDL DIMENSION Automated HDL<br />
Cholesterol<br />
ENZYGNOST Anti-Measles<br />
Virus/IgG<br />
OPENGENE DNA Sequenzing<br />
System<br />
AKL<br />
20305010311 08/03/2010 SIEMENS N Latex Lp (a)<br />
AKL<br />
10101010312 08/03/2010 SIEMENS N/T Protein Control SL/H<br />
AKL<br />
20305010317 08/03/2010 SIEMENS N Latex sTfR<br />
AKL<br />
20101010318 08/03/2010 SIEMENS N Latex HCY<br />
AKL<br />
20101010299 08/03/2010<br />
LABONACHECK Gluppy Blood<br />
Glucose Monitor<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
HUBIT INC., KOREA<br />
AKL<br />
21106011801 16/07/2010 VITROLIFE G-2 V3 Plus VITROLIFE AB., SWEDEN<br />
AKL<br />
10102604593 20/08/2010<br />
Stat Fax 3200 Microplate Reader and<br />
Accessories<br />
AKL<br />
10302401239 06/05/2010 BD BBL IsoVitaleX Enrichment<br />
AKL<br />
20305012031 09/08/2010<br />
AKL<br />
20205603422 30/07/2010<br />
PROCLEIX Ultrio Plus Tigris<br />
Controls<br />
Coatron M2, 2 Channel<br />
Coagulometer and Accessories<br />
AWARENESS TECHNOLOGY INC., USA<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
GEN PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. SETIA ANUGERAH MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10204012014 09/08/2010 PROCLEIX Assay Fluid<br />
GEN PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10303605157 28/12/2010 CORIS Rota - Strip CORIS BIOCONCEPT., BELGIUM PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20305605152 20/12/2010 Microwell Elisa HBsAb<br />
AKL<br />
20101605156 28/12/2010 Microwell Elisa T4<br />
AKL<br />
20101605155 20/12/2010 Microwell Elisa T3<br />
AKL<br />
20101605154 28/12/2010 Microwell Elisa Free T4<br />
DIAGNOSTICS AUTOMATION / CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTICS AUTOMATION/CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTICS AUTOMATION / CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTICS AUTOMATION / CORTEZ<br />
DIAGNOSTICS INC., USA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10302601273 27/05/2010 T.C.B.S CHOLERA MEDIUM OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302601272 27/05/2010 OXOID Tetrathionate Broth Base OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303010455 30/03/2010 HEPAVASE A-96 (TMB) EIA<br />
AKL<br />
20303010456 30/03/2010 HEPAVASE MA-96 (TMB) EIA<br />
AKL<br />
10102010351 23/03/2010 BN II System and Accessories<br />
AKL<br />
10204010352 23/03/2010 N Suplementary Reaget/Precipitation<br />
AKL<br />
10102010353 23/03/2010<br />
BN ProSpec System and<br />
Accessories<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
GENERAL BIOLOGICALS CORP., TAIWAN -<br />
ROC<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101010354 23/03/2010 HDI Precision Ovulation Test<br />
RUNBIO BIOTECH CO. LTD., CHINA melalui<br />
MACROSERVE PTE. LTD., SINGAPORE<br />
PT. HARMONI DINAMIK<br />
INDONESIA<br />
AKL<br />
20101010355 23/03/2010 HDI Precision Pregnancy Test<br />
AKL<br />
20101010356 23/03/2010<br />
AKL<br />
20305010362 23/03/2010<br />
BD Vacutainer SST Tubes with<br />
Hemogard Closure<br />
RUNBIO BIOTECH CO. LTD., CHINA melalui<br />
MACROSERVE PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON AND<br />
COMPANY, SINGAPORE<br />
PT. HARMONI DINAMIK<br />
INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
COBAS Elecsys PreciControl<br />
ThyroAB ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20305010363 23/03/2010 COBAS Elecsys PreciControl HIV ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20305010364 23/03/2010<br />
AKL<br />
20205010365 23/03/2010<br />
AKL<br />
20205010366 23/03/2010<br />
AKL<br />
20205010367 23/03/2010<br />
AKL<br />
20205010368 23/03/2010<br />
AKL<br />
20205010369 23/03/2010<br />
AKL<br />
20205010370 23/03/2010<br />
AKL<br />
20205010371 23/03/2010<br />
AKL<br />
20205010372 23/03/2010<br />
COBAS TaqMan HCV Test v2.0 For<br />
Use with The High Pure System<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
ABBOTT CELL-DYN Sapphire<br />
CELL_DYN 4000 System WBC<br />
Reagent - Part A & B ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 3000, 3500<br />
and 3700 Systems Diluent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 3500 and 3700<br />
System Detergent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 1300 System<br />
Rapid Lyse ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN Sapphire<br />
CELL_DYN 4000 System CN - Free<br />
HGB ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 1700, 1800<br />
Systems Detergent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT Cell-Dyn 1700 System<br />
Lytic Agent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 3200 System<br />
Diluent/Sheath ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20205010373 23/03/2010<br />
AKL<br />
20205010374 23/03/2010<br />
AKL<br />
20205010375 23/03/2010<br />
ABBOTT CELL-DYN 3500 and 3700<br />
System CN - Free HGB/WIC Lyse ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 1700, 1800<br />
System Diluent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 3000, 3500<br />
and 3700 Systems Sheeth ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20205010376 23/03/2010<br />
AKL<br />
20205010377 23/03/2010<br />
AKL<br />
20205010378 23/03/2010<br />
AKL<br />
20205010404 29/03/2010<br />
AKL<br />
20101010439 30/03/2010 ADVIA Centaur CsA<br />
AKL<br />
20101010440 30/03/2010 ADVIA Centaur T4 Diluent<br />
ABBOTT CELL-DYN Sapphire CELL-<br />
DYN 4000 System Reticulocte ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 3200 System<br />
WBC Lyse ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 1200 System<br />
Diluent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT CELL-DYN 3000 System<br />
HGB Lyse ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302010441 30/03/2010 OXOID V - FACTOR DISCS OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301010442 30/03/2010 OXOID Tigecycline - TGC 15 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302010443 30/03/2010 OXOID Disc Dispenser MK III OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305010444 30/03/2010 ABX CRP 100 HORIBA ABX S.A.S., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20102010445 30/03/2010<br />
AKL<br />
10302010446 30/03/2010<br />
ALFA WASSERMAN Starlyte III<br />
Electrolyte Analyzer<br />
BBL CRYSTAL GP Gram Positive ID<br />
System<br />
AKL<br />
20209010452 30/03/2010 INTEC RhD Blood Grouping Kit<br />
AKL<br />
20209010453 30/03/2010<br />
INTEC ABO & RhD lood Grouping<br />
Kit<br />
AKL<br />
20209010454 30/03/2010 INTEC ABO Blood Grouping Kit<br />
ALFA WASSERMANN INC., USA melalui<br />
ALFA WASSERMANN BV. NETHERLAND<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. MARADA PHARMA MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20207600137 24/03/2010 COBAS HbA1c Hemolysis Reagent ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20305600135 24/03/2010 COBAS INTEGRA CRPT Control N ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20101600139 24/03/2010<br />
COBAS INTEGRA Serum Proteins<br />
T Standard ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
30305600409 24/03/2010 MUREX HIV Ag/Ab Combination MUREX BIOTECH LIMITED., ENGLAND PT. ABBOTT INDONESIA<br />
AKL<br />
20101010438 30/03/2010 ADVIA Centaur HCY<br />
AKL<br />
20101010475 31/03/2010<br />
AKL<br />
20306010495 31/03/2010<br />
ne Step Troponin I serum Rapicard<br />
Insta Test<br />
ACON AFT Alpha Fetoprotein Rapid<br />
Test Device<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
DIAGNOSTIC AUTOMATION/CORTEZ<br />
DIAGNOSTIC INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. MACROCITRA<br />
ACON BIOTECH (HANGZHOU) CO., CHINA ARDANASEJATI<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
10101010497 30/03/2010 ARKRAY Ammonia test Kit II ARKRAY GLOBAL BUSINESS INC., JAPAN<br />
AKL<br />
20101010496 30/03/2010 AUTION Stick 10 PA ARKRAY GLOBAL BUSINESS INC., JAPAN<br />
AKL<br />
10101011404 25/06/2010 ABBOTT Apoliprotein B ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101011403 25/06/2010 ABBOTT Triglyceride ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20208600369 25/06/2010<br />
LIQUICHECK Hematology-16<br />
Control Level 1 Low<br />
AKL<br />
20101010515 06/04/2010 ADVIAChemistry calibrator<br />
AKL<br />
10303010516 06/04/2010 N Latex ASL<br />
AKL<br />
10305010517 06/04/2010 N Antigen to Human RBP<br />
BIORAD LABORATORIES, USA melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCT GmbH., GERMANY melalui<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LIMITED<br />
SIEMENS HEALTHCRE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY melalui<br />
HEALTHCARE DIAGNOSTICS LIMITED.,<br />
HONGK<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10102010518 06/04/2010<br />
AKL<br />
20208010519 06/04/2010<br />
AKL<br />
10305010520 06/04/2010<br />
AKL<br />
20102010521 06/04/2010<br />
Startus CS STAT Fluorometic<br />
Analyzer and Accessories<br />
DCA System Hemoglobin A1c<br />
Control Kit<br />
N Antiserum to Human 1-Acid<br />
Glycoprotein<br />
Dimension RxL Max Clinical<br />
Chemistry System<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY melalui<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LIMITE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20208010522 06/04/2010 ABX Difftrol 2H HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20208010523 06/04/2010 ABX Difftrol 2N HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101010524 06/04/2010 AMOR HCG Test<br />
AKL<br />
20206600747 05/11/2010<br />
AKL<br />
20206600746 05/11/2010<br />
AKL<br />
20206600743 05/11/2010<br />
HELMER Platelet Incubator<br />
PC3200h<br />
HELMER Platelet Incubator<br />
PC2200h<br />
HELMER Platelet Incubator<br />
PC1200h<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., P.R. CHINA<br />
HELMER INC., USA<br />
HELMER INC., USA<br />
HELMER INC., USA<br />
AKL<br />
20206600742 05/11/2010 HELMER Platelet Incubator PC900h HELMER INC., USA<br />
AKL<br />
20206600744 05/11/2010 HELMER Platelet Agitator PF 96i HELMER INC., USA<br />
AKL<br />
20206600745 05/11/2010 HELMER Platelet Agitator PF 48i HELMER INC., USA<br />
AKL<br />
20305011863 30/07/2010<br />
AKL<br />
20301605034 06/08/2010<br />
PT. INDOMED BERKAT JAYA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
ARCHITECT Pepsinogen I<br />
Calibrator ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
OXOID Tetracyclin Susceptibility<br />
Disc (TE 5 dan TE 10) OXOID LIMITED, ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101604956 03/08/2010 PTS CARDIOCHEK P.A Analyzer<br />
AKL<br />
10302605042 16/08/2010<br />
POLYMER TECHNOLOGY SYSTEMS<br />
INC,USA<br />
PT. MITRAASA PRATAMA<br />
OXOID Gamma Irradiated Tryptone<br />
Soya Broth OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
POLYMER TECHNOLOGY SYSTEMS<br />
10101604950 03/08/2010 PTS PANELS Lipid Panel Test Strip INC,USA<br />
AKL<br />
20301401241 12/04/2010<br />
AKL<br />
20301010572 26/04/2010<br />
BD BBL SENSI DISC Netilmicin 30<br />
ug<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
PT. MITRAASA PRATAMA<br />
PT. ANUGRAH ARGON MEDICA<br />
VITEK 2 Compact 60 Configuration<br />
+ Accessories BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010569 26/04/2010 VITEK 2 YST Identification card BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010570 26/04/2010 VITEK 2 GN Identification Card BIOMERIEUX INC., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301010571 26/04/2010 VITEK 2 GP Identification card BIOMERIEUX INC., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20205010568 26/04/2010<br />
ZENIK - 244 Full-Auto Hematology<br />
Analyzer<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
AKL<br />
20301601195 30/04/2010 OXOID Metronidazole MT 25 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20205602739 06/05/2010<br />
MYTHIC Fully Automated 22 -<br />
Parameter Haematology Analyzer ORPHEE SA., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10302010614 29/04/2010 Bactident Oxidase MERCK KGaA, Germany PT. MERCK Tbk<br />
AKL<br />
20205010616 29/04/2010<br />
AKL<br />
10102010617 29/04/2010<br />
APACT 4004,4 Channel<br />
Aggregometer Analyzer and<br />
Accessories<br />
AKL<br />
20206010618 29/04/2010 TEClot APPTS - S<br />
AKL<br />
20208010619 29/04/2010 TEControl A Plus<br />
AKL<br />
20208010620 29/04/2010 TEControl A<br />
LABITEC ( LABOR BIOMEDICAL<br />
TECHNOLOGIES ) GmbH, Germany<br />
PT. SETIA ANUGERAH MEDIKA<br />
JOLLY 103T Analyzer and<br />
Accessories CRONY INSTRUMENTS SRL, Italy PT. SETIA ANUGERAH MEDIKA<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GmbH..germany<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GmbH..germany<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GmbH..germany<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208010621 29/04/2010<br />
AKL<br />
10208010622 29/04/2010<br />
ORPHEE Mythic 18 new Formula<br />
Diluent ORPHEE SA, Switzerland PT. INDOLABTEK DINAMIKA<br />
ORPHEE Lythic Reagent Cyanide<br />
Free ORPHEE SA, Switzerland PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20102010627 29/04/2010<br />
AKL<br />
10101010628 29/04/2010<br />
AKL<br />
20102010629 29/04/2010<br />
ALFA WASSERMAN ACE Clinical<br />
Chemistry Analyzer System<br />
ALFA WASSERMAN PLD 951 Semi<br />
automated Photometer<br />
ALFA WASSERMAN Starlyte V<br />
Electrolyte Analyzer<br />
ALFA WASSERMANN ANC, USA melalui AFA<br />
WASSERMANN BV, Netherlands<br />
ALFA WASSERMANN ANC, USA melalui AFA<br />
WASSERMANN BV, Netherlands<br />
ALFA WASSERMANN ANC, USA melalui AFA<br />
WASSERMANN BV, Netherlands<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
AKL<br />
20205010633 30/04/2010 ABX PENTRA 80 XL HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20103010632 30/04/2010<br />
ABX PENTRA C 200 and<br />
Accessories HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20301010638 30/04/2010 OXOID Metronidazole Disc OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20207010748 06/05/2010 TECHROM Protein C<br />
AKL<br />
20207010749 06/05/2010 TECLOT Protein S<br />
AKL<br />
20101010750 06/05/2010 ARCHITECT B12 Reagent Kit<br />
AKL<br />
10204010751 06/05/2010 AXSYM Probe Cleaning Solution<br />
AKL<br />
20101010752 06/05/2010 ARCHITECT B12 Calibrator<br />
AKL<br />
20101602116 06/05/2010<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
FISHER DIAGNOSTICS, USA untuk ABBOTT<br />
LABORATORIES DIAGNOSTICS DIVISION,<br />
USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
Prolactin II Calset Elecsys and<br />
Cobas e analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20301010630 30/04/2010 OXOID Sulpha Methoxazole RL 25 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302010615 29/04/2010 Anaerotest for Microbiology MERCK KgaA, Germany PT. MERCK Tbk<br />
AKL<br />
20305010828 06/05/2010<br />
AKL<br />
20305010829 06/05/2010<br />
AKL<br />
20305010830 06/05/2010<br />
AKL<br />
20305010831 06/05/2010<br />
ARCHITECT HBsAg Qualitative<br />
Confirmatory Reagent Kit<br />
ARCHITECT HBsAg Qualitative<br />
Calibrators<br />
ARCHITECT HBsAg Qualitative<br />
Reagent Kit<br />
ARCHITECT HBsAg Qualitative<br />
Confirmatory Manual Diluent<br />
AKL<br />
10101010832 06/05/2010 ARCHITECT MPO Controls<br />
AKL<br />
10103010833 06/05/2010 ARCHITECT B12 Controls<br />
AKL<br />
20101010834 06/05/2010 ARCHITECT MPO Calibrator<br />
AKL<br />
20305010835 06/05/2010 CRP Vario<br />
AKL<br />
20101010836 06/05/2010<br />
AKL<br />
10103010838 06/05/2010<br />
ARCHITECT Creatinine ( Enzymatic<br />
)<br />
ABBOTT Immunosuppresant-MCC<br />
Level 1,2 dan 3<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION, USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION, USA<br />
SENTINEL CH SpA, ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
SENTINEL CH, SPA., ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
BIO-RAD LABORATORIES, USA untuk<br />
ABBOTT LABORATORIES,USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20303010816 06/05/2010 ADVANTAGE P.f Malaria Card J.Mitra & CO.LTD, INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20303010817 06/05/2010 DENGUCHECK WB ZEPHYR BIOMEDICAL, India PT. ADIWARA WORLDWIDE<br />
AKL<br />
20101010821 06/05/2010<br />
BD VACUTAINER Buffered Sodium<br />
Citrate ( 9NC) Plus Blood Collection<br />
Tubes BECTON DICKINSON & CO, USA PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20303010818 06/05/2010 TUBEX TF IDL BIOTECH AB, SWEDEN<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
20101010824 06/05/2010 ACCU-CHECK PerformaTest Strip ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20305010825 06/05/2010<br />
THERASCREEN K-RAS Mutation<br />
Kit DXS DIAGNOSTIC LTD,UK PT. ROCHE INDONESIA<br />
AKL<br />
20101010826 06/05/2010 ACCU-CHECK Sensor Comfort ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305010820 06/05/2010<br />
AKL<br />
20101010791 06/05/2010<br />
AKL<br />
20101010792 06/05/2010<br />
PROLEIX HC + LUMINOMETER<br />
and Accessories<br />
CARE One Step Pregnency Test<br />
Cassette<br />
LAMSON hCG Pregnancy Test<br />
Midstream<br />
AKL<br />
20306011030 06/05/2010 ARCHITECT ProGRP Control<br />
AKL<br />
10101010852 06/05/2010<br />
AKL<br />
10101010853 06/05/2010<br />
AKL<br />
20101010854 06/05/2010<br />
AKL<br />
10101010837 06/05/2010<br />
ABBOTT AXSYM 3rd Generation<br />
TSH Controls<br />
ABBOTT AXSYM System Troponin-I<br />
ADV Controls<br />
ABBOTT AXSYM Free T3 Standart<br />
Calibrator<br />
ABBOT ARCHITECT Free T4 ( Free<br />
Thyroxine ) Control<br />
GEN-PROBE INCORPORATED ,USA untuk<br />
NOVARTIS VACCINES AN DIAGNOSTICS,<br />
INC, USA<br />
SHANGHAI HUAGUAN BIOCHIP CO.LTD.,<br />
China<br />
SHANGHAI HUAGUAN BIOCHIP CO.LTD.,<br />
China<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, ireland<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. PRIMA BUMI MANDIRI<br />
PT. PRIMA BUMI MANDIRI<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101010851 06/05/2010<br />
ABBOTT AXSYM System Troponin-<br />
1 ADV Standard Calibrator<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20303601844 06/05/2010 OXOID Rifampicin RD5 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305402880 06/05/2010 ETI-MAK-4 ( HbsAg EIA ) DIASORIN S.P.A., Italy<br />
AKL<br />
30305010819 06/05/2010 BIOTEST Anti HIV Tetra ELISA DIASORIN, Italy<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
AKL<br />
10305601706 06/05/2010 ROCHE Cardiac ProBNP ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20305100823 06/05/2010 ROCHE System sTfR Control Set ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10101010827 05/06/2010 REFLOTRON Precinorm HDL ROCHE DIAGNOSTICS GmbH ,Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101010797 06/05/2010<br />
CARE One Step Pregnency Test<br />
Cassette<br />
SHANGHAI HUAGUAN BIOCHIP CO.LTD.,<br />
China<br />
PT. PRIMA BUMI MANDIRI<br />
AKL<br />
20305010850 06/05/2010 ROCHE/HITACHI Preciset s TfR ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20205010889 06/05/2010 BIO SOLEA 4 BIOLABO SA, France<br />
AKL<br />
20102010888 06/05/2010 KENZA MAX Biochemistry Analyser BIOLABO SA, France<br />
AKL<br />
20205010887 06/05/2010 BIO SOLEA 2 BIOLABO SA, France<br />
AKL<br />
20101010877 06/05/2010<br />
ARCHITECT I Phenytoin Reagent<br />
Kit<br />
AKL<br />
20101010875 06/05/2010 ARCHITECT I MPO Reagent Kit<br />
DENKA SEIKEN CO., LTD., Japan untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION, USA<br />
FISHER SCIENTIFIC COMPANY, USA untuk<br />
ABBOTT LABORATORIES DIAGOSTICS<br />
DIVISION, USA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10304010886 06/05/2010 I-CHROMA Reader BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20207010885 06/05/2010 I-CHROMA HbA1C BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20305010884 06/05/2010 I CHROMA hsCRP BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20305010883 06/05/2010 I CHROMA Microalbumin BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20306010882 06/05/2010 I CHROMA PSA BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20205010881 06/05/2010 HEMOCHROMA Reader BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20207010880 06/05/2010 HEMOCHROMA Hemoglobin Test BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20305010879 06/05/2010 I CHROMA CRP BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20306010878 06/05/2010 I CHROMA AFP BODITECH MED INC., Republic of Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20301010872 06/05/2010 OXOID Sensitest Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102010870 06/05/2010 DIRUI Urine Analyzer H-50 DIRUI INDUSTRIAL CO. LTD., China PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
10201010871 06/05/2010 ADVIA Autoslide Methanol<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101010874 06/05/2010 THYROCHEK TSH Adult<br />
AKL<br />
20101010873 06/05/2010 THYROCHEK TSH NeoNatal<br />
AKL<br />
10102010613 29/04/2010<br />
AKL<br />
10102010612 29/04/2010<br />
SYNTHRON BIORESEARCH INC, USA<br />
Untuk SCREENING DEVICE CANADA INC,<br />
Canada<br />
SYNTHRON BIORESEARCH INC, USA<br />
Untuk SCREENING DEVICE CANADA INC,<br />
Canada<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
MERCKKOPLATE Candida Elective<br />
Agaracc To Nickerson MERCK KGaA, Germany PT. MERCK Tbk<br />
MERCKOPLATE Sabouraud - 2%<br />
Glucose Agar MERCK KGaA, Germany PT. MERCK Tbk<br />
AKL<br />
20306011032 06/05/2010 ARCHITECT ProGRP Calibrator<br />
AKL<br />
20306011033 06/05/2010 ARCHITECT ProGRP Reagent Kit<br />
AKL<br />
20305011021 06/05/2010<br />
AKL<br />
20305011022 06/05/2010<br />
Procleix HCV Discriminatory Probe<br />
Reagents<br />
Procleix HCV Discriminatory Probe<br />
Reagents<br />
AKL<br />
20305011023 06/05/2010 Procleix Assay Fluids<br />
AKL<br />
20305011024 06/05/2010 Procleix Ultrio Assay Calibrators<br />
AKL<br />
20305011025 06/05/2010 Procleix Ultrio Tigris Control<br />
AKL<br />
30305011029 06/05/2010<br />
PROCLEIX HIV-1 Discriminatory<br />
Probe Reagents<br />
AKL<br />
10101010991 06/05/2010 TRIAGE NGAL - Control Set<br />
AKL<br />
10101010992 06/05/2010 TRIAGE NGAL - Test<br />
AKL<br />
20306010989 06/05/2010<br />
AKL<br />
20306010990 06/05/2010<br />
AKL<br />
20101011027 06/05/2010<br />
AKL<br />
10208011028 06/05/2010<br />
CYTOACTIVE HPV L1 Screening<br />
Set<br />
CYTOACTIVE HPV L1 High Risk<br />
Set<br />
ADVIA Chemistry ISE Serum<br />
Standard Set<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
BIOSITE INC., USA untuk INVERNESS<br />
MEDICAL INOVATIONS INC., UK<br />
BIOSITE INC., USA untuk INVERNESS<br />
MEDICAL INOVATIONS INC., UK<br />
CYTOIMMUN DIAGNOSTICS GMBH.,<br />
GERMANY<br />
CYTOIMMUN DIAGNOSTICS GMBH.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEGA UTAMA MEDICA<br />
PT. MEGA UTAMA MEDICA<br />
PT. SIEMENS INDONESIA<br />
STROMATOLYZER - 4DL (FFD -<br />
200A & FFD - 220A)) SYSMEX CORPORATION PT. SYSMEX INDONESIA<br />
AKL<br />
10302605057 06/08/2010 OXOID Microbact Reagent VP I OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101604948 03/08/2010<br />
AKL<br />
10102011125 14/05/2010<br />
PTS PANELS LDL Cholesterol Test<br />
Strip<br />
DIMENSION XPAND Plus Clinical<br />
Chemistry System and Accessories<br />
POLYMER TECHNOLOGY SYSTEMS<br />
INC,USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
PT. MITRAASA PRATAMA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302601265 06/05/2010 OXOID Saburaud Dextrose Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20209600122 07/06/2010 KIRSCH Laboratory Refrigerator PHILIPP KIRSCH GMBH., GERMANY<br />
AKL<br />
10102603826 25/10/2010 ARKRAY Aution Eleven ARKRAY GLOBAL BUSINESS INC., JAPAN<br />
PT.PARMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20101011152 21/05/2010<br />
AKL<br />
10101011157 21/05/2010<br />
OPTIUM EXCEED Diabetes<br />
Monitoring System and Accessories ABBOTT DIABETES CARE INC, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Activated ALT ABBOTT LABORATIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101011156 21/05/2010 ABBOTT Clinical Chemistry A-AST ABBOTT LABORATIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101011155 21/05/2010<br />
AKL<br />
10204011154 21/05/2010<br />
MEDISENSE Glucose and Ketone<br />
Control Solution ABBOTT LABORATIES, USA PT. ABBOTT INDONESIA<br />
ARCHITECT Probe Conditioning<br />
Solution ABBOTT LABORATIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10302011226 27/05/2010 OXOID Brain Heart Infusion Broth OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302011227 27/05/2010 OXOID Brain Heart Infusion Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10201011143 21/05/2010 SHANDON EZ Double Cylofunel<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305011131 14/05/2010<br />
ACON HBsAG Hepatitis B Surface<br />
Antigen Rapid Test Device (Whole<br />
Blood/Serum/Plasma)<br />
ACON BIOTECH (HANGZHOU) CO, LTD,<br />
China<br />
AKL<br />
20303011137 21/05/2010 ONSITE Typhoid IgG/IgM Rapid CTK BIOTECH INC., USA<br />
AKL<br />
20203011138 21/05/2010<br />
ONSITE Pf/Pan Malaria Ag Rapid<br />
Test<br />
CTK BIOTECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10204011160 21/05/2010<br />
MERCK Phosphate Buffer Tablets<br />
acc. To Weise ph 6.4, 6.8.and 7.2 MERCK KgaA., Germany PT. MERCK Tbk<br />
AKL<br />
10201011159 21/05/2010 MERCK Hematognost Fe MERCK KgaA., Germany PT. MERCK Tbk<br />
AKL<br />
10201011158 21/05/2010<br />
MERCK Gram-Color Satining Set for<br />
Gram Staining MERCK KgaA., Germany PT. MERCK Tbk<br />
AKL<br />
20102011144 21/05/2010 SELECTRA PRO XS VITAL SCIENTIFIC, Netherlands PT. MRK DIAGNOSTICS<br />
AKL<br />
10101011151 21/05/2010<br />
Testosterone II Elecsys and Cobas e<br />
analyzers ROCHE DIAGNOSTICS GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20208011148 21/05/2010 e-CHECK XE & XS SYSMEX CORPORATION, Japan PT. SYSMEX INDONESIA<br />
AKL<br />
20301600533 27/05/2010 OXOID Flumequine OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10208601104 21/05/2010 LABNOVATION 4DS-Lyser<br />
AKL<br />
10208601103 21/05/2010 LABNOVATION FD-I Lyser<br />
AKL<br />
10208601102 21/05/2010 LABNOVATION Lyser WH<br />
AKL<br />
10208601110 21/05/2010 LABNOVATION FB Lyser<br />
AKL<br />
10208601101 21/05/2010 LABNOVATION 4DL-Lyser<br />
AKL<br />
10208601105 21/05/2010 LABNOVATION FD-II Lyser<br />
AKL<br />
10208601099 21/05/2010 LABNOVATION Lyser 3-WP<br />
AKL<br />
10208601126 21/05/2010 LABNOVATION Lyser HGB<br />
AKL<br />
10208601134 21/05/2010 LABNOVATION Diluent - ST<br />
AKL<br />
10204011150 21/05/2010 LABNOVATION Cell Clener<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
AKL<br />
20305403595 06/05/2010 ETI-AB-HCVK-4 (Anti ACV EIA) DIASORIN S.P.A., ITALY<br />
AKL<br />
20101601943 06/05/2010<br />
PULSE Febrile Antigen O-A<br />
Paratyphi<br />
PULSE SCIENTIFIC INC., CANADA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
10102011378 18/06/2010 DIALAB Photometer DTN - 410 K DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101011373 18/06/2010<br />
AKL<br />
20101011374 18/06/2010<br />
AKL<br />
20101011375 18/06/2010<br />
AKL<br />
20101011376 18/06/2010<br />
ALFA WASSERMANN Inorganic<br />
Phosphorus UV Reagent<br />
ALFA WASSERMANN Calcium<br />
Arsenazo<br />
ALFA WASSERMANN Alkaline<br />
Phosphatase<br />
ALFA WASSERMANN Total<br />
Bilirubin Reagent<br />
AKL<br />
20101011377 18/06/2010 ALFA WASSERMANN Albumin<br />
AKL<br />
20305010876 06/05/2010<br />
ARCHITECT HBsAg Qualitative<br />
Controls<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20302602686 06/05/2010 REMEL RapID NH System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302010959 06/05/2010<br />
REMEL ERIC Electronic RapID<br />
Compendium REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302010958 06/05/2010 REMEL RapID STR System REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101010961 06/05/2010 ARCHITECT Cyclosporin Calibrator<br />
AKL<br />
ARCHITECT Cyclosporin Reagent<br />
20101010962 06/05/2010 Kit<br />
PDF Creator - PDF4Free v3.0<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES, USA<br />
http://www.pdf4free.com<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA
AKL<br />
20101010963 06/05/2010 ADVIA Chemistry ISE Buffer<br />
AKL<br />
10201010964 06/05/2010 ADVIA Autoslide Rinse<br />
AKL<br />
10201010965 06/05/2010 ADVIA Autoslide Giemsa Stain<br />
AKL<br />
20250705659 07/06/2010<br />
DIAGON D-Cell Hematology<br />
Analyzer<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
DIAGON KFT, hungaria<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. DEC HEALTHCARE<br />
INDONESIA<br />
AKL<br />
10302602688 06/05/2010 REMEL RapID Nitrate A/B Reagent REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10208601109 11/06/2010 LABNOVATION - Lyse LABNOVATION TECHNOLOGIES INC., USA PT. SARANA MAJU SEJAHTERA<br />
AKL<br />
10208601131 11/06/2010 LABNOVATION - Lyse BC3 LABNOVATION TECHNOLOGIES INC., USA PT. SARANA MAJU SEJAHTERA<br />
AKL<br />
10208601127 11/06/2010 LABNOVATION - Lyse M<br />
AKL<br />
10208601130 11/06/2010 LABNOVATION Rinse & HGB Ref<br />
AKL<br />
10208601139 11/06/2010 LABNOVATION Cleaner M<br />
AKL<br />
10208601137 11/06/2010 LABNOVATION Diluent M<br />
AKL<br />
10208601128 11/06/2010 LABNOVATION Diluent BC-3<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
AKL<br />
20305603596 11/06/2010 SD Bioline hCG STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101501429 11/06/2010 QCA Urea UV QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20207801428 11/06/2010 QCA Hemoglobin QUIMICA CLINICA APLICADA S.A., SPAIN PT. DUTA INDO OMNITRON<br />
AKL<br />
20101011309 14/06/2010 DG Gel Solution DIAGNOSTICS GRIFOLS S.A., SPANYOL<br />
AKL<br />
10204011312 14/06/2010 LABNOVATION Cleaner P<br />
AKL<br />
10204011313 14/06/2010 LABNOVATION CE Clean<br />
AKL<br />
10204011314 14/06/2010 LABNOVATION Cleaner C<br />
AKL<br />
10204011315 14/06/2010 LABNOVATION PB Cleaner<br />
AKL<br />
10102011316 14/06/2010 BOECO Rotator<br />
AKL<br />
20101011317 14/06/2010 Fluitest UREA Col<br />
AKL<br />
20101011318 14/06/2010 Fluitest Ca.CPC<br />
AKL<br />
20101011319 14/06/2010 Fluitest CI<br />
AKL<br />
20101011320 14/06/2010 Fluitest GOT-ASAT<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
BOECKEL & CO (GmbH + CO) KG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
10302011321 14/06/2010 OXOID Tryptone Soya Agar OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302011323 14/06/2010 OXOID Triple Sugar Iron Agar OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302011324 14/06/2010 OXOID Nutrient Broth OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303101264 11/06/2010<br />
HISENS One Step Rapid Malaria<br />
P.f/P.V Ag card<br />
AKL<br />
20101011265 11/06/2010 CARE hCG Pregnancy Test Strip<br />
HBI CO. LTD., KOREA melalui GEECO &<br />
ROOTIZ LTD., KOREA<br />
SHANGHAI HUAGUAN BIOCHIP CO. LTD.,<br />
CHINA<br />
PT. NEXTWAY ELECTRONICS<br />
PT. PRIMA BUMI MANDIRI<br />
AKL<br />
20301011271 11/06/2010 OXOID Lincomycin MY10 OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10201011272 11/06/2010<br />
AKL<br />
10201011275 11/06/2010<br />
AKL<br />
10101011277 11/06/2010<br />
AKL<br />
10101011278 11/06/2010<br />
AKL<br />
10101011279 11/06/2010<br />
SHANDON Rapid Chrome Kwik-Diff<br />
Staining Kits<br />
SHANDON Cytospin 4<br />
Cytocentrifuge<br />
ALFA WASSERMANN Chemistry<br />
Control 2<br />
ALFA WASSERMANN Cholesterol<br />
Reagent<br />
ALFA WASSERMANN Direct LDL<br />
Cholesterol Reagent<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101011280 11/06/2010<br />
AKL<br />
10101011281 11/06/2010<br />
ALFA WASSERMANN Direct HDL<br />
Cholesterol Reagent<br />
ALFA WASSERMANN Uric Acid<br />
Reagent<br />
AKL<br />
10208601106 11/06/2010 LABNOVATION BA-Lyse2<br />
AKL<br />
10208601107 11/06/2010 LABNOVATION BA-Lyse<br />
AKL<br />
10208601278 11/06/2010 LABNOVATION Cleaner DA<br />
AKL<br />
10208601129 11/06/2010 LABNOVATION Diluent NK<br />
AKL<br />
10208601135 11/06/2010 LABNOVATION Lyse NK<br />
AKL<br />
10208601279 11/06/2010 LABNOVATION Diluent DA<br />
AKL<br />
10208601277 11/06/2010 LABNOVATION Lyse DA<br />
AKL<br />
10208601132 11/06/2010 LABNOVATION Rinse NK<br />
AKL<br />
10208601108 11/06/2010 LABNOVATION EO-Lyse<br />
AKL<br />
10208601112 11/06/2010 LABNOVATION Diluent P<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN B.V., THE<br />
NETHERLANDS<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC.,<br />
CHINA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
AKL<br />
20101600063 25/06/2010 DIRUI Uristik 3 DIRUI INDUSTRIAL CO. LTD., CHINA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20101600066 25/06/2010 DIRUI Uristik H11 DIRUI INDUSTRIAL CO. LTD., CHINA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20101600064 25/06/2010 DIRUI Uristik A10 DIRUI INDUSTRIAL CO. LTD., CHINA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20208600367 25/06/2010<br />
AKL<br />
20208600368 25/06/2010<br />
AKL<br />
20208600366 25/06/2010<br />
LIQUICHECK Hematology-16<br />
Control Level 2 Normal<br />
LIQUICHECK Hematology-16<br />
Control Trilevel<br />
LIQUICHECK Hematology-16<br />
Control Level 3 High<br />
BIORAD LABORATORIES, USA melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIORAD LABORATORIES, USA melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIORAD LABORATORIES, USA melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10302011400 25/06/2010 OXOID Mac Conkey Agar No. 3 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101011407 28/06/2010 RANDOX Creatinine RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20102011408 28/06/2010<br />
AKL<br />
20102011409 28/06/2010<br />
RANDOX RX IMOLA Clinical<br />
Chemistry Analyzer RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
RANDOX RX DAYTONA Clinical<br />
Chemistry Analyzer RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
10208011410 28/06/2010 ZENIX Diluent M<br />
AKL<br />
10208011411 28/06/2010 ZENIX Lyse<br />
AKL<br />
20101011412 28/06/2010 ALFA WASSERMANN Urea (Lyo)<br />
AKL<br />
20101011413 28/06/2010<br />
AKL<br />
20101011414 28/06/2010<br />
ALFA WASSERMANN Glucose<br />
(HK)<br />
ALFA WASSERMANN Direct HDL /<br />
LDL Calibrators<br />
AKL<br />
20101011415 28/06/2010 ALFA WASSERMANN Glucose<br />
AKL<br />
20101011416 28/06/2010<br />
ALFA WASSERMANN AST (GOT) -<br />
IFCC<br />
AKL<br />
20101011417 28/06/2010 ALFA WASSERMANN Urea (Liquid)<br />
AKL<br />
20101011418 28/06/2010 ALFA WASSERMANN Creatinine<br />
AKL<br />
20101011419 28/06/2010<br />
ALFA WASSERMANN Gemcal<br />
Serum calibrator<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
ALFA WASSERMANN BV., THE<br />
NETHERLANDS<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SUMIFIN CITRA ABADI<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
PT. MARADA PHARMA MEDICA<br />
AKL<br />
10302011420 28/06/2010 OXOID V-Factor Disc OXOID LIMITED., ENGLAND PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101011421 28/06/2010 SYSMEX Ca Buffer (R1)<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101011422 28/06/2010 SYSMEX LD (L) - L.R1 and PL.R.1<br />
AKL<br />
20101011423 28/06/2010 SYSMEX TP and TP-P Reagent<br />
AKL<br />
10101011424 28/06/2010<br />
AmniSure ROM (Rupture of Fetal<br />
Membrane) Test<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
AMNISURE INTERNATIONAL LLC., USA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
20102011425 28/06/2010<br />
JOKOH EX-D Fully Automated<br />
Electrolyte Analyzer and Accessories<br />
AKL<br />
20101011426 28/06/2010 COBAS Roche CARDIAC Pro BNP<br />
AKL<br />
10102011427 28/06/2010 RIELE Photometer 5010<br />
AKL<br />
10209011428 28/06/2010<br />
AKL<br />
20205011429 28/06/2010<br />
AKL<br />
10101011282 11/06/2010<br />
AKL<br />
10101011283 11/06/2010<br />
AKL<br />
10101011284 11/06/2010<br />
AKL<br />
10101011285 11/06/2010<br />
AKL<br />
10101011287 11/06/2010<br />
PROCLEIX System Vortexer and<br />
Accessories<br />
PROCLEIX Reagent Preparation<br />
Incuvator (RPI)<br />
JOKOH CO. LTD., JAPAN<br />
ROCHE DIAGNOSTICS GmbH., GERMANY<br />
melalui ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND<br />
ROBERT RIELE GmbH & CO. KG.,<br />
GERMANY<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. ROCHE INDONESIA<br />
PT. RAJAWALI NUSINDO<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
ALFA WASSERMANN Triglycerides<br />
Reagent ALFA WASSERMANN B.V., The Netherlands PT. MARADA PHARMA MEDICA<br />
ALFA WASSERMANN Gamma-GT<br />
Reagent ALFA WASSERMANN B.V., The Netherlands PT. MARADA PHARMA MEDICA<br />
ALFA WASSERMANN Chemistry<br />
Control 1 ALFA WASSERMANN B.V., The Netherlands PT. MARADA PHARMA MEDICA<br />
ALFA WASSERMANN Direct<br />
HDL/LDL Control 1 ALFA WASSERMANN B.V., The Netherlands PT. MARADA PHARMA MEDICA<br />
ALFA WASSERMANN HDL<br />
Cholesterol Precipitant ALFA WASSERMANN B.V., The Netherlands PT. MARADA PHARMA MEDICA<br />
AKL<br />
10102600764 30/06/2010 ACURA Manual 825 SOCOREX ISBA S.A., SWITZERLAND<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20303011445 28/06/2010<br />
ABBOTT AXSYM SYSTEM Anti<br />
TPO Reagent Pack<br />
AXIS SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT Gmbh & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305011446 28/06/2010 ARCHITECT Anti CCP Calibrator<br />
AXIS SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT Gmbh & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305011447 28/06/2010 ARCHITECT Anti CCP Control<br />
AXIS SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT Gmbh & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305011448 28/06/2010 ARCHITECT Anti CCP Reagent Kit<br />
AKL<br />
20101011449 28/06/2010<br />
AEROSET/ARCHITECT Cystatin C<br />
calibrator<br />
AKL<br />
20101011450 28/06/2010 ARCHITECT Urine NGAL Calibrator<br />
AKL<br />
10101011451 28/06/2010 ARCHITECT Urine NGAL Control<br />
AKL<br />
20101011452 28/06/2010<br />
ARCHITECT Urine NGAL Reagent<br />
Kit<br />
AKL<br />
20101011453 29/06/2010 AEROSET/ARCHITECT Cystatin C<br />
AKL<br />
20101011454 28/06/2010<br />
HARBIN TIAN DIREN Heart-type<br />
Acid-binding Protein<br />
AKL<br />
10101011455 28/06/2010 CLINITEK Atlas Negative Control<br />
AKL<br />
10101011456 28/06/2010 CLINITEK Atlas Rinse Additive<br />
AKL<br />
10101011457 28/06/2010 CLINITEK Atlas Positive Control<br />
AKL<br />
10101011458 28/06/2010 FLUITEST UA<br />
AKL<br />
10101011459 28/06/2010 FLUITEST HDL-CHOL<br />
AKL<br />
10101011460 28/06/2010 FLUITEST GGT<br />
AKL<br />
10101011461 28/06/2010 FLUITEST TG<br />
AXIS SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT Gmbh & CO. KG., GERMANY<br />
SENTINEL CH SPA, ITALY untuk ABBOTT<br />
GmbH & CO. KG., GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
SENTINEL CH SPA, ITALY untuk ABBOTT<br />
GmbH & CO. KG., GERMANY<br />
HARBIN TIAN DIREN MEDICAL SCIENCE<br />
AND TECHNOLOGY CO. LTD., CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. NELTA MULTI GRACIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101011462 28/06/2010 FLUITEST GPT-ALAT<br />
AKL<br />
10201011943 06/08/2010<br />
AKL<br />
20102011572 05/07/2010<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
MERCK Microcopy Lactophenol Blue<br />
Solution MERCK KGaA., GERMANY PT. MERCK Tbk<br />
LIASYS Clinical Chemistry Random<br />
Access Analyzer<br />
AMS srl - ANALYZER MEDICAL SYSTEM,<br />
ITALY<br />
AKL<br />
AMS srl - ANALYZER MEDICAL SYSTEM,<br />
20102011573 05/07/2010 ELLIPSE Clinical Chemistry Analyzer ITALY<br />
AKL<br />
10102011574 05/07/2010 BOECO Photometer PM 51<br />
AKL<br />
10303602738 02/07/2010<br />
BOECKEL + CO (GMBH+ CO) KG.,<br />
GERMANY<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
DIALAB ASO Latex ( Antistreptolysin-<br />
O ) DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101602725 02/07/2010 DIALAB APO A1/A2/B Control DIALAB GES M.B.H., Austria PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101603994 02/07/2010 MULTIGENT Direct LDL Calibrator<br />
GENZYME DIAGNOSTICS,USA untuk<br />
ABBOTT LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10302605040 02/07/2010 OXOID Amies Transport Medium OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302604101 02/07/2010<br />
OXOID Oxacillin Resistance<br />
Screening agar Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302604730 02/07/2010<br />
AKL<br />
20305603989 02/07/2010 MULTIGENT Direct LDL<br />
OXOID C. Difficile A Stool Filtration<br />
Systems Toxin Detection Kit OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
GENZYME DIAGNOSTICS,USA untuk<br />
ABBOTT LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20208603987 02/07/2010 CELL-DYN HemCal plus Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101703814 02/07/2010 proDia HDL Cholesterol LS PRODIA INTERNATIONAL ,U.A.E PT. SUMIFIN CITRA ABADI<br />
AKL<br />
10101600373 02/07/2010<br />
AKL<br />
10101600370 02/07/2010<br />
AKL<br />
10101600371 02/07/2010<br />
AKL<br />
10101600372 02/07/2010<br />
AKL<br />
20102011526 02/07/2010<br />
LYPOCHECK Assayed Chemistry<br />
Control Level 2 Abnormal<br />
LYPOCHECK Unassayed Chemistry<br />
Control Level 2<br />
LYPOCHECK unassayed Chemistry<br />
Control Level 2 Abnormal<br />
LYPOCHECK Assayed Chemistry<br />
Control Level 1 Abnormal<br />
K-Lite3 Electrolyet Analyzer and<br />
accessories<br />
BIORAD LABORATORIES , USA melalui<br />
BIORAD LABORATORIES ( SINGAPORE )<br />
PTE,LTD , Singapore<br />
BIORAD LABORATORIES , USA melalui<br />
BIORAD LABORATORIES ( SINGAPORE )<br />
PTE,LTD , Singapore<br />
BIORAD LABORATORIES , USA melalui<br />
BIORAD LABORATORIES ( SINGAPORE )<br />
PTE,LTD , Singapore<br />
BIORAD LABORATORIES , USA melalui<br />
BIORAD LABORATORIES ( SINGAPORE )<br />
PTE,LTD , Singapore<br />
MEIZHOU CORNLEY HI-TECH CO, LTD ,<br />
P.R CHINA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10303011533 02/07/2010 SD Bioline RSV STANDARD DIAGNOSTICS INC, Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303011532 02/07/2010 SD Bioline Cholera Ag 01/0139 STANDARD DIAGNOSTICS INC, Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305011531 02/07/2010 SD Bioline Hantaan Virus STANDARD DIAGNOSTICS INC, Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305011530 02/07/2010 SD Bioline Tetanus STANDARD DIAGNOSTICS INC, Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303602700 12/07/2010<br />
AKL<br />
20102011525 02/07/2010<br />
REMEL Aglutinating serum<br />
salmonella Polyvalent O Group A-G REMEL EUROPE LTD., ENGLAND PT. DIPA PUSPA LABSAINS<br />
K-Lite5 Electrolyet Analyzer and<br />
accessories<br />
MEIZHOU CORNLEY HI-TECH CO, LTD ,<br />
P.R CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101011524 02/07/2010 PHOENIX 7.3/Co.Ox Zero PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
AKL<br />
20101011523 02/07/2010 PHOENIX Cal-Pack M-644 PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
AKL<br />
20101011522 02/07/2010 ABBOTT PAIb Cal ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20306011521 02/07/2010 AXSYM AFP Reagent Pack<br />
AKL<br />
20306011520 02/07/2010 AXSYM Total PSA Reagent Pack<br />
ABBOTT IRELAND DIAGNOSTICS DIVISION<br />
, sligo-ireland PT. ABBOTT INDONESIA<br />
ABBOTT IRELAND DIAGNOSTICS DIVISION<br />
, sligo-ireland PT. ABBOTT INDONESIA<br />
AKL<br />
20101011519 02/07/2010 ABBOTT APO A1/APO B Cal ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20102011757 12/07/2010<br />
AKL<br />
20102011758 12/07/2010<br />
Convergys Automatic EL-Reader<br />
ELISA Analysis System<br />
Convergys ISE NG Electrolyte<br />
Analysis<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
CO. KG., GERMANY<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
CO. KG., GERMANY<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20205011759 12/07/2010<br />
AKL<br />
20205011760 12/07/2010<br />
AKL<br />
20102011761 12/07/2010<br />
AKL<br />
20305011581 05/07/2010<br />
Convergys x3 Haematology<br />
Analyzers<br />
Convergys X5 Haematology<br />
Analyzers<br />
Convergys Automatic EL-washer<br />
ELISA Analysis System<br />
OnSite Chikungunya IgM Combo<br />
Rapid Test<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
CO. KG., GERMANY<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
CO. KG., GERMANY<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
CO. KG., GERMANY<br />
CTK BIOTECH INC., USA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20101011582 05/07/2010 Speedytest-Pregnancy Test Strip IND DIAGNOSTICS INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
10201011583 05/07/2010<br />
AKL<br />
10201011584 05/07/2010<br />
AKL<br />
10201011585 05/07/2010<br />
AKL<br />
10201011586 05/07/2010<br />
AKL<br />
10201011587 05/07/2010<br />
AKL<br />
10201011588 05/07/2010<br />
AKL<br />
30305011589 05/07/2010<br />
AKL<br />
20208011590 05/07/2010<br />
AKL<br />
20305011591 05/07/2010<br />
AKL<br />
20305011592 05/07/2010<br />
AKL<br />
20102011593 05/07/2010<br />
AKL<br />
20102011594 05/07/2010<br />
PREVI Color Gram Acetone Fuschin<br />
Solution - RA3 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PREVI Color Gram Crystal Violet<br />
Solution - RC BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PREVI Color Gram Safranin Solution<br />
- RA2 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PREVI Color Gram Fuschin Solution -<br />
RA4 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PREVI Color Gram Lodine Solution -<br />
RB BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PREVI Color Gram Acetone Safranin<br />
Solution - RA1 BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
COBAS TaqMan HIV-1 Test (HIV-1<br />
HPS)<br />
ROCHE MOLECULAR SYSTEM INC, USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
COBAS D-Dimer Gen.2 Calibrators<br />
Set ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
BEIJING KINGHAWK HbsAg ELISA<br />
Test<br />
BEIJING KINGHAWK HCV ELISA<br />
Test<br />
SHENZHEN GENIUS Fully<br />
Automatical Chemistry Analyzer GS<br />
400<br />
SHENZHEN GENIUS Fully<br />
Automatical Chemistry Analyzer GS<br />
200<br />
AKL<br />
10203011649 05/07/2010 BenchMark XT and Accessories<br />
AKL<br />
10102011650 05/07/2010<br />
AKL<br />
10101011636 05/07/2010<br />
AKL<br />
10204011637 05/07/2010<br />
BEIJING KINGHAWK PHARMACEUTICAL<br />
CO. LTD., CHINA<br />
BEIJING KINGHAWK PHARMACEUTICAL<br />
CO. LTD., CHINA<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
PT. ALIZAH<br />
PT. ALIZAH<br />
PT. ALIZAH<br />
PT. ALIZAH<br />
PT. ROCHE INDONESIA<br />
RX MONZA Clinical Chemmistry<br />
Analyzer and Accessories RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
PHOENIX Tri Level Blood Gas / ISE<br />
Control PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
PHOENIX Wash Zero Reagent<br />
M800 Series PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
AKL<br />
10204011638 05/07/2010 PHOENIX Buffer Pack M348 PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
AKL<br />
10204011639 05/07/2010 PHOENIX 6.8 Buffer M800 Series PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
AKL<br />
10204011640 05/07/2010 PHOENIX Wash / CD Pack M348 PHOENIX DIAGNOSTICS INC., USA PT. CITRA MEDIKA LESTARI<br />
AKL<br />
20101011641 05/07/2010 DIMENSION Iron Calibrator<br />
AKL<br />
20101011642 05/07/2010 DIMENSION TBI/DBI calibrator<br />
AKL<br />
20101011643 05/07/2010<br />
AKL<br />
20101011645 05/07/2010<br />
DIMENSION Direct Bilirubin (DBI)<br />
Flex Reagent Catridge<br />
DIMENSION Total Bilirubin (TBI)<br />
Flex Reagent Catridge<br />
AKL<br />
20101011646 05/07/2010 DIMENSION LIPL Calibrator<br />
AKL<br />
20101011647 05/07/2010 Clinitek Atlas Calibration Kit<br />
AKL<br />
20305011654 05/07/2010<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS Hematest Reagent<br />
Tablets<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, Hongkong<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
20101603142 29/06/2010<br />
AKL<br />
10102504508 15/07/2010<br />
ABBOTT Axsym Ferritin Standard<br />
Calibrators<br />
JOKOH EX-D Electrolyte Analyzer &<br />
Accessories / Spare Part Main<br />
Consumable<br />
AKL<br />
20207011790 16/07/2010 Blue D-Dimer Kit-60<br />
AKL<br />
20208011791 16/07/2010 TEControl D-Dimer Low<br />
AKL<br />
20208011792 16/07/2010 TEControl D-Dimer High<br />
AKL<br />
20102011793 16/07/2010<br />
AKL<br />
20102011794 16/07/2010<br />
AKL<br />
20101011795 16/07/2010<br />
AKL<br />
20101011796 16/07/2010<br />
AKL<br />
10205011797 16/07/2010<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD - IRELAND<br />
JOKOH CO.Ltd., Japan<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
SATURNO 150 Chemistry Analyzer<br />
and Accessories CRONY INSTRUMENTS S.R.L., ITALY PT. SETIA ANUGERAH MEDIKA<br />
SATURNO 300 Plus Chemistry<br />
Analyzer and Accessories CRONY INSTRUMENTS S.R.L., ITALY PT. SETIA ANUGERAH MEDIKA<br />
AXSYM SYSTEM Cyclosporine<br />
Reagent Accessories Kit<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. ABBOTT INDONESIA<br />
NAPSA Check 6 in 1 (AMP, BZO,<br />
COC, MAMP, OPI, THC) LUMIQUICK DIAGNOSTICS INC., USA PT. INDOMED BERKAT JAYA<br />
PRECISION Circulating Water Baths<br />
and Accessories<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
AKL<br />
21106011798 16/07/2010 VITROLIFE G-Oocyte VITROLIFE AB., SWEDEN<br />
AKL<br />
21106011799 16/07/2010 VITROLIFE G-Fert Plus VITROLIFE AB., SWEDEN<br />
AKL<br />
21106011800 16/07/2010 VITROLIFE Gamete 30 VITROLIFE AB., SWEDEN<br />
AKL<br />
20101604947 09/08/2010 PTS PANELS Creatinine Test Strips<br />
AKL<br />
20305602678 09/08/2010<br />
AKL<br />
20101602726 09/08/2010<br />
POLYMER TECHNOLOGY SYSTEMS INC.,<br />
USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. MITRAASA PRATAMA<br />
DIALAB CRP (C-Reactive Protein)<br />
Latex Reagent DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
DIALAB APO A1/A2/B Calibrator<br />
High DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601858 09/08/2010 DIALAB Salmonella Paratyphi AO DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601850 09/08/2010 DIALAB Salmonella Paratyphi O DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602737 09/08/2010<br />
DIALAB RF (Rheumatoid Factors)<br />
Latex Reagent DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601851 09/08/2010 DIALAB Salmonella Typhi H DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601852 09/08/2010 DIALAB Salmonella Paratyphi AH DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101602723 09/08/2010<br />
DIALAB APO A1/A2/B Calibrator<br />
Low DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303602734 09/08/2010 DIALAB Syphilis TPHA DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602679 09/08/2010<br />
DIALAB CRP (C-Reactive Protein)<br />
Liquid Reagent DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303602733 09/08/2010 DIALAB Syphilis RPR DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602677 09/08/2010<br />
DIALAB CRP Uhs (Universal high<br />
sensitivity) DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601849 06/08/2010 DIALAB Salmonella Paratyphi CO DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601859 06/08/2010 DIALAB Salmonella Paratyphi BO DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601853 06/08/2010 DIALAB Salmonella Paratyphi CH DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303601861 06/08/2010 DIALAB Salmonella Paratyphi BH DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20207601860 06/08/2010 DIALAB HbA1c Direct DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303011936 06/08/2010<br />
Binax NOW Legionella Urinary<br />
Antigen Test<br />
BINAX INC., USA melalui INVERNESS<br />
MEDICAL INNOVATIONS AUSTRALIA PTY<br />
LTD., AUSTRALIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
10303011922 06/08/2010 MICROGEN E.Coli 0157 MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10302604126 06/08/2010 MICROGEN Oxidase Strips MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
10303603122 06/08/2010 MICROGEN Staph ID MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302603484 06/08/2010 MICROGEN Mineral Oil MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
AKL<br />
20201012000 09/08/2010<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
VENTANA Rabbit anti-DNP Antibody GERMANY<br />
AKL<br />
10201012016 09/08/2010 VENTANA LCS (Pre-dilute)<br />
AKL<br />
10201012017 09/08/2010 VENTANA CC2 (Pre-dilute)<br />
AKL<br />
10201012018 09/08/2010 VENTANA CC1 (Pre-dilute)<br />
AKL<br />
10201012019 09/08/2010 VENTANA Antibody Diluent<br />
AKL<br />
10201012020 09/08/2010 VENTANA Amplification Kit<br />
AKL<br />
10204011932 06/08/2010<br />
AKL<br />
10204011934 06/08/2010<br />
VENTANA Reaction Buffer<br />
Concentrate (10x)<br />
VENTANA EZ Prep Concentrate<br />
(10x)<br />
AKL<br />
10204011935 06/08/2010 VENTANA SSC Concentrate (10x)<br />
AKL<br />
10201011937 06/08/2010 VENTANA Hematoxylin II<br />
AKL<br />
10201011938 06/08/2010 VENTANA Bluing Reagents<br />
AKL<br />
10201011940 06/08/2010 VENTANA Hematoxylin<br />
AKL<br />
20303603775 06/08/2010<br />
AKL<br />
20303603777 06/08/2010<br />
AKL<br />
20305012002 09/08/2010 ENZYGNOST HBsAg 6.0<br />
AKL<br />
20101012003 09/08/2010 IMMULITE PAPP-A<br />
AKL<br />
20101012004 09/08/2010 ADVIA Centaur HCY Calibrator<br />
AKL<br />
20101012005 09/08/2010 ADVIA Chemistry Phenobarbital<br />
AKL<br />
20101012006 09/08/2010 DIMENSION AHDL Calibrator<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
Toxo IgM Elecsys and Cobas e<br />
analyzer ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
Toxo IgG Elecsys and Cobas e<br />
analyzer ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GmbH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101012007 09/08/2010<br />
AKL<br />
20101012008 09/08/2010<br />
DIMENSION Automated HDL<br />
Cholesterol Flex Reagent Cartridge<br />
ADVIA Chemistry Microalbumin 2<br />
calibrator<br />
AKL<br />
20101012009 09/08/2010 ADVIA Chemistry Lactate Reagent<br />
AKL<br />
20101012010 09/08/2010 ADVIA CENTAUR TSH3-UL<br />
AKL<br />
20101012011 09/08/2010<br />
AKL<br />
20102012032 09/08/2010<br />
AKL<br />
20101012033 09/08/2010<br />
ADVIA Centaur HCY Homocysteine<br />
Diluent<br />
SIEMENS Clinitek Status + Analyzer<br />
and Accessories<br />
DIMENSION Creatine Kinase Flex<br />
Reagent Catridge / CKI<br />
AKL<br />
20101012034 09/08/2010 DIMENSION ENZ I Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101012035 09/08/2010<br />
DIMENSION Creatine Kinase MB<br />
Flex Reagent Catridge/MBI<br />
AKL<br />
20305012036 09/08/2010 IMMULITE ALB<br />
AKL<br />
20101012037 09/08/2010 IMMULITE CAL<br />
AKL<br />
10101011941 06/08/2010 ADVIA Chemistry APO B<br />
AKL<br />
10101011942 06/08/2010<br />
DADE Monitrol Lyophilized Assayed<br />
Chemistry Control (L1)<br />
AKL<br />
20102011945 06/08/2010 SIEMENS Clinitek Atlas Pro 12<br />
AKL<br />
20101011946 06/08/2010<br />
AKL<br />
20101011947 06/08/2010<br />
ADVIA Chemistry APO A1/B<br />
Calibrator<br />
DIMENSION LDI Flex Reagent<br />
Catridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20102011948 06/08/2010<br />
SIEMENS Clinitek Atlas 10 (Color,<br />
Glucose, Bilirubin, Protein, PH,<br />
Ketone, Occult Blood, Urobilinoge<br />
AKL<br />
20101011949 06/08/2010 IMMULITE ACTH<br />
AKL<br />
20102011877 02/08/2010 STREAMLAB Analytical Workcell<br />
AKL<br />
20101011878 02/08/2010 BIOSUB UREA / EK<br />
AKL<br />
20207011879 02/08/2010 BIODIAB HbA1<br />
AKL<br />
20207011880 02/08/2010 In2it Quality Control<br />
AKL<br />
20207011881 02/08/2010 In2it Test cartridge<br />
AKL<br />
10102011894 06/08/2010<br />
AKL<br />
10303011895 06/08/2010<br />
CALLEGAR CR 3000T Diagnostics<br />
Analyzer<br />
BINAX NOW Streptococcus<br />
Pneumonia Test<br />
AKL<br />
20303011896 06/08/2010 ARCHITECT Rubella IgG Controls<br />
AKL<br />
20303011897 06/08/2010<br />
AKL<br />
20303011898 06/08/2010<br />
AKL<br />
10101011899 06/08/2010<br />
AKL<br />
10101011900 06/08/2010<br />
AKL<br />
10101011901 06/08/2010<br />
AKL<br />
10101011902 06/08/2010<br />
AKL<br />
10101011903 06/08/2010<br />
ARCHITECT Rubella IgG<br />
Calibrators<br />
ARCHITECT Rubella IgG Reagent<br />
Kit<br />
DIMENSION Iron Flex Reagent<br />
Cartridge<br />
IMMULITE Systems Gastrin Control<br />
Module/GAS<br />
IMMULITE Systems C-Peptide<br />
Control/CPE<br />
IMMULITE Systems ACTH<br />
Control/ACT<br />
DIMENSION LIPL Flex Reagent<br />
Cartridge<br />
AKL<br />
10101011904 06/08/2010 IMMULITE Androstenedion (AND)<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
BIO-RAD LABORATORIES INC, USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES INC, USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
CALLEGARI S.p.A., ITALY<br />
BINAX INC, USA melalui INVERNESS<br />
MEDICAL INNOVATION PTY LTD.,<br />
AUSTRALIA<br />
FISHER DIAGNOSTICS, USA untuk ABBOTT<br />
GmbH & CO. KG., GERMANY<br />
FISHER DIAGNOSTICS, USA untuk ABBOTT<br />
GmbH & CO. KG., GERMANY<br />
FISHER DIAGNOSTICS, USA untuk ABBOTT<br />
GmbH & CO. KG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. PHARMINDO RIMPANG<br />
KOKOH<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
10101011905 06/08/2010 ADVIA Chemistry GGT<br />
AKL<br />
10101011906 06/08/2010<br />
DADE Monitor Lyophilized Assayed<br />
Chemistry Control (L2)<br />
AKL<br />
10101011907 06/08/2010 DADE Tru Liquid Monitror I<br />
AKL<br />
10101011908 06/08/2010 DADE Tru Liquid Monitrol 2<br />
AKL<br />
10204012089 10/08/2010 ARCHITECT Trigger Solution<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui MICROGENICS CORPORATION,<br />
USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui MICROGENICS CORPORATION,<br />
USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD-IRELAND<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305011864 30/07/2010 ARCHITECT Pepsinogen I Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305011865 30/07/2010 ARCHITECT Pepsinogen II Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305011866 30/07/2010<br />
AKL<br />
20305011867 30/07/2010<br />
AKL<br />
20305012088 10/08/2010<br />
ARCHITECT Pepsinogen II Reagent<br />
Kit ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ARCHITECT Pepsinogen I Reagent<br />
Kit ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ARCHITECT Pepsinogen II<br />
Calibrators ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
21106012077 10/08/2010 VITROLIFE HAS solution VITROLIFE AB., SWEDEN<br />
AKL<br />
21106012076 10/08/2010 VITROLIFE IVF VITROLIFE AB., SWEDEN<br />
AKL<br />
21106012075 10/08/2010 VITROLIFE G-Sperm Plus VITROLIFE AB., SWEDEN<br />
AKL<br />
21106012074 10/08/2010 VITROLIFE G-1 V3 Plus VITROLIFE AB., SWEDEN<br />
AKL<br />
21106012073 10/08/2010 VITROLIFE Freeze Kit 2 VITROLIFE AB., SWEDEN<br />
AKL<br />
10102605052 30/07/2010 TPC Drilling Pipettes<br />
AKL<br />
10102605053 30/07/2010 TPC Cutting Pipettes<br />
THE PIPETTE COMPANY (TPC_ PTY. LTD.,<br />
AUSTRALIA<br />
THE PIPETTE COMPANY (TPC_ PTY. LTD.,<br />
AUSTRALIA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20301604820 30/07/2010 OXOID Erythromycin E30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301605024 30/07/2010<br />
OXOID Fosfomycin / Trometamol<br />
FOT 200 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301605026 30/07/2010 OXOID Fusidic Acid FD 10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301605025 30/07/2010 OXOID Framycetin FY 100 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301605027 30/07/2010 OXOID Rifampicin RD 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303011869 30/07/2010 SD Bioline H. Pylori Ag STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101012086 10/08/2010<br />
SD Codefree Blood Glucose Test<br />
Strip STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101012087 10/08/2010 SD Codefree Blood Glucose Meter STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101504871 30/07/2010 STANBIO Fertitell Direct Pregnancy STANBIO LABORATORY., USA<br />
AKL<br />
10101504870 30/07/2010 STANBIO ALT/SGPT Liqui-UV STANBIO LABORATORY., USA<br />
AKL<br />
20101504866 30/07/2010 STANBIO AST/GOT Liqui-UV STANBIO LABORATORY., USA<br />
AKL<br />
20101504869 30/07/2010 STANBIO Urea Nitrogen (BUN) STANBIO LABORATORY., USA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
10205011868 30/07/2010 ALIFAX Roller 20 LC ALIFAX S.p.A., ITALY PT. MULTISERA INDOSA<br />
AKL<br />
20102011871 30/07/2010<br />
DIAMOND Prolyte Electrolyte<br />
Analyzer DIAMOND DIAGNOSTICS INC., USA PT. MULTISERA INDOSA<br />
AKL<br />
20101011872 30/07/2010 RANDOX Urea RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20101011873 30/07/2010 RANDOX Urea RX-eries RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20101011874 30/07/2010 RANDOX Albumin (ALB) RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305011876 30/07/2010 RANDOX Albumin ALB RX series RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20101011875 30/07/2010<br />
AKL<br />
20303012069 10/08/2010<br />
AKL<br />
20303012068 10/08/2010<br />
AKL<br />
10201011870 30/07/2010<br />
AKL<br />
20304012078 10/08/2010<br />
RANDOX Triglycerides TRIGS RX<br />
series RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
PreciControl Toxo IgG Elecsys and<br />
Cobas e analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
PreciControl Toxo IgM Elecsys and<br />
Cobas e analyzers ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
VENTANA Ultra View Universal DAB<br />
Detection Kit<br />
VENTANA Ultra View Alkaline<br />
Phosphotase Red ISH Detection Kit<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10201012079 10/08/2010<br />
VENTANA CONFIRM anti-Estrogen<br />
Receptor (ER) (SP1) Rabbit<br />
Monoclonal Primary Antibody<br />
AKL<br />
10204012080 10/08/2010 ultraView Silver Wash II (Pre-dilute)<br />
AKL<br />
10201012081 10/08/2010<br />
AKL<br />
10203012082 10/08/2010<br />
AKL<br />
10201012083 10/08/2010<br />
AKL<br />
20304012084 10/08/2010<br />
CONFIRM anti-Progesterone<br />
Receptor (PR) (1E2) Rabbit<br />
Monoclonal Primary Antibody<br />
VENTANA HER2 3-in-1 Xenograft<br />
Control Slides<br />
VENTANA PATHWAY anti-HER-<br />
2/neu (4B5) Rabbit Monoclonal<br />
Primary Antibody<br />
VENTANA ultra view SISH Detection<br />
Kit<br />
AKL<br />
20205603421 30/07/2010 TECHROM IV Plus and Accessories<br />
AKL<br />
20103012070 30/07/2010 ADVIA Chemistry Digoxin<br />
AKL<br />
20103012071 10/08/2010<br />
AKL<br />
20101012072 10/08/2010<br />
AKL<br />
20101012091 10/08/2010<br />
AKL<br />
20101012092 10/08/2010<br />
AKL<br />
20305012093 10/08/2010<br />
Dimension Lidocaine Flex Reagent<br />
cartridge/LIDO<br />
Dimension Glucose Flex Reagent<br />
cartridge/GLUC<br />
Rapidpoint 350 Blood Gas Analyzer<br />
and Accessories<br />
Rapidpoint 340 Blood Gas Analyzer<br />
and Accessories<br />
IMMULITE Ferritin Control<br />
Module/FER<br />
AKL<br />
20305012094 10/08/2010 IMMULITE B-2 Microglobulin<br />
AKL<br />
10101012095 10/08/2010<br />
AKL<br />
20101012096 10/08/2010 Dimension CKI/MBI Calibrator<br />
AKL<br />
20101012085 10/08/2010 BETACHECK G5 Test Strips<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS GmbH.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
MEDICA CORPORATION., USA melalui<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
MEDICA CORPORATION., USA melalui<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
DCA Systems<br />
USA melalui SIEMENS HEALTHCARE<br />
Microalbumin/Creatinine Control (L,H) DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
NATIONAL DIAGNOSTIC PRODUCTS PTY.<br />
LTD., AUSTRALIA<br />
AKL<br />
20209703408 28/12/2010 KUBOTA Refrigerated Centrifuge KUBOTA MANUFACTURING CORP., JAPAN<br />
AKL<br />
20209701866 28/12/2010<br />
AKL<br />
20209701872 28/12/2010<br />
DIAMED-MP Test A-B-AB-D-ctl/A1-<br />
B-O<br />
DIAMED-MP Test A-B-AB-D-ctl/A1-<br />
A2-B<br />
DIAMED GmbH., SWITZERLAND<br />
DIAMED GmbH., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
10102601241 16/08/2010 DIALAB Urine Strip Analyzer DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20209700462 28/12/2010<br />
KUBOTA Large Capacity<br />
Refrigerated Centrifuge<br />
KUBOTA MANUFACTURING CORP., JAPAN<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
10102012198 19/08/2010<br />
BIOSYSTEM BTS-350 Semi Auto<br />
Clinical Analyzer and Accessories BIOSYSTEM S.A., SPAIN PT. MULTISERA INDOSA<br />
AKL<br />
10302012199 19/08/2010 MERCKOPLATE Rice Extract Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201012200 19/08/2010<br />
AKL<br />
20305012201 19/08/2010<br />
AKL<br />
20205012202 19/08/2010<br />
AKL<br />
20205012203 19/08/2010<br />
AKL<br />
20205603029 20/08/2010<br />
Sputofluol for Microbiology and<br />
Microscopy MERCK KGaA., GERMANY PT. MERCK Tbk<br />
PROCLEIX Ultrio Plus Assay<br />
Calibrators<br />
CoaDATA 4004, 4-Channel<br />
Coagulation Analyzer and<br />
Accessories<br />
CoaLAB 1000, Fully Automated<br />
Coagulation Analyzer and<br />
Accessories<br />
GEN PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
LABITEC (LABOR BIOMEDICAL<br />
TECHNOLOGIES GMBH)., GERMANY<br />
LABITEC (LABOR BIOMEDICAL<br />
TECHNOLOGIES GMBH)., GERMANY<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
DIATRON arcus Pro Human<br />
Hematology Analyzer DIATRON MI KFT., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10208012229 20/08/2010 GUAVA 1x Lysing Solution GUAVA TECHNOLOGIES INC., USA PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20303012231 20/08/2010 MBDr PanLF Rapid Test<br />
MALYSIAN BIO-DIAGNOSTICS RESEARCH<br />
SDN. BHD (MBDr)., MALAYSIA<br />
PT. RIMATANDU MOTARO<br />
PRATAMA<br />
AKL<br />
20101012230 20/08/2010 WOMAN CHOICE Pregnancy Test IND DIAGNOSTICS INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
10302604667 03/08/2010 OXOID SS Agar Modified OXOID LIMITED,UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302605031 03/08/2010<br />
OXOID Mac Conkey Agar Without<br />
Salt OXOID LIMITED,UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302605036 03/08/2010 OXOID Tryptose OXOID LIMITED,UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305601915 03/08/2010 PANASSAY IV-C ( Latex ) SEKISUI MEDICAL CO,LTD,Japan<br />
AKL<br />
10302012249 20/08/2010 PROCLEIX Auto Detect Reagent<br />
AKL<br />
10302012250 20/08/2010<br />
PROCLEIX System Fluid<br />
Preservative<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
GEN-PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305012251 20/08/2010 SD Chikungunya IgM Elisa STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305012252 20/08/2010 SD Bioline Tsutsugamushi STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101012253 20/08/2010<br />
dBest 3 parameter Urine Reagent<br />
Strip for Urinalysis (pH, Glucose,<br />
Protein) AMERITEK USA INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101012254 20/08/2010<br />
dBest 10 parameter Urine Reagent<br />
Strip for Urinalysis ( Glucose,<br />
Bilirubin, Keton, Specific Gravity, AMERITEK USA INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101012255 20/08/2010<br />
dBest 2 parameter Urine Reagent<br />
Strip for Urinalysis ( Glucose, Keton) AMERITEK USA INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101012256 20/08/2010<br />
dBest 1 parameter Urine Reagent<br />
Strip for Urinalysis ( Glucose Test) AMERITEK USA INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10101012232 20/08/2010 RANDOX Direct LDL Cholesterol RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
10101012233 20/08/2010 RANDOX Uric Acid RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
10101012234 20/08/2010 RANDOX Cholesterol RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20101012235 20/08/2010 RANDOX Direct Bilirubin RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20101012236 20/08/2010<br />
RANDOX Glucose GOD - PAP<br />
(GLUC-PAP) RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
10102604594 20/08/2010 Stat Fax 2600 Microplate Washer AWARENESS TECHNOLOGY INC., USA<br />
AKL<br />
10102604595 20/08/2010<br />
Stat Fax 1904 Plus Chemistry<br />
Analyzer and Accessories<br />
AWARENESS TECHNOLOGY INC., USA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10102604596 20/08/2010<br />
AKL<br />
20205012260 20/08/2010<br />
AKL<br />
20208505027 20/08/2010<br />
AKL<br />
20305600011 20/08/2010<br />
AKL<br />
20305600010 20/08/2010<br />
Stat Fax 2200 Incubator/Shaker and<br />
and Accessories<br />
ADVIA 2120 Hematology System &<br />
Accessories<br />
ADVIA 120 3 IN 1 Test Point<br />
Hematology Control (L, N, H)<br />
VERSANT HCV Amplification 2.0<br />
(LiPA)<br />
VERSANT HCV Genotype Assay 2.0<br />
(LiPA)<br />
AKL<br />
20101602858 20/08/2010 ADVIA Centaur Calibrator B<br />
AKL<br />
10101012336 27/08/2010<br />
AWARENESS TECHNOLOGY INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED, HONGKONG<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
dBest One Step LH Ovulation Card<br />
Test AMERITEK USA, USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20205012337 27/08/2010 HEMOCUE Hb 201 + Microcuvette HEMOCUE AB., SWEDEN PT. PUTERA SEGARA ANAKAN<br />
AKL<br />
20205012338 27/08/2010 HEMOCUE Hb 201 + Analyzer HEMOCUE AB., SWEDEN PT. PUTERA SEGARA ANAKAN<br />
AKL<br />
10302012339 27/08/2010<br />
TECHLAB C. Diff Quik Chek<br />
Complate<br />
TECHLAB INC, USA melalui INVERNESS<br />
MEDICAL, AUSTRALIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
10101012340 27/08/2010 SYSMEX UIBC-L,R1 & PL.R1 SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101012341 27/08/2010 SYSMEX UIBC-L,R2 & PL.R2 SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101012342 27/08/2010 SYSMEX Fe-L.R2 & Fe-PL.R2 SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101012343 27/08/2010 SYSMEX CHE-L.R1 dan CHE-L.R2 SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101012344 27/08/2010 SYSMEX Fe-L.R1 & Fe-PL.R1 SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
10101012345 27/08/2010<br />
SYSMEX HDL.C.KL.R2 &<br />
HDL.C.P.KL.R2 SYSMEX CORPORATION, JAPAN PT. SYSMEX INDONESIA<br />
AKL<br />
20102012362 01/09/2010 ABL 90 FLEX ANALYZER RADIOMETER MEDICAL ApS., DENMARK<br />
AKL<br />
10102011528 02/07/2010 GRIFOLS DG Therm<br />
AKL<br />
10102011529 02/07/2010 GRIFOLS DG Spin<br />
DIAGNOSTIC GRIFOLS S.A., SPIN melalui<br />
GRIFOLS ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
DIAGNOSTIC GRIFOLS S.A., SPIN melalui<br />
GRIFOLS ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20205012367 01/09/2010 MEDONIC M-Series M16 BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20305012369 01/09/2010 BIOLAND NanoSign HCV BIOLAND LTD., REPUBLIC OF KOREA PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
20305012370 01/09/2010 BIOLAND NanoSign HBs BIOLAND LTD., REPUBLIC OF KOREA PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
20303012371 01/09/2010 BIOLAND nanoSign Syphilis BIOLAND LTD., REPUBLIC OF KOREA PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
20303012372 01/09/2010<br />
BIOLAND nanoSign malaria Pf/Pv<br />
Device BIOLAND LTD., REPUBLIC OF KOREA PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
20205012375 01/09/2010 MEDONIC M-Series M20M-GP MPA BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20101012474 03/09/2010 VACUPRO 3.8% Sodium Citrate<br />
AKL<br />
20101012475 03/09/2010 VACUPRO Plain<br />
AKL<br />
20101012395 03/09/2010 VACUPRO Lithium Heparin<br />
AKL<br />
20101012396 03/09/2010 VACUPRO Clot Activator<br />
AKL<br />
20101012397 03/09/2010 VACUPRO Gel Clot Activator<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101012398 03/09/2010 VACUPRO Sodium Heparin<br />
AKL<br />
20101012399 03/09/2010<br />
VACUPRO Sodium Flouride<br />
Potassium Oxalate<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
FUZHOU CHANGGENG MEDICAL DEVICE<br />
CO. LTD., CHINA melalui SUNPHORIA CO.<br />
LTD., TAIWAN<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
AKL<br />
10302012499 06/09/2010 RapID SS/u Panel REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302012500 06/09/2010 RapID Staph Plus Panel REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101012400 03/09/2010<br />
AKL<br />
20101012401 03/09/2010<br />
AKL<br />
20101012402 03/09/2010<br />
AKL<br />
20101012403 03/09/2010<br />
AKL<br />
20101012404 03/09/2010<br />
INSEPACK Vacuum Blood<br />
Collection Tube Cap Yellow - Gel +<br />
Clot Activator<br />
INSEPACK Vacuum Blood<br />
Collection Tube Cap Blue - 3.8%<br />
Sodium Citrate<br />
INSEPACK Vacuum Blood<br />
Collection Tube Cap Green - Lithium<br />
Heparin<br />
INSEPACK Vacuum Blood<br />
Collection Tube Cap Purple - EDTA-<br />
3K<br />
INSEPACK Vacuum Blood<br />
Collection Tube Cap Red - Clot<br />
Activator<br />
SEKISUI MEDICAL TECHNOLOGY (CHINA)<br />
LTD., CHINA<br />
SEKISUI MEDICAL TECHNOLOGY (CHINA)<br />
LTD., CHINA<br />
SEKISUI MEDICAL TECHNOLOGY (CHINA)<br />
LTD., CHINA<br />
SEKISUI MEDICAL TECHNOLOGY (CHINA)<br />
LTD., CHINA<br />
SEKISUI MEDICAL TECHNOLOGY (CHINA)<br />
LTD., CHINA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
AKL<br />
20101012462 03/09/2010 BPC BIOSED Keylab Albumine BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012463 03/09/2010 BPC BIOSED Keylab Creatinine BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012464 03/09/2010<br />
BPC BIOSED Keylab Alkaline<br />
Phosphatase BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012465 03/09/2010 BPC BIOSED Keylab Direct Bilirubin BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012466 03/09/2010 BPC BIOSED Keylab Glucose BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012467 03/09/2010 BPC BIOSED Keylab Urinary Protein BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012468 03/09/2010 BPC BIOSED Keylab GPT/ALT BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012469 03/09/2010 BPC BIOSED Keylab Tryglicerides BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012509 06/09/2010 KEYLAB Uric Aid BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012510 06/09/2010 KEYLAB Gamma GT BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012511 06/09/2010 KEYLAB Magnesium BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012512 06/09/2010 KEYLAB HDL Cholesterol BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012513 06/09/2010 KEYLAB Cholesterol BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012451 03/09/2010 KEYLAB BUN UV (UREA) BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012452 03/09/2010 KEYLAB total Protein BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012453 03/09/2010 KEYLAB Multicalibrator-e BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101012454 03/09/2010 KEYLAB GOT/AST BPC BIOSED S.R.L., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303012460 03/09/2010<br />
AKL<br />
20305012461 03/09/2010<br />
ACON Syphilis Ultra Rapid Test<br />
Strip<br />
On Site Filariasis IgG/IgM Combo<br />
Rapid Test<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
CTK BIOTECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303012506 06/09/2010 SD BIOLINE JEV IgM STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20103012507 06/09/2010<br />
SD BIOLINE DOA MULTI-3<br />
(MET/THC/MOP) STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303012508 06/09/2010 SD BIOLINE Toxoplasma IgG/IgM STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303012504 06/09/2010 IMMUNOQUICK MALARIA + 4<br />
AKL<br />
BIOSYNEX S.A.R.L, FRANCE atas<br />
penunjukan ALLDIAG, FRANCE<br />
10101504865 06/09/2010 STANBIO Triglyceride Liquicolor STANBIO LABORATORIES, USA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com<br />
PT. MITRAASA PRATAMA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA
AKL<br />
10101504863 06/09/2010 STANBIO Uric Acid Liquicolor STANBIO LABORATORIES, USA<br />
AKL<br />
10101504864 06/09/2010 STANBIO Cholesterol Liquicolor STANBIO LABORATORIES, USA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20205012505 06/09/2010 MEDONIC M-SERIES M16SBD BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20305602718 06/09/2010<br />
DIALAB CRP Uhs Calibrator Series<br />
(High sensitivy range) DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602720 06/09/2010 DIALAB CRP Calibrator super High DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602681 06/09/2010 DIALAB CRP Calibrator High DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602721 06/09/2010 DIALAB CRP Control High DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602680 06/09/2010<br />
AKL<br />
20208012458 03/09/2010<br />
AKL<br />
20208012459 03/09/2010<br />
DIALAB CRP Calibrator 5 Level<br />
series DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
DIALAB HbA1c Direct Calibrator 4<br />
Level Series DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
DIALAB HbA1c Direct Control Set (2<br />
level) DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305602722 03/09/2010 DIALAB CRP Calibrator Low DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20207604592 03/09/2010<br />
DIALAB HEMOGLOBIN A1c Ion<br />
Exchange DIALAB GES.M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20902012470 03/09/2010 ACCU-CHECK safe-T-Pro-Uno ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20201012501 06/09/2010<br />
AKL<br />
20201012502 06/09/2010<br />
AKL<br />
20201012503 06/09/2010<br />
CONFIRM anti-Topoisomerase II<br />
(J55B4) Rabbitt Monoclonal Primary<br />
Antibody<br />
CONFIRM anti-Vimentin (V9)<br />
Primary Antibody<br />
CONFIRM anti-Ki-67 (30-9) Rabbit<br />
Monoclonal Primary Antibody<br />
AKL<br />
20301012646 16/09/2010 BD BBL MGIT AST SIRE Kit<br />
AKL<br />
20102012609 08/09/2010<br />
VENTANA MEDICAL SYSTEMS INC, USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC, USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
VENTANA MEDICAL SYSTEMS INC, USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
BECTON DICKINSON & COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
ABX PENTRA C 200 and<br />
Accessories HORIBA ABX DIAGNOSTICS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10201012657 21/09/2010 VENTANA ISH Protease 2<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10201012658 21/09/2010 VENTANA ISH Protease 1<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10201012659 21/09/2010<br />
VENTANA Endogenous Biotin<br />
Blocking Kit<br />
AKL<br />
20305012660 21/09/2010 SURE-TEZ HBsAg RAPID Test<br />
AKL<br />
20305012661 21/09/2010 SURE-TEZ HCV Ab rapid Test<br />
AKL<br />
20101012662 21/09/2010 SURE-TEZ HCG Combo rapid Test<br />
AKL<br />
10101012604 08/09/2010<br />
IMMULITE 2000 System<br />
Uncojugated Estriol UE3<br />
AKL<br />
10101012605 08/09/2010 ADVIA Centaur DHEAS<br />
AKL<br />
10204012606 08/09/2010 MD1355 sample Preparation System<br />
AKL<br />
10204012608 08/09/2010<br />
AKL<br />
20303012607 08/09/2010<br />
MD2005 Sample Preparation System<br />
DNA<br />
VIROSEQ HIV-1 Genotyping System<br />
Pack 2<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
BEIJING GENESEE BIOTECH., CHINA untuk<br />
CTK BIOTECH INC., USA<br />
BEIJING GENESEE BIOTECH., CHINA untuk<br />
CTK BIOTECH INC., USA<br />
BEIJING GENESEE BIOTECH., CHINA untuk<br />
CTK BIOTECH INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PROMEGA CORPORATION., USA melalui<br />
ABBOTT MOLECULAR INC., USA<br />
PROMEGA CORPORATION., USA melalui<br />
ABBOTT MOLECULAR INC., USA<br />
CELERA CORPORATION., USA melalui<br />
ABBOTT MOLECULAR INC., USA<br />
PT. ROCHE INDONESIA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
10205012602 08/09/2010 ALIFAX Test 1 THL ALIFAX S.p.A., ITALY PT. MULTISERA INDOSA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10304012601 08/09/2010<br />
VEDA-LAB Easy READER / Rapid<br />
Test Reader VEDA-LAB., FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20305012673 29/09/2010 FOOD DETECTIVE Professional<br />
AKL<br />
20805012762 07/10/2010<br />
BAXTER Solution Concentrate Basic<br />
for Hemodialisa<br />
CAMBRIDGE NUTRITIONAL SCIENCES<br />
LTD., UNITED KINGDOM<br />
INDUSTRIAL Y COMERCIAL BAXTER DE<br />
CHILE LTDA., CHILE<br />
PT. MULTIREDJEKI KITA<br />
PT. MENDJANGAN<br />
AKL<br />
20306012770 07/10/2010 BRAHMS CEA K-CAL B.R.A.H.M.S., A.G., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20306012771 07/10/2010 BRAHMS Total PSA K-CAL B.R.A.H.M.S., A.G., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20306012772 07/10/2010 BRAHMS CEA K-100 B.R.A.H.M.S., A.G., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20306012773 07/10/2010 BRAHMS Total PSA K-100 B.R.A.H.M.S., A.G., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20209011527 02/07/2010 GRIFOLS DG Gel Coombs<br />
AKL<br />
10101012724 04/10/2010<br />
AKL<br />
20101012725 04/10/2010<br />
AKL<br />
20205012721 04/10/2010<br />
AKL<br />
20205012720 04/10/2010<br />
SUNOSTIK Auto Biochemistry<br />
Analyzer SBA-733<br />
SUNOSTIK Fully Automatic Bio<br />
Chemistry Analyzer 6008<br />
DIAGNOSTICS GRIFOLS S.A., SPAIN melalui<br />
GRIFOLS ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SUNOSTIK MEDICAL TECHNOLOGY CO.<br />
LTD., CHINA<br />
SUNOSTIK MEDICAL TECHNOLOGY CO.<br />
LTD., CHINA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. DKSH INDONESIA<br />
PT. DKSH INDONESIA<br />
GUAVA Auto CD4/CD4% Antibody<br />
Coctail GUAVA TECHNOLOGIES., USA PT. INDOLABTEK DINAMIKA<br />
GUAVA Auto CD4/CD4% Reagent<br />
Kit GUAVA TECHNOLOGIES., USA PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20101012731 04/10/2010 RIA-gnost T4 CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101012727 04/10/2010 RIA-gnost hTSH CIO BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101012728 04/10/2010 RIA-gnost T3 CIO BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101012730 04/10/2010 RIA-gnost T4 CIO BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305012729 04/10/2010 THYROGLOBULINE IRMA CIO BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10201012723 04/10/2010 MERCK Microscopy Tb-color MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201012726 04/10/2010 VENTANA ISH Protease 3<br />
AKL<br />
20305604606 29/10/2010<br />
Sensatest One-Step Anti -HCV Test<br />
Cassette<br />
VENTANA MEDICAL SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS LT PT. ROCHE INDONESIA<br />
ATLAS LINK ( BEIJING ) tECHNOLOGY<br />
Co.,Ltd., China untuk NEOPHARMA BIOTECH<br />
ASIA Sdn.,Bhd.,Malaysia<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101705732 29/10/2010<br />
AKL<br />
20102012947 26/10/2010<br />
AKL<br />
20101013000 01/11/2010 KONELAB / T series eCal<br />
AKL<br />
20101013001 01/11/2010<br />
AKL<br />
20101013002 01/11/2010<br />
CERP2 Creatinine Plus Ver 2<br />
COBAS INTEGRA Cobas c Systems ROCHE DIAGNOSTICS Gmbh., Germany PT. ROCHE INDONESIA<br />
i-Smart 30 Electrolyte Analyzer and<br />
Accessories I-SENS INC., SOUTH KOREA PT. BEHRINDO NUSA PERKASA<br />
KONELAB / T series Total Protein<br />
Plus<br />
KONELAB / T series HDL/LDL<br />
Calibrator<br />
AKL<br />
10204013005 01/11/2010 Cytorich Red Collection Fluid<br />
AKL<br />
10204013006 01/11/2010 TBD - 2 Decalcifier<br />
AKL<br />
10204013007 01/11/2010 SHANDON Flo-Texx<br />
AKL<br />
10204013008 01/11/2010 SHANDON Reagent Alcohol<br />
AKL<br />
10204013010 01/11/2010 SHANDON Cryomatrix<br />
AKL<br />
10204013011 01/11/2010 TBD - 1 Decalcifier<br />
PDF Creator - PDF4Free v3.0<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
http://www.pdf4free.com<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA
AKL<br />
10204013012 01/11/2010 Glyo-Fixx<br />
AKL<br />
10201013013 01/11/2010 SHANDON Bluing Reagent<br />
AKL<br />
10201013014 01/11/2010 SHANDON Orange G-6<br />
AKL<br />
10201013015 01/11/2010 SHANDON EA-50 Stain<br />
AKL<br />
10201013016 01/11/2010 SHANDON Instant Eosin Alcoholic<br />
AKL<br />
10201013017 01/11/2010 SHANDON EA-65 Stain<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203013018 01/11/2010<br />
AKL<br />
10201013019 01/11/2010<br />
SHANDON Gross-Star Pathology<br />
Workstation and Accessories<br />
SHANDON RAPID CHROME<br />
Papanicolaou Staining Kit<br />
AKL<br />
10201013020 01/11/2010 SHANDON RAPID-FIXX<br />
AKL<br />
10201013021 01/11/2010 SHANDON Instatn Hematoxylin<br />
AKL<br />
10204013022 01/11/2010 CELL-FIXX<br />
AKL<br />
10201013023 01/11/2010 SHANDON INSTATN EOSIN<br />
AKL<br />
10101012985 01/11/2010<br />
AKL<br />
10101012986 01/11/2010<br />
KONELAB /T Series Uric Acid<br />
(AOX)<br />
KONELAB /T Series HDL<br />
CHOLESTEROL<br />
AKL<br />
10101012987 01/11/2010 KONELAB /T Series GAMMA GT<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203012988 01/11/2010 ClearVue Coverslipper THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101012989 01/11/2010 KONELAB /T Series Cholesterol<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203012990 01/11/2010 HISTOPLAST Paraffin Pellet THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204012991 01/11/2010 SHANDON Hypermount (EZ-Mount)<br />
AKL<br />
10204012992 01/11/2010<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
SHANDON Enviro-Tech Freezing<br />
Spray THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204012993 01/11/2010 SHANDON Xylene Substitute THERMO FISHER SCIENTIFIC., USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203012994 01/11/2010<br />
AKL<br />
10203012995 01/11/2010<br />
AKL<br />
10203012996 01/11/2010<br />
AKL<br />
10203012997 01/11/2010<br />
AKL<br />
10203012998 01/11/2010<br />
SHANDON CRYOTOME E<br />
Cryostate and Accessories THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
SHANDON CRYOTOME FSE<br />
Cryostate and Accessories THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
SHANDON CRYOTOME SME<br />
Cryostate and Accessories THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
HM 325 ROTARY MICROTOME<br />
and Accessories<br />
AKL<br />
10203012980 01/11/2010 LEICA CM1850UV Cryostat<br />
AKL<br />
10203012981 01/11/2010<br />
PDF Creator - PDF4Free v3.0<br />
MICROM INTERNATIONAL GMBH.,<br />
GERMANY untuk THERMO FISHER<br />
SCIENTIFIC INC., GERMANY<br />
PT. ENSEVAL MEDIKA PRIMA<br />
SHANDON PATHCENTRE And<br />
Accessories THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
LEICA RM2125RTS - Rotary<br />
Microtome<br />
LEICA BIOSYSTEM (NUSSLOCH) GMBH.,<br />
GERMANY melalui LEICA BIOSYSTEM PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM (NUSSLOCH) GMBH.,<br />
GERMANY melalui LEICA BIOSYSTEM PTE.<br />
LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC
AKL<br />
10203012982 01/11/2010<br />
AKL<br />
10203012983 01/11/2010<br />
LEICA TP1020 Automated Tissue<br />
Processor<br />
LEICA EG1150 Tissue Embedding<br />
Center<br />
AKL<br />
10203012984 01/11/2010 LEICA BOND MAX<br />
LEICA BIOSYSTEM (NUSSLOCH) GMBH.,<br />
GERMANY melalui LEICA BIOSYSTEM PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM (NUSSLOCH) GMBH.,<br />
GERMANY melalui LEICA BIOSYSTEM PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM (MELBOURNE) PTY.<br />
LTD AUSTRALIA melalui LEICA BIOSYSTEM<br />
PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
20201012897 25/10/2010<br />
VENTANA Ultraview Universal<br />
lkaline Phosphatase Red Detection<br />
Kit<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20201012898 25/10/2010 VENTANA Protease 3<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20201012899 25/10/2010 VENTANA Protease 2<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20201012900 25/10/2010 VENTANA Protease 1<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS L<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20205012883 25/10/2010 HEMOFAST Lecteur ALL DIAG S.A., FRANCE PT. MITRA ASA PRATAMA<br />
AKL<br />
20101012884 25/10/2010 ELSA-TSH Neonatal CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101012885 25/10/2010 CIS PSA-RIACT CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20102012886 25/10/2010 BIOLIS 12i<br />
AKL<br />
20102012887 25/10/2010 BIOLIS 24i Premium<br />
AKL<br />
20305012888 25/10/2010<br />
ABBOTT m2000rt Instrument and<br />
Accessories<br />
TOKYO BOEKI MEDICAL SYSTEM LTD.,<br />
JAPAN melalui DIALINE DIAGNOSTICS<br />
SYSTEM PTE. LTD., SINGAPORE<br />
TOKYO BOEKI MEDICAL SYSTEM LTD.,<br />
JAPAN melalui DIALINE DIAGNOSTICS<br />
SYSTEM PTE. LTD., SINGAPORE<br />
ABBOTT MOLECULAR INC., USA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
10101012889 25/10/2010 OPTIUM B-Ketone Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
AKL<br />
20306012890 25/10/2010 ARCHITECT SCC calibrator<br />
AKL<br />
20306012891 25/10/2010 ARCHITECT SCC Reagent Kit<br />
AKL<br />
20306012892 25/10/2010 ARCHITECT SCC Control<br />
AKL<br />
10204012893 25/10/2010 SYSMEX CellCheck - 400<br />
AKL<br />
20101012894 25/10/2010<br />
AKL<br />
20102012895 25/10/2010<br />
AKL<br />
20102012896 25/10/2010<br />
RADIOMETER ABL 800 Flex and<br />
Accessories<br />
XUNDA Electrolyte Analyzer XD 683<br />
(K/Na/Ci) and Accessories<br />
XUNDA Electrolyte Analyzer XD 685<br />
(K/Na/Ci/Ca/PH) and Accessories<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
RADIOMETER MEDICAL ApS., DENMARK<br />
SHANGHAI XUNDA MEDICAL INSTRUMENT<br />
CO. LTD., P.R. CHINA<br />
SHANGHAI XUNDA MEDICAL INSTRUMENT<br />
CO. LTD., P.R. CHINA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ABADINUSA USAHA<br />
SEMESTA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
AKL<br />
20208012821 08/10/2010 PreciControl HbA1c Path ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20208012822 08/10/2010 ROCHE HBA1C Control P ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20208012816 08/10/2010 ROCHE HBA1C Control N ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20208012820 08/10/2010<br />
AKL<br />
20101012902 25/10/2010<br />
PreciControl HbA1C Norm Roche<br />
System<br />
ARCHITECT Cyclosporine Whole<br />
Blood Precipitation Reagent Kit<br />
AKL<br />
20306012901 25/10/2010 ABBOTT Axsym CA 125 Controls<br />
AKL<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
AMERICAN BIOLOGICAL TECHNOLOGIES<br />
INC., USA untuk ABBOTT LABORATORIES.,<br />
USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, SLIGO-IRELAND<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
20102013153 05/11/2010 Selectra Pro M VITAL SCIENTIFIC B.V., THE NETHERLAND PT. MRK DIAGNOSTICS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101013086 01/11/2010 ARCHITECT T-Uptake Controls<br />
AKL<br />
20101013087 01/11/2010 ARCHITECT T-Uptake Reagent Kit<br />
AKL<br />
20306013118 02/12/2010 ARCHITECT HE4 Reagent Kit<br />
FISHER DIAGNOSTICS.,USA for ABBOTT<br />
LABORATORIES.,USA<br />
FISHER DIAGNOSTICS.,USA for ABBOTT<br />
LABORATORIES.,USA<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT GmbH & CO.KG., Germany<br />
AKL<br />
20303013136 05/11/2010 CORE malaria Pan/Pv/Pf CORE DIAGNOSTICS LIMITED., UK<br />
PT.ABBOTT INDONESIA<br />
PT.ABBOTT INDONESIA<br />
PT.ABBOTT INDONESAI<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
AKL<br />
20101013127 05/11/2010 EliTech LDH - L SL SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101013128 05/11/2010 EliTech Total Protein Plus SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101013120 05/11/2010 JOKOH Urine Calibrator Set JOKOH CORPORATION LTD., JAPAN<br />
AKL<br />
20101013165 05/11/2010<br />
AKL<br />
30305013204 05/11/2010<br />
MEDI-TEST Combi 11 (Blood,<br />
Urobilinogen, Bilirubin, Protein, Nitrite, MACHEREY-NAGEL GMBH & CO. KG.,<br />
Ketones, Assorbic Acid, Glucos GERMANY<br />
PROCLEIX ULTRIO PLUS HIV-1<br />
Discriminatory Probe Reagents<br />
GEN PROBE INCORPORATED, USA untuk<br />
NOVARTIS VACCINESS AND DIAGNOSTICS<br />
INC., USA<br />
AKL<br />
20101013121 05/11/2010 JOKOH standard Solution 2 JOKOH CORPORATION LTD., JAPAN<br />
AKL<br />
20101013122 05/11/2010 JOKOH Standard Solution 1 JOKOH CORPORATION LTD., JAPAN<br />
AKL<br />
10204013073 01/11/2010 JOKOH Washing Solution JOKOH CORPORATION LTD., JAPAN<br />
AKL<br />
10204013074 01/11/2010 JOKOH Conditioning Solution No. 2 JOKOH CORPORATION LTD., JAPAN<br />
AKL<br />
20101013272 05/11/2010 KONELAB / T Series Bilirubin Total<br />
AKL<br />
10203013273 15/11/2010 OVUTEST Scope<br />
AKL<br />
20305013274 15/11/2010 ROCHE C3 Fluid Pack<br />
AKL<br />
20305013275 15/11/2010 INFORM Chromosome 17 Probe<br />
AKL<br />
20201013276 15/11/2010<br />
CONFIRM anti-HER-2 / neu (4B5)<br />
Primary Antibody<br />
AKL<br />
20304013277 15/11/2010 INFORM HER2 DNA Probe<br />
AKL<br />
20101013278 15/11/2010 ACCU-CHEK Performa Test Strips<br />
AKL<br />
20304013279 15/11/2010<br />
COBAS s 201 System and<br />
Accessories<br />
AKL<br />
10101013262 15/11/2010 KONELAB / T Series ABTROL<br />
AKL<br />
10101013263 15/11/2010<br />
AKL<br />
10101013264 15/11/2010<br />
AKL<br />
10101013265 15/11/2010<br />
KONELAB / T series CK-MB Control<br />
High<br />
KONELAB / T Series LIPOTROL<br />
HDL/LDL Abnormal<br />
KONELAB / T Series LIPOTROL<br />
HDL/LDL Abnormal<br />
AKL<br />
10101013266 15/11/2010 KONELAB / T Series NORTROL<br />
AKL<br />
20102013267 15/11/2010<br />
KONELAB PRIME 30 and<br />
Accessories<br />
AKL<br />
20102013268 15/11/2010 KONELAB 20 and Accessories<br />
AKL<br />
10204013009 01/11/2010 SHANDON Formal - Texx<br />
AKL<br />
20102013269 15/11/2010<br />
PDF Creator - PDF4Free v3.0<br />
KONELAB PRIME 60 and<br />
Accessories<br />
THERMOFISHER SCIENTIFIC OY.,<br />
FINLAND<br />
CHENG DU DING HAO MEI LIAN INDUSTRY<br />
CO. LTD., CHINA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
RICHARD ALLEN SCIENTIFIC CO., USA<br />
untuk THERMO FISHER SCIENTIFIC INC.,<br />
USA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
http://www.pdf4free.com<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. MRK DIAGNOSTICS<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. DANPAC PHARMA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA
AKL<br />
20101013270 15/11/2010<br />
KONELAB / T Series Alkaline<br />
Phosphatase (IFCC) Plus<br />
AKL<br />
20101013271 15/11/2010 KONELAB / T Series lucose (HK)<br />
AKL<br />
10203013303 19/11/2010<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
SHANDON EXCELSIOR ES and<br />
Accessories THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203013302 19/11/2010 SHANDON Varistain Gemini ES THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203013305 19/11/2010 SHANDON Finesse E+ THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10203013304 19/11/2010 SHANDON Finesse ME+ THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL 001<br />
15 Desember<br />
2010 warming bed - -<br />
AKL<br />
10302013088 01/11/2010 DIALAB ASO Calibrator Low DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101013090 01/11/2010 DIALAB Apolipoprotein B DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10302013091 01/11/2010 DIALAB ASO Calibrator Super High DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10303013089 01/11/2010 DIALAB ASO calibrator High DIALAB GES M.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20306013117 05/11/2010 ARCHITECT HE4 Control<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20306013116 05/11/2010 ARCHITECT HE4 Calibrator<br />
AKL<br />
20301604155 22/12/2010<br />
AKL<br />
20101012948 28/12/2010<br />
AKL<br />
10201013772 28/12/2010<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
BD BBL SENSI-DISC<br />
Spectinonmycin SPT-100 ug BECTON DICKINSON & COMPANY.,USA PT.ANUGRAH ARGON MEDICA<br />
MISSION Urine Analysis Protein<br />
Test Strip ( 1 Parameter)<br />
ACON LABORATORIES., CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
MERCK Leucognost Mixture of<br />
Fixatives MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201013773 28/12/2010 MERCK Tb-Flour Phenol-Free MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201013774 28/12/2010 MERCK PAS Kit MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20101013768 28/12/2010 UF II Calibrator<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10204013763 28/12/2010 CLEAN Cobas C III ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
10207013764 28/12/2010 NaCi Diluent 9% Cobas C III ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20101013765 28/12/2010 GESAN Urea GESAN PRODUCTION S.R.L., ITALY PT. LABTAMA ADISINDO<br />
AKL<br />
10204013766 28/12/2010 Alkaline Washing Solution<br />
CLEAN CHEMICAL CO. LTD., JAPAN untuk<br />
TOKYO BOEKI MACHINERY LTD., JAPAN<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
AKL<br />
10204013767 28/12/2010 Acid Washing Solution<br />
AKL<br />
20101013762 28/12/2010 ADVIA entaur Tni-Ultra Ready Pack<br />
AKL<br />
20207013759 28/12/2010<br />
AKL<br />
20207013760 28/12/2010<br />
AKL<br />
20207013761 28/12/2010<br />
AKL<br />
20301013758 28/12/2010<br />
HELENA Actalyke Clotting Time Test<br />
System K-ACT<br />
HELENA Actalyke Clotting Time Test<br />
System C-ACT<br />
HELENA Actalyke Clotting Time Test<br />
System MAX-ACT<br />
AKL<br />
20101013757 28/12/2010 ARCHITECT Insulin Calibrator<br />
CLEAN CHEMICAL CO. LTD., JAPAN untuk<br />
TOKYO BOEKI MACHINERY LTD., JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
HELENA LABORATORIES., USA<br />
HELENA LABORATORIES., USA<br />
HELENA LABORATORIES., USA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SIEMENS INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
BD BBL SENSI-DISC Minocycline<br />
MI 30 ug BECTON DICKINSON & COMPANY., USA PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101013755 28/12/2010 ARCHITECT Cortisol Calibrator<br />
AKL<br />
20101013756 28/12/2010 ARCHITECT Cortisol Reagent Kit<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT LABORATORIES., USA<br />
FISHER DIAGNOSTICS a DIVISION of<br />
THERMO SCIENTIFIC CO. LLC., USA untuk<br />
ABBOTT LABORATORIES INC., USA<br />
FISHER DIAGNOSTICS., USA untuk<br />
ABBOTT LABORATORIES., USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303013754 28/12/2010 BIORAD Platelia Aspergillus EIA<br />
AKL<br />
10102403593 28/12/2010 PLATE WASHER<br />
AKL<br />
10302013309 19/11/2010 HERACELL CO2 Incubator<br />
AKL<br />
10201013130 05/11/2010<br />
BIORAD LABORATORIES., FRANCE atas<br />
penunjukan BIORAD LABORATORIES<br />
(SINGAPORE) PTE. LTD., SINGAPORE<br />
DIGITAL AND ANALOG SYSTEM (DAS)<br />
S.R.L., ITALY<br />
THERMO ELECTRON LED GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE<br />
PT. DOS NI ROHA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NEW MODULE<br />
INTERNATIONAL<br />
MERCK Tb-fluor Staining kit for<br />
fluorescence microscopic detection<br />
of acid fast bacteria MERCK KGaA.,Germany PT.MERCK Tbk.,<br />
AKL<br />
20305013311 19/11/2010 LightCyclear SeptiFast Kit M ROCHE DIAGNOSTICS GmbH,Germany PT.ROCHE INDONESIA,<br />
AKL<br />
20305013310 19/11/2010 LightCyclear Septifast mecA Kit M ROCHE DIAGNOSTICS Gmbh, Germany PT.ROCHE INDONESIA<br />
AKL<br />
10101013071 01/11/2010 DIAGON HDL Cholesterol Direct DIAGON KFT,Hungary PT.INTI DIAGONTAMA SELARAS<br />
AKL<br />
10102013072 01/11/2010 JOKO Diluent for Urine JOKOH CORPORATION LTD.japan PT SUMBERMITRA AGUNGJAYA<br />
AKL<br />
20102013084 01/11/2010 HumaSens Pluse HUMAN GmbH.,Germany PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101013085 01/11/2010 ARCHITECT T-Uptake Calibrator<br />
FISHER DIAGNOSTICS., USA for ABBOTT<br />
LABORATORIES., USA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20208013081 01/11/2010 HEMOFAST Controles ALL DIAG S.A.,France PT.MITRA ASA PRATAMA.,<br />
AKL<br />
10101013082 01/11/2010<br />
PreciControl Universal Elecsys and<br />
cobas e analyzers<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS Intl,<br />
Ltd.,Switzerland<br />
AKL<br />
20101013063 01/11/2010 SENTINEL urea Liquid SENTINEL CH.SpA.,Italy<br />
AKL<br />
20101013064 01/11/2010 SENTINEL Clin Chem Cal SENTINEL CH.SpA.,Italy<br />
AKL<br />
20101013065 01/11/2010 SENTINEL Glucose Liquid SENTINEL CH.SpA.,Italy<br />
AKL<br />
20101013066 01/11/2010 SENTINEL Creatinine liquid SENTINEL CH.SpA.,Italy<br />
AKL<br />
20101013067 01/11/2010 SENTINEL AST UV Liquid SENTINEL CH.SpA.,Italy<br />
AKL<br />
20101013068 01/11/2010 PAXgene Blood RNA Tube<br />
DECTON DICKINSON AND<br />
COMPANY,Singapore<br />
PT.ROCHE INDONESIA<br />
PT TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT.TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT.TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT.TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT.ANUGRAH ARGONE MEDIKA<br />
AKL<br />
20207013069 01/11/2010 RONDOX Hemoglobin assay RANDOX LABORATORIES LTD.,UK PT.RAFA TOPAZ UTAMA<br />
AKL<br />
20101013070 01/11/2010 RANDOX Total Bilirubin RANDOX LABORATORIES LTD.,UK PT.RAFA TOPAZ UTAMA<br />
AKL<br />
10101013159 05/11/2010 IMMULITE Estradiol Sample Diluent<br />
AKL<br />
10103013160 05/11/2010<br />
AKL<br />
10101013157 05/11/2010<br />
SIEMENS serum Drug Control<br />
Module<br />
SIEMENS Rapid QC Complete Level<br />
1<br />
AKL<br />
10101013158 05/11/2010 ADVIA Chemistry Cholesterol 2<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT.SIEMENS INDONESIA<br />
PT.SIEMENS INDONESIA<br />
PT.SIEMENS INDONESIA<br />
PT.SIEMENS INDONESIA<br />
AKL<br />
20306013138 05/11/2010 RIA - gnost AFP CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306013140 05/11/2010 ELSA CA 19-9 CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306013137 05/11/2010 ELSA CA 125 II CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20303013132 05/11/2010<br />
Sure-Tez Dengue IgG/IgM Combo<br />
Rapid Test<br />
BEIJING GENESSE BIOTECH INC melalui<br />
CTK BIOTECH INC., USA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101013075 01/11/2010 ESTR - CTRIA CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101013076 01/11/2010 TESTO - CT2 CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101013077 01/11/2010 PROG - CTRIA CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306013080 01/11/2010 CEA - RIACT CIS BIO INTERNATIONAL., FRANCE PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305013119 05/11/2010<br />
AKL<br />
SPEEDYTEST HBsAg (Hepatitis B<br />
Surface Antigen) Serum/Plasma Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
20305013079 01/11/2010 HEMASCREEN POOL DIAGAST., FRANCE PT. MEDQUEST JAYA GLOBAL<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101013142 05/11/2010 MEDI-TEST Control N & P<br />
AKL<br />
10102013162 05/11/2010 GENARRAYT 200+Food IgG Kit<br />
AKL<br />
10302013146 05/11/2010 HERACELL 240i CO2 Incubator<br />
AKL<br />
10302013145 05/11/2010<br />
HERASAFE Biological Safety<br />
Cabinet KS 12<br />
AKL<br />
20305013148 05/11/2010 Cardio Detect Lab<br />
AKL<br />
20305013149 05/11/2010 Cardio Detect Combi<br />
AKL<br />
20103013124 05/11/2010<br />
AKL<br />
20103013125 05/11/2010<br />
AKL<br />
20103013126 05/11/2010<br />
AKL<br />
20102013131 05/11/2010<br />
AKL<br />
20103013123 05/11/2010<br />
AKL<br />
20303013163 05/11/2010 ADVIA Centaur Toxo G QC<br />
AKL<br />
20303013164 05/11/2010 ADVIA Centaur Toxo G<br />
AKL<br />
20303013141 05/11/2010 ADVIA Centaur Rub G<br />
AKL<br />
20305013152 05/11/2010<br />
AKL<br />
20305013154 05/11/2010<br />
AKL<br />
20305013155 05/11/2010<br />
AKL<br />
20305013156 05/11/2010<br />
AKL<br />
20303013150 01/11/2010<br />
AKL<br />
20303013151 05/11/2010<br />
MACHEREL NAGEL GMBH & CO. KG.,<br />
GERMANY<br />
GENESIS DIAGNOSTICS LTD., UNITED<br />
KINGDOM<br />
THERMO ELECTRON LED GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LED., SINGAPORE<br />
THERMO ELECTRON LED GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE<br />
RENNESENS GMBH., GERMANY atas<br />
penunjukan TRIMEDIS CARDIO PVT. LTD.,<br />
INDIA<br />
RENNESENS GMBH., GERMANY atas<br />
penunjukan TRIMEDIS CARDIO PVT. LTD.,<br />
INDIA<br />
PT. MRK DIAGNOSTICS<br />
PT. MULTIREDJEKI KITA<br />
PT. NEW MODULE<br />
INTERNATIONAL<br />
PT. NEW MODULE<br />
INTERNATIONAL<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
RANDOX Cannabinoid (THC) RX<br />
Series RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
RANDOX Amphetamines (AMPH)<br />
RX Series RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
RANDOX Benzodiazepine (BENZ)<br />
RX Series RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
RANDOX Evidence Investigator<br />
Analyzer RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
RANDOX Opiates (OPIAT) RX<br />
series RANDOX LABORATORIES LTD., UK PT. RAFA TOPAZ UTAMA<br />
ABBOTT RealTime HCV Calibrator<br />
Kit<br />
ABBOTT RealTime HIV-1<br />
Amplification Reagent Kit<br />
ABBOTT RealTime High Risk HPV<br />
Amplification Reagent Kit<br />
ABBOTT RealTime High Risk HPV<br />
Control Kit<br />
ABBOTT RealTime CT/NG<br />
Amplification Reagent Kit<br />
ABBOTT RealTime CT/NG Control<br />
Kit<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20305013114 05/11/2010 ABBOTT RealTime HIV-1 Control Kit ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20305013115 05/11/2010<br />
ABBOTT RealTime HIV-1 Calibrator<br />
Kit<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20303013134 05/11/2010 CORE Malaria PF CORE DIAGNOSTICS LIMITED., UK<br />
AKL<br />
20303013135 05/11/2010 CORE Dengue (IgG + IgM) CORE DIAGNOSTICS LIMITED., UK<br />
AKL<br />
10102013147 05/11/2010 Verify Urinalyzer U120<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
AKL<br />
10101013109 05/11/2010 SENTINEL ALT UV Liquid SENTINEL CH. SpA., ITALY<br />
AKL<br />
10101013110 05/11/2010 SENTINEL Cholesterol Liqiud SENTINEL CH. SpA., ITALY<br />
AKL<br />
10101013111 05/11/2010 SENTINEL Triglycerides Liquid SENTINEL CH. SpA., ITALY<br />
AKL<br />
10101013112 05/11/2010 SENTINEL Uric Acid Liquid SENTINEL CH. SpA., ITALY<br />
AKL<br />
10101013113 05/11/2010 SENTINEL Clin Chem Control 1 SENTINEL CH. SpA., ITALY<br />
AKL<br />
20209701868 05/11/2010 ID-DiaCell I-II-III Asia (Mia+) DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20209701869 05/11/2010 ID-DiaPanel DIAMED GMBH., SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. SUMBER MANDIRI<br />
<strong>ALKES</strong>TRON<br />
PT. TIRTA MEDILAB<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. TRIPATRIA ANDALAN<br />
MEDIKA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20101600472 05/11/2010 SENSITIF Compact SURESCREEN DIAGNOSTICS LTD., UK PT. DANPAC PHARMA<br />
AKL<br />
10201012722 04/10/2010<br />
PDF Creator - PDF4Free v3.0<br />
MERCK Schiff's Reagent for<br />
Microscopy and for Electrophoresis MERCK KGaA.,Germany PT. MERCK Tbk<br />
http://www.pdf4free.com
AKL<br />
20209701865 05/11/2010 ID-DiaCell I-II DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20303013741 28/12/2010 INDEC BIO Cytolisa IgG<br />
AKL<br />
20306013729 28/12/2010 INDEC BIO Carsinolisa CA 125<br />
AKL<br />
20101013544 20/12/2010 PAXgene Blood RNA Tube<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, JAKARTA<br />
BECTON DICKINSON AND COMPANY.,<br />
UNITED KINGDOM<br />
AKL<br />
10102703410 15/11/2010 EFK Hemo - Control Cuvette EFK DIAGNOSTICS GMBH., GERMANY<br />
AKL<br />
10102703409 15/11/2010 EFK Hemo - Control EFK DIAGNOSTICS GMBH., GERMANY<br />
AKL<br />
10209703407 15/11/2010 CENTRON Blood Collection Mixer CENTRON TECHNOLOGIES INC., USA<br />
AKL<br />
20305013693 22/12/2010<br />
AKL<br />
20305013694 22/12/2010<br />
AKL<br />
20102013691 22/12/2010<br />
AKL<br />
20102013690 22/12/2010<br />
ABBOTT RealTime HBV<br />
Amplification Reagent Kit<br />
ABBOTT RealTime HCV<br />
Amplification Reagent Kit<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
BIOLABO Automatic Biochemistry<br />
Analyzer BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIOLABO Automatic Biochemistry<br />
Analizer BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20102013689 22/12/2010 KENZA MAX Biochemistry Analizer BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20205013687 22/12/2010<br />
AKL<br />
20205013688 22/12/2010<br />
AKL<br />
20101013433 22/12/2010<br />
AKL<br />
20101013432 15/12/2010<br />
BIO SOLEA 2 Semi Automatic<br />
Analizer for Haemostatis BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIO SOLEA 4 Semi Automatic<br />
Analizer for Haemostatis BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
WOMAN CHOICE Pregnancy Test<br />
Cassette IND DIAGNOSTICS INC., CANADA PT. LANIROS DIAN PHARMA<br />
WOMAN CHOICE Pregnancy Test<br />
Strip IND DIAGNOSTICS INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
10302013531 20/12/2010 VITEK 2 AST-P612 TEST CARD<br />
AKL<br />
10302013532 20/12/2010 VITEK 2 ANC TEST CARD<br />
AKL<br />
10302013533 20/12/2010 VITEK 2 AST-YS01 TEST CARD<br />
AKL<br />
10302013534 20/12/2010 VITEK 2 BCL TEST CARD<br />
AKL<br />
10102013535 20/12/2010<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
Liquid-bsed PopSpin Gynaecological<br />
Test THERMO FISHER SCIENTIFIC INC., UK PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20301013536 20/12/2010 VITEK 2 AST-P561 TEST CARD<br />
AKL<br />
20301013537 20/12/2010 VITEK 2 AST-N100 TEST CARD<br />
AKL<br />
20301013538 20/12/2010 VITEK 2 AST-NO89 TEST CARD<br />
AKL<br />
20301013539 20/12/2010 VITEK 2 AST-N156 TEST CARD<br />
AKL<br />
20301013540 20/12/2010 VITEK 2 AST-GP67 TEST CARD<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
BIOMERIEUX INC., USA untuk BIOMERIEUX<br />
SA., FRANCE<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20205013541 20/12/2010 AMPULAB Coagulation Tube SOYA GREENTEC., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20209013542 20/12/2010<br />
AMPULAB SSGT (Serum<br />
Separation Gel) Tube SOYA GREENTEC., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20305013543 20/12/2010 DiaMRSA Kit DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101013545 20/12/2010<br />
AKL<br />
10101013546 20/12/2010<br />
AKL<br />
20207013547 20/12/2010<br />
PreciControl ClinChem Multi 1<br />
Roche Systems<br />
PreciControl ClinChem Multi 2<br />
Roche Systems<br />
HBA1C III Tina-quant [a]<br />
Roche/hitachi<br />
AKL<br />
20305013548 20/12/2010 FERR4 Tina-quant [a] Feritin Gen-4<br />
AKL<br />
20305013549 20/12/2010<br />
PDF Creator - PDF4Free v3.0<br />
Ferritin Gen.4 Tina-quant<br />
Roche/Hitachi<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20305013551 20/12/2010<br />
DIAEXTRACT MICROBIAL DNA<br />
Isolation Kit DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20305013425 15/12/2010 INNOTEST HCV Ab IV INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
20305013692 22/12/2010 ABBOTT RealTime HBV Control Kit ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20101013696 22/12/2010<br />
KONELAB / T Series ALT/GPT<br />
(IFCC)<br />
AKL<br />
20101013697 22/12/2010 KONELAB / T eries Bilirubin Direct<br />
AKL<br />
20101013698 22/12/2010 KONELAB / T Series Creatinine<br />
THERMO FISHER SCIENTIFIC , OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC , OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC , OY.,<br />
FINLAND<br />
AKL<br />
THERMO FISHER SCIENTIFIC , OY.,<br />
20101013699 22/12/2010 KONELAB / T Series Albumin (BCG) FINLAND<br />
AKL<br />
20101013700 22/12/2010 KONELAB / T Series UREA<br />
AKL<br />
20101013695 22/12/2010<br />
KONELAB / T Series AST/GOT<br />
(IFCC)<br />
THERMO FISHER SCIENTIFIC , OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC , OY.,<br />
FINLAND<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305013709 22/12/2010 LG HBsAg ELISA LG Life Scientific Ltd., Korea PT. DKSH INDONESIA<br />
AKL<br />
20305013710 22/12/2010 LG HCD 3.0 Plus LG Life Scientific Ltd., Korea PT. DKSH INDONESIA<br />
AKL<br />
20306013728 28/12/2010 INDEC BIO Carsinolisa CA 19-9<br />
AKL<br />
20101013730 28/12/2010 INDEC BIO Pregnolisa hCG<br />
AKL<br />
20101013731 28/12/2010 INDEC BIO Thyrolisa Free T4<br />
AKL<br />
20303013732 28/12/2010 INDEC BIO Cytolisa IgM<br />
AKL<br />
20303013733 28/12/2010 INDEC BIO Toxolisa IgG<br />
AKL<br />
20303013734 28/12/2010 INDEC BIO Toxolisa IgM<br />
AKL<br />
20101013735 28/12/2010 INDEC BIO Thyrolisa T3<br />
AKL<br />
20101013736 28/12/2010 INDEC AGD Alkaline Phosphatase<br />
AKL<br />
20101013737 28/12/2010 INDEC AGD Bilirubin Direct<br />
AKL<br />
20101013738 28/12/2010 INDEC AGD Bilirubin Total<br />
AKL<br />
20101013739 28/12/2010 INDEC AGD Albumin<br />
AKL<br />
20101013740 28/12/2010 INDEC AGD Creatinine<br />
AKL<br />
20101013782 28/12/2010<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS., JAKARTA<br />
AGAPPE DIAGNOSTICS., INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS., INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS., INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS., INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS., INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
HUMASENS Plus Glucose Test<br />
Strips HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101013639 20/12/2010 HumaSens Uric Acid Test Strips HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101013640 20/12/2010<br />
AKL<br />
10201013161 05/11/2010<br />
AKL<br />
20301014015 29/12/2010<br />
AKL<br />
10101013615 20/12/2010<br />
HumaSens Total Cholesterol Test<br />
Strips HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
ADVIA Autoslide Wright Giemsa<br />
Stain<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
VITEK 2 Compact 30 Configuration<br />
and Accessories BIOMERIEUX INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
Konelab T series LDL-<br />
CHOLESTEROL<br />
AKL<br />
10101013616 20/12/2010 Konelab T series TRIGLYCERIDES<br />
AKL<br />
20101013004 11/01/2010 KONELAB T Series eCal<br />
AKL<br />
10101013973 28/12/2010 Architect Insulin Reagent<br />
AKL<br />
10101013974 28/12/2010 Architect Insulin Control<br />
AKL<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
DENKA SEIKEN., JAPAN for ABBOTT<br />
LABORATORIES., USA<br />
DENKA SEIKEN., JAPAN for ABBOTT<br />
LABORATORIES., USA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
10101013779 28/12/2010 BIOLABO EXATROL-N Level 1 BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101013778 28/12/2010 BIOLABO EXATROL-P Level 2 BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20101013922 28/12/2010 BIOLABO Multicalibrator BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20208013921 28/12/2010 BIOLABO Plasma Normal Value BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20305013634 20/12/2010 Immulite Cytokine Control Moduls<br />
AKL<br />
20303013635 20/12/2010 ADVIA Centaur Rubella M<br />
AKL<br />
20101013985 28/12/2010 ADVIA Chemistry prealbumin<br />
AKL<br />
10203013984 28/12/2010<br />
AKL<br />
10203013983 28/12/2010<br />
LEICA ASP300S - Automated<br />
Vacuum Tissue Processor<br />
LEICA HI1220 Flattening Table for<br />
Clinical Histopathology<br />
AKL<br />
10203013981 28/12/2010 LEICA HI1210 Water Bath<br />
AKL<br />
10203013982 28/12/2010<br />
LEICA EG1160 - Programmable<br />
tissue embedding station<br />
AKL<br />
10203013980 28/12/2010 LEICA RM2245<br />
AKL<br />
10102013743 28/12/2010 URIT Automatic Chemistry Analyzer<br />
AKL<br />
10102013742 28/12/2010 URIT Urine Analyzer<br />
AKL<br />
10203013979 28/12/2010<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK<br />
LEICA BIOSYSTEM EMS (NUSSLOCH)<br />
GMBH., GERMANY melalui LEICA<br />
BIOSYSTEMS PTE. LTD., SINGAPORE<br />
LEICA BIOSYSTEM EMS (NUSSLOCH)<br />
GMBH., GERMANY melalui LEICA<br />
BIOSYSTEMS PTE. LTD., SINGAPORE<br />
LEICA BIOSYSTEM EMS (NUSSLOCH)<br />
GMBH., GERMANY melalui LEICA<br />
BIOSYSTEMS PTE. LTD., SINGAPORE<br />
LEICA BIOSYSTEM EMS (NUSSLOCH)<br />
GMBH., GERMANY melalui LEICA<br />
BIOSYSTEMS PTE. LTD., SINGAPORE<br />
LEICA BIOSYSTEM EMS (NUSSLOCH)<br />
GMBH., GERMANY melalui LEICA<br />
BIOSYSTEMS PTE. LTD., SINGAPORE<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
Methenamine Silver Plating Kit acc.<br />
to Gomon MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10203013978 28/12/2010 Entellan new for Cover Slippers MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10201013977 28/12/2010 Masson-Goldner Staining Kit MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10203013976 28/12/2010 Elastica Van Gieson Staining Kit MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302013975 28/12/2010 Cult-Dip Plus Merck MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20208013968 28/12/2010 PRECINORM HBA1C<br />
AKL<br />
20101013969 28/12/2010 ACCU-CHECK Advantage II<br />
AKL<br />
10204013967 28/12/2010 ROCHE / HITACHI Hitergent<br />
AKL<br />
20101011503 29/06/2010<br />
AKL<br />
10101011286 11/06/2010<br />
AKL<br />
10101011286 11/06/2010<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
Testosterone II Calset II Elecsys and<br />
Cobas e Analyzers ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
ALFA WASSERMANN Direct<br />
HDL/LDL/ Cholesterol control 3 ALFA WASSERMANN B.V.,The Netherlands PT MARADA PHARMA MEDICA<br />
ALFA WASSERMANN Direct<br />
HDL/LDL/ Cholesterol control 3 ALFA WASSERMANN B.V.,The Netherlands PT MARADA PHARMA MEDICA<br />
AKL<br />
10102013752 28/12/2010 SD Urometer 720 STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10102013924 28/12/2010<br />
Humalyzer Primus (Semi Automatic<br />
Microprocessor Controlled<br />
Photometer) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20301013550 20/12/2010 OXOID S25 STREPTOMYCIN OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301604159 22/12/2010<br />
AKL<br />
20101605151 20/12/2010 MICROWELL ELISA TSH<br />
AKL<br />
20305605153 20/12/2010 MICROWELL ELISA HBsAg<br />
AKL<br />
20103013747 28/12/2010<br />
BD BBL SENSI-DISC Bacitracin B-<br />
10 BECTON DICKINSON & COMPANY., USA PT. ANUGRAH ARGON MEDICA<br />
NARCOTESR 3 Amphetamine<br />
(Shabu-shabu, ekstasi)<br />
DIAGNOSTIC AUTOMATION / COTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTIC AUTOMATION / COTEZ<br />
DIAGNOSTICS INC., USA<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGAPORE<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. DANPAC PHARMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208110229 11/02/2010<br />
CONVERGYS Dil Diff (D50) Diluent<br />
Solution<br />
AKL<br />
10101013963 28/12/2010 Indec, Agd Uric Acid<br />
AKL<br />
10101013961 28/12/2010 Indec, Agd Triglycerides<br />
AKL<br />
20101013740 28/12/2010 INDEC AGD Creatinine<br />
AKL<br />
10101013956 28/12/2010 Indec, Agd Cholesterol<br />
AKL<br />
20101013631 20/12/2010<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
Co. KG, Germany<br />
AGAPPE DIAGNOSTICS, INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS, INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS, INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
AGAPPE DIAGNOSTICS, INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
AMPULAB PLASMA Tube (Sodium<br />
Heparin) SOYAGREENTEC CO. LTD., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20101013633 20/12/2010 AMPULAB PLAIN Tube SOYAGREENTEC CO. LTD., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20101013630 20/12/2010<br />
AMPULAB GLUCOSE Preservation<br />
Tube (Sodium Flouride Potassium<br />
Oxalate) SOYAGREENTEC CO. LTD., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20101013632 20/12/2010 AMPULAB K-2 EDTA Tube SOYAGREENTEC CO. LTD., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20101013629 20/12/2010 AMPULAB K-3 EDTA Tube SOYAGREENTEC CO. LTD., KOREA PT. NEXTWAY ELECTRONICS<br />
AKL<br />
20301013617 20/12/2010 ETEST XM Cefuroxime AB BIOMERIEUX, SWEDEN PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103504547 28/12/2010 NARCOTEST 6 THC (Cannabis)<br />
AKL<br />
30305014001 28/12/2010 ENZYGNOST Anti HIV 1/2 Plus<br />
AKL<br />
20305013971 28/12/2010 BIOCARE HBsAg Rapid Test<br />
AKL<br />
20303013965 28/12/2010 BIORAD Platelia CMV IgM<br />
AKL<br />
20303013966 28/12/2010 BIORAD Platelia Candida Ag Plus<br />
AKL<br />
20303013964 28/12/2010 BIORAD Platelia CMV IgG<br />
AKL<br />
20303013960 28/12/2010<br />
AKL<br />
20303013959 28/12/2010<br />
AKL<br />
20303013958 28/12/2010<br />
Indec, Nova Herpelisa 2 IgG<br />
(Recombinant)<br />
Indec, Nova Herpalisa 2 IgM<br />
(Recombinant)<br />
Indec, Nova Herpalisa 1 IgM<br />
(Recombinant)<br />
AKL<br />
20303013957 28/12/2010 Indec, Nova Entamoebalisa IgG<br />
ATIMA INTERNATIONAL INC., CANADA atas<br />
penunjukan APOTHECA MARKETING PTE.<br />
LTD., SINGPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA<br />
BIO-RAD LABORATORIES LTD., FRANCE<br />
melalui BIO-RAD LABORATORIES<br />
(SINGAPORE) PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES LTD., FRANCE<br />
melalui BIO-RAD LABORATORIES<br />
(SINGAPORE) PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES LTD., FRANCE<br />
melalui BIO-RAD LABORATORIES<br />
(SINGAPORE) PTE. LTD., SINGAPORE<br />
NOVATEC IMMUNODIAGNOSTICA GMBH.,<br />
GERMANY dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, JAKARTA<br />
NOVATEC IMMUNODIAGNOSTICA GMBH.,<br />
GERMANY dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, JAKARTA<br />
NOVATEC IMMUNODIAGNOSTICA GMBH.,<br />
GERMANY dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, JAKARTA<br />
NOVATEC IMMUNODIAGNOSTICA GMBH.,<br />
GERMANY dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, JAKARTA<br />
PT. DANPAC PHARMA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BIOCARE SEJAHTERA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. DOS NI ROHA<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
AKL<br />
30305014002 28/12/2010 CARESTART HIV - 1/2 3 Lines ACCESS BIO INC., USA PT. INDOCORE PERKASA<br />
AKL<br />
10303013972 28/12/2010<br />
AKL<br />
10102013986 28/12/2010<br />
DALF PS Antistreptolysin-O<br />
Antibody Test<br />
AKL<br />
10101013923 28/12/2010 RANDOX ALT (GPT)<br />
AKL<br />
20208013970 28/12/2010 PRECIPATH HBA1C<br />
PULSE SCIENTIFIC., CANADA repacking by<br />
PT. JAFAREL MEDIATICS., JAKARTA<br />
PT. JAFAREL MEDIATICS<br />
METERTECH UV-Vis<br />
Spectrophotometer METERTECH INC., TAIWAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10204013625 20/12/2010 Cobas Taq-Screen Wash Reagent<br />
RANDOX LABORATORIES LTD., UNITED<br />
KINGDOM<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. RAFA TOPAZ UTAMA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20103013636 20/12/2010 COZART DDS Deluxe CONCATENO PLC, UK PT. ARYASA TEKAD PRATAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103013637 20/12/2010<br />
AKL<br />
20103013638 20/12/2010<br />
AKL<br />
20303013920 28/12/2010 BIOCARE Syphilis Rapid Test<br />
AKL<br />
20303013744 28/12/2010<br />
COZART DDS Drug Test Kits 6<br />
Panel<br />
(MTD/OPI/COC/AMP/MAMP/BZO) CONCATENO PLC, UK PT. ARYASA TEKAD PRATAMA<br />
COZART DDS Drug Test Kits 6<br />
Panel<br />
(THC/OPI/COC/AMP/MAMP/BZO) CONCATENO PLC, UK PT. ARYASA TEKAD PRATAMA<br />
ATLAS LINK ( BEIJING) TECHNOLOGY CO,<br />
LTD., China<br />
PT. BIOCARE SEJAHTERA<br />
CARESTART Malaria Device<br />
(Combo 3 Line, PAN-PLDH, P.f-<br />
HRP2 Test) ACCESS BIO INC, USA PT. INDOCORE PERKASA<br />
AKL<br />
10101013746 28/12/2010 MULTICARE IN Cholesterol Strip<br />
AKL<br />
10101013745 28/12/2010 MULTICARE IN Triglycerides Strip<br />
BIOCHEMICAL SYSTEMS INTERNATIONAL<br />
SrI,. Italy<br />
BIOCHEMICAL SYSTEMS INTERNATIONAL<br />
SrI,. Italy<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20305013750 28/12/2010 DALF PS C-Reactive Protein Test<br />
PULSE SCIENTIFIC INC, Canada Dikemas<br />
Ulang Oleh PT. JAFAREL MEDIATICS, Jakarta PT. JAFAREL MEDIATICS<br />
AKL<br />
20305013751 28/12/2010 DALF PS Rheumatoid Factor Test<br />
PULSE SCIENTIFIC INC, Canada Dikemas<br />
Ulang Oleh PT. JAFAREL MEDIATICS, Jakarta PT. JAFAREL MEDIATICS<br />
AKL<br />
10101013919 28/12/2010 GESAN ALT-GPT LR GESAN PRODUCTION s.r.I., Italy PT. LABTAMA ADISINDO<br />
AKL<br />
20305013783 28/12/2010 SD Bioline Tnl / Myo STANDARD DIAGNOSTICS INC, Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10102013753 28/12/2010 SD Urometer 120 STANDARD DIAGNOSTICS INC, Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20205013776 28/12/2010 HEMOFAST Carte Test ALL DIAG S.A, France PT. MITRAASA PRATAMA<br />
AKL<br />
20101013780 28/12/2010 RANDOX Total Protein RANDOX LABORATORIES LTD, UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
20101013781 28/12/2010 RANDOX AST RANDOX LABORATORIES LTD, UK PT. RAFA TOPAZ UTAMA<br />
AKL<br />
10102403594 28/12/2010 PLATE READER<br />
AKL<br />
20209211831 15/11/2010<br />
AKL<br />
20209211832 15/11/2010<br />
HELMER Blood Blank Refrigerator<br />
HB 111<br />
HELMER Blood Blank Refrigerator<br />
HB 125<br />
AKL<br />
20305013628 20/12/2010 Cobas Taq-Screen MPX Test<br />
AKL<br />
20303011138 21/05/2010<br />
ONSITE Pf / Pan Malaria AG Rapid<br />
Test<br />
DIGITAL AND ANALOG SYSTEM (DAS)<br />
S.r.l., ITALY<br />
HELMER INC., USA<br />
HELMER INC., USA<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
CTK BIOTECH INC., USA<br />
AKL<br />
10101113625 28/12/2011 SEKISUI Apo B Auto N "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101803595 22/05/2011<br />
AKL<br />
20101604795 07/02/2011<br />
AKL<br />
10203113031 22/11/2011<br />
PRECICONTROL Troponin T<br />
Elecsys and Cobas e analyzers<br />
SIEMENS CAL Calibrator C Advia<br />
Centaur Systems, ACS:180 Systems<br />
AKL<br />
20101801604 28/12/2011 DSI Urinary Calculi Analysis<br />
AKL<br />
20305801547 28/12/2011 DSI LDL Precipitant FS<br />
AKL<br />
30305800808 06/10/2011<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
MAGNUS MLXi Micro LED<br />
(fluorescence) Microscope and<br />
Accessories MAGNUS ANALYTICS, INDIA PT. C.V. KHARISMA UTAMA<br />
ONCOPROBE HIV 1&2 Antibody<br />
Rapid Test 4th Generation Card<br />
AKL<br />
20101800703 28/12/2011 DSI a-Amylase CC FS<br />
AKL<br />
10101800593 28/12/2011 Precinorm CK-MB Roche System<br />
AKL<br />
10101800592 21/12/2011<br />
AKL<br />
20305800545 28/12/2011<br />
PDF Creator - PDF4Free v3.0<br />
LIPC cobas c 111 Lipase<br />
Colorimetric assay<br />
AMPLICOR HCV Controls Kit<br />
Version 2.0 (HCV CTL)<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
GERMANY<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM, INC, USA<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ONCOPROBE UTAMA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20103113539 28/12/2011<br />
ABON AMP One Step Amphetamine<br />
Test Device (Urine)<br />
AKL<br />
10101800548 28/12/2011 Lactate Roche/Hitachi<br />
AKL<br />
10101800571 21/11/2011 DSI LIPASE DC FS<br />
AKL<br />
20101800564 22/11/2011 DSI Total Protein UC FS<br />
AKL<br />
10101800561 29/11/2011 DSI Trulab P<br />
AKL<br />
10102800562 14/11/2011<br />
STARDUST MC-15 and<br />
Accessories<br />
AKL<br />
10101800563 29/11/2011 DSI Magnesium XL FS<br />
AKL<br />
20101800566 22/11/2011 DSI Alkaline Phosphatase FS IFCC<br />
AKL<br />
20101800565 22/11/2011 DSI TRUCAL U<br />
AKL<br />
10101112933 21/11/2011 DSI LDL-C SELECT FS<br />
AKL<br />
20101800569 22/11/2011 DSI Pancreatic Amylase CC FS<br />
AKL<br />
10101112932 21/11/2011 DSI HDL-C IMMUNO FS<br />
AKL<br />
20101800570 22/11/2011 DSI TRUCAL HDL/LDL<br />
AKL<br />
20101800443 21/11/2011 DSI LDH FS<br />
AKL<br />
20101800444 29/11/2011 DSI Calcium AS FS<br />
AKL<br />
20101800439 21/11/2011 DSI Bilirubin Auto Direct FS<br />
AKL<br />
20101800441 21/11/2011 DSI Albumin FS<br />
AKL<br />
20101800434 21/11/2011 DSI Glucose GOD FS<br />
AKL<br />
20101800435 21/11/2011 DSI Creatinine FS<br />
AKL<br />
20101800438 21/11/2011 DSI Bilirubin Auto Total FS<br />
AKL<br />
10102800440 14/11/2011 STARDUST FC<br />
AKL<br />
10101112934 21/11/2011 DSI HDL Precipitant FS<br />
AKL<br />
10101800447 24/10/2011 DSI TruLab N<br />
AKL<br />
10101800442 21/11/2011 DSI Gamma GT FS<br />
AKL<br />
20101800454 24/10/2011 DSI Urea CT FS<br />
AKL<br />
10101800437 24/10/2011 DSI Triglycerides FS<br />
AKL<br />
10101800436 24/10/2011 DSI Cholesterol FS<br />
AKL<br />
10101800452 24/10/2011 DSI Uric Acid FS TBHBA<br />
AKL<br />
10101800451 24/10/2011 DSI ALAT (GPT) FS<br />
AKL<br />
20101800448 21/11/2011 DSI CK-NAC FS<br />
AKL<br />
20101800453 21/11/2011 DSI Urea FS<br />
AKL<br />
20101800449 21/11/2011 DSI Alkaline Phosphtase FS DGKC<br />
AKL<br />
20101800450 21/11/2011 DSI ASAT (GOT) FS (FCC Mod)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA untuk ALERE SAN DIEGO INC., USA<br />
melalui INVERNESS MEDICAL I<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS (DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ROCHE INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
20101800264 01/12/2011 DIALAB Bilirubin Total DIALAB GMBH., AUSTRALIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101604591 28/12/2011 DIALAB Bilirubin Direct Total DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101800278 01/12/2011 DIALAB Alkaline Phosphatase DIALAB GMBH., AUSTRALIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800276 01/12/2011 DIALAB Alpha Amylase DIALAB GMBH., AUSTRALIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800275 28/12/2011 DIALAB Iron DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800281 28/12/2011 DIALAB Phosphorus DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800279 28/12/2011 DIALAB Cholesterol LDL Direct DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800273 28/12/2011 DIALAB magnesium DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800272 28/12/2011 DIALAB LDH - P Opt. DGKC DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800233 01/12/2011<br />
AKL<br />
10101800223 28/12/2011<br />
CREA Plus Roche/Hitachi Creatinine<br />
Plus<br />
LIP Roche/Hitachi Lipase<br />
Colorimetric Assay<br />
AKL<br />
20101800234 01/12/2011 UREA/BUN Roche/Hitachi<br />
AKL<br />
20205702667 14/06/2011<br />
AKL<br />
20305800030 21/12/2011<br />
AKL<br />
20305705592 01/12/2011<br />
AKL<br />
20207705593 29/11/2011<br />
AKL<br />
20305705594 01/12/2011<br />
AKL<br />
20101705595 21/11/2011<br />
AKL<br />
20305705591 21/11/2011<br />
CELL-DYN Immuno T-Cell<br />
(CD3/4/8) Reagent<br />
Anti-HCV Elecsys and Cobas e<br />
Analyzer<br />
MYOGLOBIN Control Set Roche<br />
Systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
BD BIOSCIENCES, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
A1CD2 Hemolyzing Reagent Gen.2 ROCHE DIAGNOSTICS GMBH., GERMANY<br />
Tina-quant [a] Hemoglobin A1c Gen.2 melalui ROCHE DIAGNOSTICS<br />
cobas c system<br />
INTERNATIONAL LTD., SWITZERLAND<br />
Prealbumin/Ceruloplasmin Control<br />
Set roche Systems<br />
ISE Reference Electrolyte Roche /<br />
Hitachi cobas e system<br />
c.f.a.s. Myoglobin Calibrator for<br />
automated system<br />
AKL<br />
20101705536 21/11/2011 MICRAL-TEST Roche Systems<br />
AKL<br />
20306705537 01/12/2011<br />
CA 15-3 ll Elecsys and Cobas e<br />
analyzer<br />
AKL<br />
10102705538 29/11/2011 COBAS b 121 Accessories<br />
AKL<br />
20207705534 21/11/2011 CARDIAC D-Dimer<br />
AKL<br />
10101705530 28/10/2011<br />
AKL<br />
20101705531 21/11/2011<br />
AKL<br />
20101705533 21/11/2011<br />
ALTL Alanine Aminotransferase<br />
COBAS INTEGRA cobas c systems<br />
CKMBL Creatine Kinase - MB<br />
COBAS INTEGRA cobas c systems<br />
UREAL Urea/Bun COBAS<br />
INTEGRA cobas c systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101113285 01/12/2011 TOSOH AIA-PACK B12 TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113685 28/12/2011<br />
SEKISUI Lp(a) Latex Standard<br />
Serum L,M,H<br />
SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
20305705514 28/12/2011 SEKISUI CRP Calibrator (SS Type) SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
20305705513 28/12/2011<br />
SEKISUI Pureauto S CRP Latex (SS<br />
Type)<br />
SEKISUI MEDICAL CO. LTD., JAPAN<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101705511 28/12/2011 SEKISUI Cholesterol N HDL SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705512 28/12/2011 SEKISUI Cholesterol LDL SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705490 28/12/2011 SEKISUI Pureauto S CHO-N 1 & 2 SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
20101705508 28/12/2011 SEKISUI Cholestest N Calibrator SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101113635 28/12/2011 SEKISUI Apo A-I Auto N "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705507 28/12/2011 SEKISUI Autosera S TG-N 1 & 2 SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705487 28/12/2011<br />
AKL<br />
20101705486 28/12/2011<br />
SEKISUI Lp (a) Control Serum<br />
"DAIICHI" Low & High<br />
SEKISUI Apo Auto N DAIICHI<br />
Calibrator<br />
SEKISUI MEDICAL CO. LTD., JAPAN<br />
SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705485 28/12/2011 SEKISUI Lp (a) Latex "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705484 28/12/2011 SEKISUI Apo C-ll Auto N "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705480 28/12/2011 SEKISUI Apo E Auto N "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
AKL<br />
20303705455 01/12/2011 ARCHITECT Toxo IgG Control ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20102705457 01/12/2011<br />
ARCHITECT i-Theophylline<br />
Calibrator<br />
BIOKIT SA, SPAIN for ABBOTT GMBH. CO &<br />
KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20303705454 01/12/2011 ARCHITECT Toxo IgG Reagent ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20103705452 01/12/2011 ARCHITECT i-Theopylline Reagent<br />
BIOKIT S.A, SPAIN for ABBOTT GMBH &<br />
CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20303705453 01/12/2011 ARCHITECT Toxo IgG Calibrator ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
10204113677 28/12/2011 FURUNO Washing Solution No. 3<br />
AKL<br />
20101705138 21/11/2011<br />
AKL<br />
20101705137 21/11/2011<br />
AKL<br />
20101705136 19/10/2011<br />
AKL<br />
10101705135 19/10/2011<br />
AKL<br />
10101705133 28/10/2011<br />
AKL<br />
20103705132 28/10/2011<br />
AKL<br />
20101705134 19/10/2011<br />
AKL<br />
20101705131 21/11/2011<br />
AKL<br />
20103705128 28/10/2011<br />
HCG+B Calset Elecsys and Cobas e<br />
Analyzer<br />
HCG+B Elecsys and Cobas e<br />
Analyzers<br />
DHEA-S Calset Elecsys and Cobas<br />
e Analyzer<br />
DHEA-S Elecsys and Cobas e<br />
Analyzer<br />
B - CROSSLAPS/SERUM Elecsys<br />
and cobas e Analyzer<br />
DIGITOXIN Elecsys and Cobas e<br />
Analyzer<br />
ACCU-CHECK Active and<br />
Accessories<br />
CORTISOL CalSet Elecsys and<br />
Cobas e analyzers<br />
DIGOXIN Elecsys and Cobas e<br />
Analyzer<br />
AKL<br />
10303112900 14/11/2011 ASSURE H.Pylori Rapid Test<br />
NIHON KEMIKOTO CO. LTD., JAPAN untuk<br />
FURUNO ELECTRIC CO. LTD., JAPAN<br />
melalui SYSMEX ASIA PASIFIC PTE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
MP BIOMEDICALS ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20207705116 21/12/2011 TECHNOPLASTIN HIS TECHNOCLONE GMBH., AUSTRIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20207705119 21/12/2011 NYCOCard D-DIMER Single Test AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20305705117 21/12/2011 NYCOCard CRP Single Test AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20101705118 21/12/2011 NYCOCard U-Albumin AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20205112950 21/11/2011 HUMACOUNT 5 L and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303705072 21/11/2011 BIO-RAD PASTOREX Staph Plus<br />
AKL<br />
20205112948 21/11/2011<br />
AKL<br />
20102112600 17/10/2011<br />
AKL<br />
20305705057 21/11/2011<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
PT. DOS NI ROHA<br />
HUMACOUNT 60 TS and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
ARCHITECT 12000 SR and<br />
Accessories<br />
PreciControl HBeAg Elecsys and<br />
Cobas e Analyzer<br />
AKL<br />
20306705053 21/11/2011 NSE Elecsys and Cobas e Analyzer<br />
AKL<br />
20305705039 21/11/2011<br />
AKL<br />
20305705037 19/10/2011<br />
AKL<br />
20306705051 19/10/2011<br />
AKL<br />
20305705038 21/11/2011<br />
PreciControl Anti-HBe Elecsys and<br />
Cobas e Analyzer<br />
HbeAg Elecsys and Cobas e<br />
Analyzers<br />
CA-72-4 Calset Elecsys and Cobas e<br />
Analyzers<br />
Anti-Hbe Elecsys and Cobas e<br />
Analyzers<br />
FLEXTRONICS MANUFACTURING<br />
(SINGAPORE) PTE. LTD., SINGAPORE untuk<br />
ABBOTT LABORATORIES., USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20207705048 01/12/2011 AXIS SHIELD Calcium Chloride AXIS SHIELD P.O.C AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20208705046 21/12/2011 NYCOCard U-Albumin Control AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20205705045 21/12/2011<br />
NYCOCard Reader ll dan<br />
Accessories AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20208705043 01/12/2011 NYCOCARD HbA1C Control AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
10207705042 01/12/2011 AXIS SHIELD Thrombotest AXIS SHIELD PoC. AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20208705044 01/12/2011<br />
AKL<br />
20101705031 21/11/2011<br />
AXIS SHIELD Control Plasma<br />
Normal AXIS SHIELD P.O.C AS., NORWEGIA PT. ALAM PUTRA SAKTI<br />
KETO-DIABOR-TEST 5000<br />
Glucose/Ketones<br />
AKL<br />
20101705034 19/10/2011 ACCU-CHECK Softclix Lancets<br />
AKL<br />
20101705033 19/10/2011 ACCU-CHECK Softclix<br />
AKL<br />
10101704886 26/09/2011<br />
AKL<br />
20101704881 21/11/2011<br />
AKL<br />
20101704883 26/09/2011<br />
AKL<br />
20101704882 21/11/2011<br />
AKL<br />
20305704884 24/10/2011<br />
AKL<br />
20306704878 26/09/2011<br />
AKL<br />
10101704880 19/10/2011<br />
Testosterone Elecsys and Cobas e<br />
Analyzers<br />
Vitamin B12 Elecsys and Cobas e<br />
Analyzer<br />
N-MID Osteocalcin Elecsys and<br />
Cobas e Analyzer<br />
N-MID Osteocalcin Calset Elecsys<br />
and Cobas e Analyzer<br />
IgE Calset Elecsys and Cobas e<br />
Analyzers<br />
CA 19-9 Elecsys and Cobas e<br />
Analyzers<br />
PRECICONTROL BONE Elecsys<br />
and Cobas e Analyzers<br />
AKL<br />
20101704876 26/09/2011 PTH Elecsys and Cobas e Analyzers<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20306704874 24/10/2011<br />
AKL<br />
20101704875 24/10/2011<br />
CA 125 ll Elecsys and e Cobas<br />
Analyzer<br />
PTH CalSet Elecsys and Cobas &<br />
Analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
AKL<br />
21106704867 03/08/2011 VITROLIFE G-1 V5 Plus VITRO LIFE AB., SWEDEN<br />
AKL<br />
21106704866 03/08/2011 VITROLIFE G - 2 V5 VITRO LIFE AB., SWEDEN<br />
AKL<br />
21106704865 03/08/2011 VITROLIFE G-2 V5 Plus VITRO LIFE AB., SWEDEN<br />
AKL<br />
21106704863 03/08/2011 VITROLIFE G - 1 v5 VITRO LIFE AB., SWEDEN<br />
AKL<br />
10101704847 26/09/2011<br />
PreciControl cardiac II Elecsys and<br />
cobas e analyzers<br />
AKL<br />
10101704839 26/09/2011 PHOS2 cobas c111 Phosphate ver.2<br />
AKL<br />
20101704848 26/09/2011<br />
AKL<br />
20101704849 26/09/2011<br />
AKL<br />
20207704837 26/09/2011<br />
ROCHE ProBNP II Calset Elecsys<br />
and Cobas e Analyzers<br />
ROCHE ProBNP ll Elecsys and<br />
Cobas e Analyzers<br />
AIC-2 cobas c111 Tina-quant [a]<br />
Hemoglobin A1c Gen..2<br />
AKL<br />
20101704838 26/09/2011 BIL-D cobas c111 Bilirubin Direct<br />
AKL<br />
20207704836 26/09/2011<br />
A1C-2 Hemoglobin A1c Gen.2<br />
COBAS INTEGRA cobas system<br />
AKL<br />
20101704835 17/10/2011 ACCUTREND Plus<br />
AKL<br />
20205704768 28/12/2011 URITEST Hemoglobin Hb Meter<br />
AKL<br />
10102704765 28/12/2011 URITEST Urine Analyzer 50<br />
AKL<br />
10102704767 28/12/2011 URITEST Urine Analyzer 300<br />
AKL<br />
10102704766 28/12/2011 URITEST Urine Analyzer 150<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
URIT MEDICAL ELECTRONIC CO. LTD.,<br />
P.R. CHINA<br />
URIT MEDICAL ELECTRONIC CO. LTD.,<br />
P.R. CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
AKL<br />
URIT MEDICAL ELECTRONIC CO. LTD.,<br />
20205704762 28/12/2011 URITEST Hematology Analyzer 3000 P.R. CHINA<br />
AKL<br />
10102704764 28/12/2011 URITEST Urine Analyzer 500<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
AKL<br />
URIT MEDICAL ELECTRONIC CO. LTD.,<br />
20205704763 28/12/2011 URITEST Hematology Analyzer 2900 P.R. CHINA<br />
AKL<br />
10101704757 24/10/2011<br />
AKL<br />
10101704758 24/10/2011<br />
AKL<br />
10101704756 24/10/2011<br />
AKL<br />
20207704759 17/10/2011<br />
AKL<br />
10101704755 24/10/2011<br />
AKL<br />
20101704740 26/09/2011<br />
AKL<br />
20209704698 29/11/2011<br />
AKL<br />
20209704699 29/11/2011<br />
PDF Creator - PDF4Free v3.0<br />
BIO-RAD LIQUICHECK Cardiac<br />
Markers Control LT Level 2<br />
BIO-RAD LIQUICHECKTM cardiac<br />
Markers Control CT Level 1<br />
BIO-RAD LIQUICHECK Cardiac<br />
Markers Control LT Level 3<br />
BIO-RAD QUANTASE Neonatal<br />
G6PD Deficiency Screaning Assay<br />
BIO-RAD LIQUICHECK Cardiac<br />
Markers Control LT Tri Level<br />
Abbott Axsym 3 rd Generation TSH<br />
Standard Calibrators<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES EUROPE LTD.,<br />
UK melalui BIO-RAD LABORATORIES<br />
(SINGAPORE) PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ABBOTT INDONESIA<br />
MINICOLLECT Tube Lithium<br />
Heparin GREINER BIO-ONE GMBH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
MINICOLLECT Serum with Clot<br />
Activator GREINER BIO-ONE GMBH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
http://www.pdf4free.com
AKL<br />
20209704697 29/11/2011 VACUTTE No-Addisive Tube GREINER BIO-ONE GMBH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
10303704700 17/10/2011 MICROGEN M80 Rotascreen MICROGEN BIOPRODUCTS LTD., UK PT. PYRIDAM FARMA<br />
AKL<br />
20103704650 30/11/2011<br />
SPEEDYTEST One StepStrip Style<br />
BZO (Benzodiazepine) Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103704649 30/11/2011<br />
AKL<br />
20103704646 29/11/2011<br />
AKL<br />
20103704647 29/11/2011<br />
SPEEDYTEST One Step Cassette<br />
BZO (Benzodiazepine) Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST One Step Style<br />
Amphetamine Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
SPEDYTEST One Step Cassette<br />
Style Amphetamine Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20305704611 30/11/2011 FOKUS AntiHBs Cassette PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20305704609 30/11/2011 FOKUS HBsAg Strip PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20305704610 30/11/2011 FOKUS Anti HBs Strip PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20101704607 30/11/2011 FOKUS hCG Strip PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20101704608 30/11/2011 FOKUS hCG Cassette PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20205704283 28/10/2011 CoaDATA 501<br />
LABITEC (LABOR BIO MEDICAL<br />
TECHNOLOGY) GMBH., GERMANY<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10302704279 18/07/2011 OXOID TTC Solution 1,0 % OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305704168 26/09/2011 COBAS AmplyScreen HCV Test<br />
AKL<br />
20305704270 26/09/2011<br />
COBAS Ampliscreen MultiPrep<br />
Specimen Preparation and Control Kit<br />
AKL<br />
20305704167 26/09/2011 COBAS AmplyScreen HBV Test<br />
ROCHE MOLECULAR SYSTEM, INC, USA<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM, INC, USA<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM, INC, USA<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20207704121 28/10/2011 TULIP FIBROQUANT TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20207704119 28/10/2011 TULIP LIQUICELIN-E TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20207704120 28/10/2011 TULIP CALCIUM CHLORIDE TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10207704115 28/10/2011 TULIP UNIPLASTIN TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20207704118 28/10/2011 TULIP FIBROSCREEN TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20207704117 28/10/2011 TULIP PLASMATROL H-II TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103704021 22/11/2011<br />
AKL<br />
20101703994 28/10/2011<br />
FOKUS Methanphetamine (Mamp<br />
Drug Cassette)<br />
BENECHEK Plus Glucose Test<br />
Strips<br />
GENIX TECHNOLOGY INC., CANADA<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN<br />
AKL<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
10101703993 28/12/2011 BeneCheck Plus Uric Acid Test Strip LTD., TAIWAN<br />
AKL<br />
20305113666 28/12/2011<br />
AKL<br />
10101703991 28/12/2011<br />
AKL<br />
20103703976 26/09/2011<br />
AKL<br />
20103703946 26/09/2011<br />
AKL<br />
20103703944 15/08/2011<br />
AKL<br />
20103703942 15/08/2011<br />
IMMULITE 2000 ystems aHB Anti-<br />
HBs<br />
BENECHEK Plus Glucose Control<br />
Solution<br />
Phenytoin ONLINE TDM Roche /<br />
Hitachi<br />
CEDIA Carbamazepine II Roche /<br />
Hitachi<br />
VANC2 ONLINE TDM Vancomycin<br />
cobas c system<br />
THE02 ONLINE TDM Theophylline<br />
Cobas c systems<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
GENERAL LIFE BIOTEKNOLOGY CO. LTD.,<br />
TAIWAN<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. MITRAASA PRATAMA<br />
PT. MITRAASA PRATAMA<br />
PT. SIEMENS INDONESIA<br />
PT. MITRAASA PRATAMA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103703943 15/08/2011<br />
VALP2 ONLINE TDM Valproic Acid<br />
cobas c systems<br />
AKL<br />
20103703941 26/09/2011 CEDIA Phenytoin II Roche / Hitachi<br />
AKL<br />
20103703940 21/07/2011<br />
PHNY2 ONLINE TDM Phenytoin<br />
cobas c system<br />
AKL<br />
20101703945 15/08/2011 Preciset DAT Plus II<br />
AKL<br />
10101111915 08/08/2011<br />
Roche CARDIAC Control Troponin T<br />
cobas h 232<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10103703947 02/08/2011<br />
AKL<br />
10102703809 18/07/2011<br />
AKL<br />
20103703567 09/08/2011<br />
AKL<br />
10305703568 21/07/2011<br />
AKL<br />
20303703563 08/08/2011<br />
AKL<br />
20103703538 08/09/2011<br />
AKL<br />
20103703537 08/08/2011<br />
CHED2 Cholinesterase/Dibucaine<br />
Gen2 COBAS INTEGRA cobas c<br />
system<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
CYBOW Reader 720 Urine<br />
Chemistry Analyzer DFI CO. LTD., KOREA PT. RAJAWALI NUSINDO<br />
CARB3 cobas c system CEDIA<br />
Carbamazepine<br />
ROCHE CARDIAC T Quantitative<br />
Troponin T Cobas h 232<br />
ONCOPROBE Syphilis Antibody<br />
Rapid Test<br />
AMPSH (Abuscreen OnLine<br />
Amphetamines High Sensitivity)<br />
cobas c systems<br />
CARB2 (ONLINE TDM<br />
Carbamazepine) cobas c systems<br />
AKL<br />
20103703536 15/08/2011 Preciset DAT Amphetamine<br />
AKL<br />
20103703535 08/08/2011<br />
AKL<br />
20103703534 08/08/2011<br />
AKL<br />
20305703482 09/08/2011<br />
AKL<br />
20101703483 21/07/2011<br />
AKL<br />
20101703478 21/07/2011<br />
AKL<br />
20101703468 21/07/2011<br />
AMPS (Abuscreen OnLine<br />
Amphetamines) cobas c systems<br />
GENT2 (ONLINE TDM Gentamicin)<br />
cobas c systems<br />
MY02 (Tina-Quant [a] Myoglobin<br />
Gen.2) COBAS INTEGRA cobas c<br />
system<br />
TPUC3 Total Protein Urine/CFS<br />
Gen.3 COBAS INTEGRA cobas c<br />
systems<br />
LDHI2 (Lactate dehydrogenase<br />
acc.IFCC ver.2) COBAS INTEGRA<br />
cobas c systems<br />
CO22-L cobas c 111 Bicarbonate<br />
Liquid<br />
AKL<br />
20103111963 09/08/2011 LI (Lithium) cobas c system<br />
AKL<br />
20101703467 21/07/2011<br />
AKL<br />
20101703466 21/07/2011<br />
AKL<br />
20101703465 02/08/2011<br />
LDHI2 cobas c 111 Lactate<br />
Dehydrogenase acc. to IFCC ver.2<br />
AMY-P cobas c 111 a-Amylase EPS<br />
Pancreatic<br />
CKMBL cobas c 111 Creatine Kinase<br />
- MB<br />
AKL<br />
20101703464 09/08/2011 CREJ2 cobas c 111 Creatinine Jaffe<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ONCOPROBE UTAMA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20102112410 26/09/2011<br />
AKL<br />
10101703480 02/08/2011<br />
AKL<br />
20206703315 01/08/2011<br />
MEGA 200 Plus With ISE and<br />
Barcode System HOSPITEX DIAGNOSTICS S.r.l., ITALY PT. AKURAT INTAN MADYA<br />
COBAS ISE Internal standard Gen.2<br />
cobas c systems<br />
ONCOPROBE Rapid Test Fecal<br />
Occult Blood<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
PT. ROCHE INDONESIA<br />
PT. ONCOPROBE UTAMA<br />
AKL<br />
20103703792 01/08/2011<br />
AKL<br />
20305703317 01/08/2011<br />
ONCOPROBE Rapid Test Multi<br />
Drug Abuse 5 in 1 (Amphetamine,<br />
Methamphetamine, Marijuana/THC,<br />
Morphine,<br />
ONCOPROBE Rapid Test HCV<br />
Antibody<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
PT. ONCOPROBE UTAMA<br />
PT. ONCOPROBE UTAMA<br />
AKL<br />
20103703793 01/08/2011<br />
AKL<br />
20305703316 24/10/2011<br />
ONCOPROBE Rapid Test Multi<br />
Drug Abuse 5 in 1 + pH<br />
(Amphetamine, Methamphetamine,<br />
Marijuana/THC, Morp<br />
ONCOPROBE Rapid Test HBa<br />
Antibody<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
PT. ONCOPROBE UTAMA<br />
PT. ONCOPROBE UTAMA<br />
AKL<br />
20401110727 05/04/2011<br />
AKL<br />
10102703252 17/10/2011<br />
AKL<br />
10102703251 17/10/2011<br />
AKL<br />
20305703175 20/07/2011<br />
AKL<br />
20305703174 20/07/2011<br />
AKL<br />
20305703172 20/07/2011<br />
AKL<br />
20305703173 20/07/2011<br />
AKL<br />
20305703171 01/07/2011<br />
AKL<br />
10305703163 21/07/2011<br />
AKL<br />
20207703162 21/07/2011<br />
AKL<br />
20108703161 01/07/2011<br />
AKL<br />
20103703179 14/06/2011<br />
AKL<br />
20103703178 14/06/2011<br />
AKL<br />
20101703169 01/07/2011<br />
AKL<br />
20103703176 14/06/2011<br />
AKL<br />
20101703170 01/07/2011<br />
AKL<br />
20103703177 14/06/2011<br />
PDF Creator - PDF4Free v3.0<br />
BEDFONT COMPACT Smokerlyzer<br />
Breath Carbon Monoxide (CO) Tester<br />
ZENIX-288 Semi-Auto Chemistry<br />
Analyzer<br />
ZENIX-188 Semi-Auto Chemistry<br />
Analyzer<br />
ALBT2 Tina-quant [a] Albumin Gen.2<br />
cobas c systems<br />
FERR3 Tina-quant [a] Ferritin Gen.3<br />
cobas c systems<br />
IGG-2 Tina-quant [a] IgG Gen.2<br />
cobas c systems<br />
IGA-2 Tina-quant [a] IgA Gen.2<br />
cobasc s ystems<br />
COBAS IGM-2 (Tina-quant [a] IgM<br />
Gen.2) cobas c systems<br />
ROCHE TROP T Sensitive,<br />
Troponin T cobas h 232<br />
ROCHE Cardiac D - Dimer Cobas h<br />
232<br />
ROCHE CARDIAC Control D -<br />
Dimer cobas h 232<br />
DIG Digoxin cobas integra cobas<br />
csystems<br />
DIGIT Digitoxin COBAS INTEGRA<br />
Cobas C Systems<br />
ROCHE INTEGRA (ALP IFCC<br />
Gen.2 Small) cobas c systems<br />
Barbiturates Plus ONLINE DAT<br />
Roche/Hitachi<br />
ROCHE INTEGRA INTEGRA<br />
ALP2L (ALP IFCC Gen.2 Large)<br />
cobas c systems<br />
SALI Saliclate COBAS INTEGRA<br />
Cobasc Systems<br />
BEDFONT SCIENTIFIC LIMITED., UNITED<br />
KINGDOM<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. MITRAASA PRATAMA<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SUMIFIN CITRA ABADI<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101703166 21/07/2011<br />
AKL<br />
20101703158 01/07/2011<br />
AKL<br />
20101703160 21/07/2011<br />
AKL<br />
10303703167 20/07/2011<br />
AKL<br />
20101703157 01/07/2011<br />
AKL<br />
10101703165 01/07/2011<br />
AKL<br />
10101703168 21/07/2011<br />
AKL<br />
10101703159 01/07/2011<br />
ROCHE Cardiac Pro BNP Cobas h<br />
232<br />
COBAS ASTLP (Aspartate<br />
Aminotransferase PyP) cobas c<br />
systems<br />
ROCHE Cardiac CK-MB Cobas h<br />
232<br />
COBAS INTEGRA ASLOT (Tinaquant<br />
[a] Antistreptolysin O) cobas c<br />
systems<br />
ROCHE INTEGRA CREJ2<br />
(creatinine Jaffe Gen.2) cobas c<br />
systems<br />
ROCHECARDIAC Control proBNP<br />
cobas h 232<br />
ALTLP (Alanine Aminotransferase<br />
PyP) cobas c system<br />
ROCHE CARDIAC Control Ck-MB<br />
Cobas h 232<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
AKL<br />
20305703144 15/08/2011 ETI-AB-AUK-3 DIASORIN S.p.A. Italy<br />
AKL<br />
20303703143 15/08/2011 ETI-Rubek-M reserve Plus DIASORIN S.p.A. Italy<br />
AKL<br />
10101703111 20/07/2011<br />
AKL<br />
20102703109 21/07/2011<br />
HDL C3 Cobas c 11 HDL<br />
Cholesterol<br />
COBAS e 411 Analyzer and<br />
Accessories<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
HITACHI HIGN TECHNOLOGIES<br />
CORPORATION, JAPAN untuk ROCHE<br />
DIAGNOSTICS GMBH., GERMANY melalui<br />
ROCHE DI<br />
AKL<br />
20303703141 15/08/2011 ETI-Rubek-G Plus DIASORIN S.p.A. Italy<br />
AKL<br />
20101703115 30/03/2011 PREGNATEST<br />
AKL<br />
10101703117 30/03/2011 FERTITEST STRIP<br />
AKL<br />
10101703116 30/03/2011 FERTITEST MIDSTREAM<br />
DIAGNOSTICS AUTOMATION / CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTICS AUTOMATION / CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTICS AUTOMATION / CORTEZ<br />
DIAGNOSTICS INC., USA<br />
AKL<br />
20303703001 15/08/2011 ETI-Toxok-G Plus DIASORIN S.p.A. Italy<br />
AKL<br />
20303703000 15/08/2011 ETI-Toxok-M reverse Plus DIASORIN S.p.A. Italy<br />
AKL<br />
20305702976 13/05/2011<br />
AKL<br />
20305702977 13/05/2011<br />
BIO-RAD Autoimmune EiA Anti-<br />
Cardiolipin IgG<br />
BIO-RAD Autoimmune EiA AntiCardiolipin<br />
IgM<br />
AKL<br />
20303702954 14/06/2011 ARCHITECT CMV IgM Reagent Kit<br />
AKL<br />
20303702953 14/06/2011 ARCHITECT CMV IgM Calibrator<br />
AKL<br />
20303702952 14/06/2011 ARCHITECT CMV IgM Controls<br />
AKL<br />
20303702951 14/06/2011 ARCHITECT CMV IgG Reagent Kit<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
PT. ANTAR MITRA SEMBADA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20306702921 15/08/2011 HUMAN CA 19-9 Ag ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306702925 15/08/2011 HUMAN CA 125 Ag ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306702923 15/08/2011 HUMAN CA 15-3 Ag ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303702924 15/08/2011 HUMAN Sypilis Screen ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20209702915 15/08/2011 HUMATYPE Anti-A HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20209112109 15/08/2011 HUMATYPE Anti-D (IgM) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101702916 24/08/2011 HUMAN Turbidos Control HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20208702920 15/08/2011 HUMAN HCS-Control HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20209702914 15/08/2011 HUMATYPE Anti - B HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10208702919 15/08/2011 HUMAN HC5-Eolyse HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101111809 08/08/2011<br />
VITROS Chemistry Products Liquid<br />
Performance Verifier II<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305112095 15/08/2011 HUMATYPE Anti-Human Globulin HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10208702918 15/08/2011 HUMAN HC5-Basolyse HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305702874 26/08/2011 HUMAN Transferrin HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305702876 09/08/2011 HUMAN COMPELMENT C3 HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305702872 09/08/2011 HUMAN COMPELMENT C4 HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101702871 26/08/2011 HUMAN Microalbumin HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20209702875 15/08/2011 HUMATYPE Anti-A,B HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101702877 09/08/2011<br />
AKL<br />
30305111633 28/07/2011<br />
AKL<br />
20101702772 28/10/2011<br />
HUMAN Pancreas Amylase<br />
Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
OraQuick Rapid HIV - 1/2 Antibody<br />
Test<br />
SMART CHECK Blood Gucose Test<br />
Strip<br />
ORASURE TECHNOLOGIES INC., USA<br />
melalui ONE-CONNECT INTERNATIONAL<br />
PTE. LTD., SINGAPORE<br />
MAYOR BIOSYSTEM CORP, TAIWAN<br />
PT. BIOTECH FARMA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
AKL<br />
20301702775 15/08/2011 OXOID CEFSULODIN CFS 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303702774 25/02/2011<br />
AKL<br />
20303702773 25/02/2011<br />
REMEL Shigella Dysenterie<br />
Polyvalent (1-10) REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
REMEL Shigella Baydii Polyvalent 1<br />
(1-6) REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102702734 11/07/2011 HOSPITEX Microplate Washer HOSPITEX DIAGNOSTICS S.r.l., ITALY PT. BAVARIA COMBININDO<br />
AKL<br />
10102702733 11/07/2011<br />
AKL<br />
20103704637 29/11/2011<br />
AKL<br />
20103704635 30/11/2011<br />
AKL<br />
20103704636 30/11/2011<br />
HOSPITEX Plate Screen Microplate<br />
Reader HOSPITEX DIAGNOSTICS S.r.l., ITALY PT. BAVARIA COMBININDO<br />
SPEEDYTEST One Strip Style<br />
Marijuana (THC) Ganja Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST One Step Cassette<br />
Style Methamphetamine (shabushabu)<br />
Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST One Step Strip Style<br />
Methampehetamine (shabu-shabu)<br />
Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103704631 29/11/2011<br />
AKL<br />
20103704639 30/11/2011<br />
AKL<br />
20103704629 29/11/2011<br />
SPEEDYTEST One Step Cassette<br />
Style Marijuana (THC) / Ganja Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST One Step Cassette<br />
Style Ecsyacy (MDMA) test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST One Step Cassette<br />
style Opiates / Morphine / Heroin Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103704626 29/11/2011<br />
SPEEDYTEST One Step Strip Style<br />
Opiates/Morphine/Heroin Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
AKL<br />
20303704613 22/11/2011 FOKUS Syphilis Cassette GENIX TECHNOLOGY INC., CANADA<br />
AKL<br />
20303704612 01/12/2011 FOKUS TPHA PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20205702685 21/07/2011 PARTEC Cyclow Counter PARTEC GMBH., GERMANY<br />
AKL<br />
10304702684 30/03/2011 CHORUS<br />
AKL<br />
20303702682 25/04/2011 ARCHITECT CMV IgG Controls<br />
AKL<br />
20303702683 25/04/2011 ARCHITECT Rubella IgM Calibrator<br />
PDF Creator - PDF4Free v3.0<br />
DIESSE DIAGNOSTICA SENECE S.p.A.,<br />
ITALY<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
http://www.pdf4free.com<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. EUROP CONTINENTS<br />
INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA
AKL<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
20303702673 25/04/2011 ARCHITECT Rubella IgM Reagen kit Ireland<br />
AKL<br />
20303702672 25/04/2011 ARCHITECT Rubella IgM Control<br />
AKL<br />
20303702671 14/06/2011 ARCHITECT CMV IgG Calibrator<br />
AKL<br />
30305702653 19/07/2011 Foresight HIV 1/2/O EIA Test Kit<br />
AKL<br />
10102702623 01/08/2011<br />
LABOTRON Microplate Washer<br />
Model LB-3200<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
Ireland<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101702001 28/04/2011 NIHON Wash Solution C-1<br />
NIHON KEMIKOTO KASEI CO. LTD., JAPAN<br />
untuk FURUNO ELECTRIC CO. LTD., JAPAN<br />
melalui SYSMEX ASIA PACIF<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10101702002 28/04/2011 NIHON Wash Solution No. 10-2<br />
AKL<br />
10102701970 15/08/2011<br />
AKL<br />
10203701960 16/08/2011<br />
NIHON KEMIKOTO KASEI CO. LTD., JAPAN<br />
untuk FURUNO ELECTRIC CO. LTD., JAPAN<br />
melalui SYSMEX ASIA PACIF<br />
PT. SYSMEX INDONESIA<br />
POINTE 2600 MPW Microplate<br />
Washer POINTE SCIENTIFIC INC., USA PT. SEGARA HUSADA MANDIRI<br />
HUMASCOPE PLUS and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102701968 15/08/2011 POINTE 180 PR EIA Plate Reader POINTE SCIENTIFIC INC., USA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
10102701962 08/08/2011 ELISYS UNO and accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20102701957 09/08/2011 HUMASTAR 600 and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102701959 16/08/2011 HUMAROLLER and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102701961 16/08/2011<br />
HUMAX 4K Bench-Top Centrifuge<br />
and accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102701958 08/08/2011 HUMALYZER 3500 and accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205701967 08/08/2011<br />
AKL<br />
10102701966 08/08/2011<br />
AKL<br />
10102701963 08/08/2011<br />
AKL<br />
10102701965 16/08/2011<br />
HUMAMETER HB PLUS and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAREADER HS and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAWASH Manual and<br />
accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAX 5K Bench-Top Centrifuge<br />
and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20209701956 07/07/2011 DIAGAST Freelys Nano DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209701955 17/06/2011<br />
DIAGAST QWALYS (8 Needles)<br />
and accessories DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10302702378 18/07/2011 OXOID Briliance Candida Agar Base OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302701925 25/02/2011<br />
AKL<br />
20306113341 01/12/2011<br />
OXOID Legion byce Suppl. w/ocysteine<br />
1 x 10 v OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
VITROS Immunodiagnostic<br />
Products Total B-hCG II Reagent<br />
Pack<br />
AKL<br />
10302702293 07/03/2011 BD Phoenix ID Broth<br />
AKL<br />
20101701772 24/04/2011 ACCU - CHEK Performa Test Strip<br />
AKL<br />
10101701773 19/04/2011 ACCU-CHEK Performa Control<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20209701789 17/06/2011 DIAGAST DuoLys DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209701788 07/07/2011<br />
DIAGAST Hemalys 1, A1, A2, B dan<br />
O DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209701787 17/06/2011 DIAGAST Hemascreen DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209701786 07/07/2011 DIAGAST Hemalys 1, A1, B DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305701790 27/04/2011<br />
PDF Creator - PDF4Free v3.0<br />
BIO-RAD Autoimmune EIA Anti -<br />
dsDNA<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. DIASTIKA BIOTEKINDO
AKL<br />
20303701649 19/07/2011<br />
Foresight Syphilis Antibody EiA Test<br />
Kit<br />
AKL<br />
20305701651 19/07/2011 Foresight HBsAg EIA Test Kit<br />
AKL<br />
10101701504 26/04/2011 CONTROL Set DAT II<br />
AKL<br />
20103701501 25/04/2011<br />
AKL<br />
10101701506 26/04/2011<br />
ACETAMINDOPHEN Roche /<br />
Hitachi<br />
ACTIVATOR Cobas Integra Cobas<br />
C System<br />
AKL<br />
10101701505 26/04/2011 CONTROL Set DAT I<br />
AKL<br />
10101701500 19/04/2011 Control Set DAT III Roche Systems<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305701482 20/04/2011 ONCOPROBE HBsAg Rapid Test CDI/BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20305701483 20/04/2011<br />
ONCOPROBE Dengue IgG/IgM<br />
Rapid Test CDI/BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103701486 27/04/2011 ONCOPROBE Morphine Rapid Test CDI BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103701485 20/04/2011<br />
ONCOPROBE Methanphetamine<br />
Rapid Test CDI/BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103701479 27/04/2011 ONCOPROBE THC Rapid Test CDI BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103701480 20/04/2011<br />
AKL<br />
20103701484 27/04/2011<br />
ONCOPROBE Amphetamine Rapid<br />
Test CDI/BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
ONCOPROBE Benzodiazepine<br />
Rapid Test CDI BIOCHEM CORPORATION., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103701481 20/04/2011 ONCOPROBE Cocaine Rapid Test CDI/BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
10208701516 30/03/2011 Orphee Cleaner For Mythic 22 ORPHEE SA., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10208701518 30/03/2011 ORPHEE Diluent for Mythic 22 ORPHEE SA., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10208701517 30/03/2011<br />
AKL<br />
20101700759 07/02/2011<br />
AKL<br />
20306704874 24/10/2011<br />
ORPHEE OnlyOne Lytic Reagent 5<br />
Diff Orphee Sa, Switzerland PT. INDOLABTEK DINAMIKA<br />
GLUC3 Glucose HK Gen.3 COBAS<br />
INTEGRA cobas e system<br />
CA 125 II Elecsys and cobas e<br />
analyzer<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20303701436 25/08/2011 DIALAB Syphilis VDRL DIALAB Ges.MB.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10303701467 13/06/2011 FOKUS Malaria Pf/PV Cassette PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20303701457 14/06/2011<br />
AKL<br />
10101700932 08/02/2011<br />
AKL<br />
20303701465 28/04/2011<br />
FOKUS Salmonella Typhi IgG/IgM<br />
Cassette<br />
IRON2 Iron Gen.2 COBAS<br />
INTEGRA cobas c system<br />
FOKUS Salmonella Typhi IgM<br />
Cassette<br />
PULSE SCIENTIFIC INC., CANADA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
CORE DIAGNOSTICS LTD., UK<br />
AKL<br />
20303701466 28/04/2011 FOKUS Leptospira IgM Cassette CORE DIAGNOSTICS LTD., UK<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
20208701445 09/08/2011 R & D CBC 3D Normal R & D SYSTEM INC., U.S.A PT. DIATRON PROMEDIKA<br />
AKL<br />
20208701444 10/08/2011 R & D CBC ST Plus Tripack R & D SYSTEM INC., U.S.A PT. DIATRON PROMEDIKA<br />
AKL<br />
20208701440 10/08/2011 R & D CBC-ST Plus Normal R & D SYSTEM INC., USA PT. DIATRON PROMEDIKA<br />
AKL<br />
20208701442 10/08/2011 R & D CD-Cal R & D SYSTEM INC., U.S.A PT. DIATRON PROMEDIKA<br />
AKL<br />
20208701439 10/08/2011 R & D CBC 3D Tripack R & D SYSTEM INC., U.S.A PT. DIATRON PROMEDIKA<br />
AKL<br />
20208701443 10/08/2011 R & D CBC 3K Tripack R & D SYSTEM INC., U.S.A PT. DIATRON PROMEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20208701438 10/08/2011 R & D CBC - SYS Tripack R & D SYSTEM INC., U.S.A PT. DIATRON PROMEDIKA<br />
AKL<br />
20101701446 18/07/2011 DIALINE BIOCAL HDL/LDL<br />
AKL<br />
20103701430 26/04/2011<br />
AKL<br />
20103701428 14/06/2011<br />
AKL<br />
20103701427 14/06/2011<br />
AKL<br />
20103701429 14/06/2011<br />
AKL<br />
20103701425 25/04/2011<br />
AKL<br />
20103701426 14/06/2011<br />
BARB ONLINE DAT Barbitarates<br />
Plus Cobas C System<br />
PCP ONLINE DAT Phencyclidine<br />
Plus cobas c system<br />
THC II ONLINE DAT Cannabinoids<br />
II Cobas Integra<br />
PPX Online DAT Propoxyphene Plus<br />
Cobas C Systems<br />
AMPS Abuscreen Online<br />
Amphetamines Cobas Integra<br />
THC2 ONLINE DAT Cannabinoids II<br />
Cobas C Systems<br />
DIASYS DIAGNOSTICS SYSTEMS GMBH.,<br />
GERMANY melalui DIASYS DIAGNOSTICS<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
AKL<br />
20101701420 26/04/2011 CROMATEST Sodium LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20101701419 26/04/2011 CROMOTEST Potassium LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
20305701378 26/04/2011<br />
AKL<br />
30305701380 07/03/2011<br />
AKL<br />
10304701377 25/04/2011<br />
COBAS AmpliPrep / Cobas TaqMan<br />
HBV Test (HBMCAP)<br />
COBAS AmpliPrep/Cobas Taqman<br />
HIV-1 Test, V2.0 (H12CAP)<br />
COBAS AmpliPrep / Cobas Taqman<br />
Wash Reagent<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC, USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. DIATRON PROMEDIKA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10303701330 27/04/2011<br />
SENSATEST Malaria Antigen Rapid<br />
Test Cassette<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD. P.R., CHINA melalui NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., MALAY<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20301701332 25/02/2011 OXOID SULBENICILIN OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20103701290 14/06/2011<br />
AKL<br />
20103701216 14/06/2011<br />
AKL<br />
20103701197 26/04/2011<br />
AKL<br />
20103701196 25/04/2011<br />
AKL<br />
20103701198 25/04/2011<br />
AKL<br />
20103701195 30/05/2011<br />
AKL<br />
20103701194 30/05/2011<br />
AKL<br />
20103701192 30/05/2011<br />
AKL<br />
20103701191 30/05/2011<br />
AKL<br />
20103701193 30/05/2011<br />
PDF Creator - PDF4Free v3.0<br />
MDN2 ONLINE DAT Methadone II<br />
cobas e systems<br />
BENZ Abuscreen Online<br />
Benzodiazepines Cobas Integra<br />
ETOH 2 Ethanol Gen.2 Cobas<br />
Integra Cobas C System<br />
OPI 2 ONLINE DAT Opiates II<br />
Cobas CSystem<br />
Cocaine II ONLINE DAT IRoche /<br />
Hitachi<br />
Methadone II ONLINE DAT ROCHE<br />
/ HITACHI<br />
MTQL Abuscreen Online<br />
Methaqualone COBAS aintegra<br />
PCP Abuscreen OnLine<br />
Phencyclidine COBAS INTEGRA<br />
MDNII ONLINE DAT Methadone II<br />
COBAS INTEGRA<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
OPI ONLINE DAT Opiates 300/2000 melalui ROCHE DIAGNOSTICS<br />
COBAS INTEGRA<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20205701151 30/03/2011<br />
AKL<br />
20205701152 30/03/2011<br />
SYSMEX CS-2100i Automated Blood<br />
Coagulation Analyzer and<br />
Accessories<br />
SYSMEX CS-2000i Fully Automated<br />
Coagulation Analyzer and<br />
Accessories<br />
AKL<br />
20103701069 25/04/2011 Preciset DAT Plus 1 Roche Systems<br />
AKL<br />
20103701068 25/04/2011<br />
AKL<br />
20103701066 26/04/2011<br />
AKL<br />
20103701065 25/04/2011<br />
Phencyclidine Plus ONLINE DAT<br />
Roche / Hitachi<br />
Cannabinoids II ONLINE DAT<br />
Roche / Hitachi<br />
PPX Abuscreen Online<br />
Propoxyphene Cobas Integra<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10102701072 19/04/2011<br />
TECO TC MATRIX Auto Chemistry<br />
Analyzer and Accessories TECO DIAGNOSTIC., USA PT. RIOCA MEDICA<br />
AKL<br />
10102701064 25/04/2011 URIXXON Relax MACHEREY-NAGEL GmbH., GERMANY PT. MRK DIAGNOSTICS<br />
AKL<br />
20101701075 09/02/2011<br />
LIFESCAN SURESTEP FLEXX<br />
Blood Glucose Meter<br />
FLEXTRONICS INDUSTRIAL (SHENZHEN) PT. JOHNSON & JOHNSON<br />
CO., LTD., CHINA untuk LIFESCAN INC., USA INDONESIA<br />
AKL<br />
20101701074 09/02/2011<br />
ONE TOUCH Ultra Blood Glucose<br />
Monitoring System + Accessories<br />
FLEXTRONICS INDUSTRIAL (SHENZHEN)<br />
CO. LTD., CHINA untuk LIFESCAN INC., USA<br />
AKL<br />
20207701076 16/08/2011 DSI One HbAic Fs DIASYS DIAGNOSTIC SYSTEM, GERMANY<br />
AKL<br />
20103700953 25/04/2011<br />
AKL<br />
20103700951 25/04/2011<br />
AKL<br />
20103700950 26/04/2011<br />
AKL<br />
20103700949 25/04/2011<br />
AKL<br />
20101700930 25/02/2011<br />
Barbiturates Plus ONLINE DAT<br />
Roche / Hitachi<br />
BARB Abuscreen Online<br />
Barbiturates COBAS Integra<br />
BENZ ONLINE DAT Benzodizene<br />
Plus Cobas C System<br />
ONLINE DAT Benzodiazepines Plus<br />
Roche / Hitachi<br />
BILTS cobas c 111 Total Bilirubin<br />
Special<br />
AKL<br />
20101700927 25/02/2011 LDL-C cobas c 111 LDL Cholesterol<br />
AKL<br />
10101700929 30/03/2011<br />
AKL<br />
10302700940 25/02/2011<br />
AKL<br />
20303700821 27/04/2011<br />
AKL<br />
20303700820 27/04/2011<br />
AKL<br />
20303700819 27/04/2011<br />
AKL<br />
20303700818 30/03/2011<br />
PreciControl Anemia Elecsys and<br />
cobas e analyzer<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
OXOID Comylabacter Growth<br />
Supplement (Liquid) OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
REMEL Vibrio Cholerae Inaba<br />
Agglutinating Serum REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
REMEL Vibrio Cholerae 01<br />
Polyvalent Agglutinating Serum REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
Remel Vibrio Cholerae Ogawa<br />
Agglutinating Serum REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
REMEL Salmonella H (L) Polyvalent<br />
for IV, IW, IZ13, IZ28, IZ38, IZ40 REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303700817 30/03/2011 REMEL Salmonella H (gm) REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303700816 27/04/2011<br />
AKL<br />
20303700814 30/03/2011<br />
AKL<br />
20303700813 27/04/2011<br />
AKL<br />
20303700812 30/03/2011<br />
PDF Creator - PDF4Free v3.0<br />
Remel Salmonella 9 - O<br />
Agglutinating Serum REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
REMEL Salmonella H Polyvalent<br />
Phases 2 (1, 2, 5, 6, 7) REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
REMEL Salmonella Polyvalent O (A-<br />
S) Agglutinating Serum REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
REMEL Salmonella H Polyvalent<br />
Plases I dan 2 REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
http://www.pdf4free.com
AKL<br />
20301700811 25/02/2011 OXOID Fluconazole FCA 25 OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20208700758 09/02/2011<br />
AKL<br />
10305700757 08/02/2011<br />
AKL<br />
10103700760 25/02/2011<br />
C.f.a.s HbA1c Calinbrator for<br />
Automated System<br />
AAGP2 Tina-Quan [a] a1-Acid<br />
Glycoprotein Gen.2<br />
CHE2 Cholinesterase Gen.2 COBAS<br />
INTEGRA cobas c systems<br />
AKL<br />
20101700620 11/02/2011 ALB2 Cobas c III Albumin Gen.2<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101700619 09/02/2011<br />
ONE TOUCH Ultra 2 Blood Glucose<br />
Monitoring System<br />
AKL<br />
20305700594 08/02/2011 ARCHITECT Anti-Tg Controls<br />
AKL<br />
20305700595 08/02/2011 ARCHITECT Anti-Tg Calibrators<br />
AKL<br />
20305700593 08/02/2011 ARCHITECT Anti-TPO Reagent Kit<br />
AKL<br />
20305700592 08/02/2011 ARCHITECT Anti-TPO Calibrators<br />
AKL<br />
20305700589 08/02/2011 ARCHITECT Anti-Tg Reagent Kit<br />
AKL<br />
20305700590 08/02/2011 ARCHITECT Anti-TPO Controls<br />
AKL<br />
20101700528 11/02/2011 SYS-Multicalib II<br />
AKL<br />
20303700530 25/02/2011<br />
AKL<br />
20101700420 08/02/2011<br />
FLEXTRONICS INDUSTRIAL (SHENZHEN)<br />
CO. LTD., CHINA untuk LIFESCAN INC., USA<br />
FISHER DIAGNOSTIC A FISHER<br />
SCIENTIFIC CO. LLC, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
FISHER DIAGNOSTIC A FISHER<br />
SCIENTIFIC CO. LLC, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
FISHER DIAGNOSTIC A FISHER<br />
SCIENTIFIC CO. LLC, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
FISHER DIAGNOSTIC A FISHER<br />
SCIENTIFIC CO. LLC, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
FISHER DIAGNOSTIC A FISHER<br />
SCIENTIFIC CO. LLC, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
FISHER DIAGNOSTIC A FISHER<br />
SCIENTIFIC CO. LLC, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SYSMEX INDONESIA<br />
REMEL Shingella Somnei Phasos 1<br />
& 2 REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
ASTL Cobas c III Aspartate<br />
Aminotransferase<br />
AKL<br />
10101700419 08/02/2011 ALTL Cobas c III<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10102700408 30/05/2011<br />
ERMA Micro Flow Cell Photometer /<br />
Biochemical Analyzer AE-600N<br />
IRMA Inc, Japan<br />
PT. MULTIMEDILAB<br />
KARYAMANDIRI<br />
AKL<br />
20101700391 12/04/2011<br />
AKL<br />
20101700254 11/02/2011 SYS-Multicalib I<br />
AKL<br />
10205700213 25/02/2011<br />
AKL<br />
10205700212 25/02/2011<br />
Combiscreen 11 Sys (Bilirubin,<br />
Urobilinogen, Ketones, Ascorbic Acid, ANALYTICON BIOTECHNOLOGIES AG.,<br />
Glucose, Protein, Blood, pH, Ni GERMANY<br />
VACUETTE Sed Rate Timer 10/II<br />
(SRT 10/II)<br />
VACUETTE Red Rate Screener 20/II<br />
(SRS 20/II)<br />
AKL<br />
20305700057 08/02/2011 IgE II Elecsys and cobas e analyzer<br />
AKL<br />
20101700069 08/02/2011 TP2 Cobas c III Total Protein Gen 2<br />
AKL<br />
20101700065 08/02/2011 UREAL Cobas c III Urea/bun<br />
AKL<br />
20101700066 08/02/2011 GLUC 2 Cobas c III Glucose HK<br />
PDF Creator - PDF4Free v3.0<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
VITAL DIAGNOSTICS S.r.l., ITALY untuk<br />
GREINER BIO-ONE GMBH., AUSTRIA<br />
VITAL DIAGNOSTICS S.r.l., ITALY untuk<br />
GREINER BIO-ONE GMBH., AUSTRIA<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. BAVARIA COMBININDO<br />
PT. SYSMEX INDONESIA<br />
PT. WIDYA MITRA PERSADA<br />
PT. WIDYA MITRA PERSADA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101700060 08/02/2011<br />
ALP2S Cobas c III Alkaline<br />
phosphate acc. To IFCC Gen.2<br />
AKL<br />
20101700062 09/02/2011 ROCHE CA Cobas c III Calcium<br />
AKL<br />
20101700053 09/02/2011<br />
ROCHE BILT2 Bilirubin Total DPD<br />
Gen2 Cobas c System2<br />
AKL<br />
10101700067 09/02/2011 UA2 Cobas c III Uric ver.2Acid<br />
AKL<br />
10101700068 09/02/2011<br />
CHOL2 Cobas c III Cholesterol Gen-<br />
2<br />
AKL<br />
10101700070 09/02/2011 TRIGL Cobas c III Triglicerides<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20301700042 25/02/2011 OXOID Ceffazidime/Clovularue Acid OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302700044 25/02/2011<br />
OXOID Legionella Species Test Kit 1<br />
x 50 Tests OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21106110448 24/10/2011 SPERM RINSE VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
20209604892 30/05/2011 CROMATEST Anti A+B Monoclonal LINEAR CHEMICALS. L., SPAIN<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
10102701969 15/08/2011 POINTE 180 II Chemistry Analyzer POINTE SCIENTIFIC INC., USA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20305604619 07/02/2011 SEPTIFAST Prep Kit M<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
untuk ROCHE MOLECULAR SYSTEM INC,<br />
USA melalui ROCHE DIAGNOSTICS INT<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10208604601 30/03/2011 MEDONIC M - Series DualPack 200 BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20305604640 27/04/2011<br />
AKL<br />
20305604634 27/04/2011<br />
AKL<br />
20303604638 27/04/2011<br />
AKL<br />
10303604637 17/06/2011<br />
AKL<br />
20101604629 27/04/2011<br />
AKL<br />
20305604680 25/02/2011<br />
SENSATEST One-Step HBsAg Test<br />
Cassette<br />
SENSATEST HBsAG One Step<br />
Diagnostic Rapid Test<br />
SENSATEST Syphilis One-Step<br />
Diagnostic Rapid Test<br />
SENSATEST One-Step Chlamydia<br />
Test Cassette<br />
SENSATEST One-Step HCGTest<br />
Cassette<br />
ABBOTT Murex HCV Ag/Ab<br />
Combination<br />
AKL<br />
20101604413 07/02/2011 ORCHID Alat Tes Kehamilan<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA untuk NEOPHARMA BIOTECH<br />
ASIA SDN. BHD., MALAYSIA<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA untuk NEOPHARMA BIOTECH<br />
ASIA SDN. BHD., MALAYSIA<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA untuk NEOPHARMA BIOTECH<br />
ASIA SDN. BHD., MALAYSIA<br />
ATLASLINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA for NEOPHARM BIOTECH ASIA<br />
SDN. BHD., MALAYSIA<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA untuk NEOPHARMA BIOTECH<br />
ASIA SDN. BHD., MALAYSIA<br />
MUREX BIOTECH S.A., (PTY) LTD., SOUTH<br />
AFRICA<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., CHINA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. ABBOTT INDONESIA<br />
PT. USAHATAMA SENTOSA MAS<br />
AKL<br />
10302604414 31/01/2011 COBAS AmpliPrep and Accessories ROCHE DIAGNOSTIC LTD., SWITZERLAND PT. ROCHE INDONESIA<br />
AKL<br />
20101604353 27/04/2011 JITU Strip Uji Kehamilan Pribadi AMGENIX INTERNATIONAL INC., USA PT. DANPAC PHARMA<br />
AKL<br />
20101604351 27/04/2011<br />
AKL<br />
20301110301 08/02/2011 BD BACTEC BGIT 960 SIRE Kit<br />
SENSITIF Strip Uji Kehamilan<br />
Pribadi AMGENIX INTERNATIONAL INC., USA PT. DANPAC PHARMA<br />
AKL<br />
10302110305 07/02/2011 BD BBL Taxo TB Niacin Test Strip<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
AKL<br />
20303604261 30/05/2011 CROMATEST Febrile Antigen Kit LINEAR CHEMICALS. L., SPAIN<br />
AKL<br />
10302110040 25/01/2011<br />
AKL<br />
10303604120 31/01/2011<br />
PDF Creator - PDF4Free v3.0<br />
BD BBL MGIT Mycrobacteria Growth<br />
Indicator<br />
BD BBL Streptocard Enzym Test<br />
Latex<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY.,<br />
SINGAPORE<br />
http://www.pdf4free.com<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA
AKL<br />
10303501310 30/03/2011 SD Bioline Rotavirus STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303113665 28/12/2011<br />
AKL<br />
20305701379 26/04/2011<br />
AKL<br />
20101702771 28/10/2011<br />
IMMULITE 2000 Systems HVG<br />
Herpes I & II IgG<br />
COBAS AmpliPrep / COBAS<br />
TaqMan HCV Test (HCMCAP)<br />
SMART CHEK Blood Glucose<br />
Monitoring System<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
MAJOR BIOSYSTEM CORP., TAIWAN<br />
AKL<br />
10101705517 28/12/2011 SEKUSUI Anasolv L CAT SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101113579 28/12/2011 SEKISUI CETP ELISA - DAIICHI SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
30305805982 23/08/2011 FOKUS AntiHIV 1/2 cassette GENIX TECHNOLOGI INC., CANADA<br />
AKL<br />
20101705688 28/11/2011<br />
AKL<br />
20101705691 29/11/2011<br />
AKL<br />
10101705690 28/11/2011<br />
AKL<br />
20305705695 29/11/2011<br />
AKL<br />
20305705694 29/11/2011<br />
AKL<br />
20101705696 28/11/2011<br />
AKL<br />
10101705692 28/11/2011<br />
Testosterone Calset ll Elecsys and<br />
Cobas e Analyzers<br />
Progesterone ll Calset Elecsys and<br />
Cobas e Analyzer<br />
Progesterone ll Elecsys and Cobas e<br />
Analyzers<br />
MYOGLOBIN CalSet Elecsys and<br />
cobas e analyzers<br />
MYOGLOBIN Elecsys and cobas e<br />
analyzers<br />
Estradiol ll Calset Elecsys and Cobas<br />
e Analyzers<br />
INSULIN Elecsys and Cobas e<br />
Analyzers<br />
AKL<br />
20101705729 21/11/2011 URISYS 2400 Calibration Strip<br />
AKL<br />
20101705730 28/11/2011 Urisys 2400 Cassette<br />
AKL<br />
20101705731 28/11/2011<br />
AKL<br />
20101705735 28/11/2011<br />
AKL<br />
20305112767 27/10/2011<br />
Amy-P a-Amylase EPS Pancreatic<br />
COBAS INTEGRA cobas C system<br />
ACCU-Check Advantage Blood<br />
Glucose Meter and Lancing Device<br />
VITROS Immunodiagnostic Products<br />
HBsAg ES Calibrator<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ORTHO-CLINICAL DIAGNOSTICS., UNITED<br />
KINGDOM<br />
AKL<br />
20207703372 08/02/2011 ID-Liss Coombs Card DIAMED GMBH., SWITZERLAND<br />
AKL<br />
20102110893 20/04/2011<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
POINTE 360 Random Access<br />
Automated Discrate Analyzer POINTE SCIENTIFIC INC., USA PT. SEGARA HUSADA MANDIRI<br />
AKL<br />
20301110303 08/02/2011 BD BACTEC MGIT 960 STR 4.0 Kit<br />
AKL<br />
20301110302 08/02/2011 BD BACTEC MGIT 960 IR Kit<br />
AKL<br />
10302604941 09/02/2011<br />
BD BBL TAXO TB Nlacin Test<br />
Control<br />
AKL<br />
20301110304 08/02/2011 BD BACTEC MGIT 960 INH 0.4 Kit<br />
AKL<br />
20101604428 07/02/2011<br />
AKL<br />
20101604432 09/02/2011<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS CAL Calibrator A ADVIA<br />
Centaur Systems, ACS:180 Systems<br />
ADVIA CENTAUR T3/T4/VB12<br />
Ready Pack<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
http://www.pdf4free.com<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
20305702929 15/08/2011 HUMATYPE Albumin 22% HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20207111088 27/04/2011<br />
BIORAD D-10 Dual Program<br />
Reorder Pack<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20306702922 15/08/2011 HUMAN f PSA ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101110214 07/02/2011<br />
AKL<br />
20101701771 26/04/2011<br />
AKL<br />
20101700809 30/05/2011<br />
AKL<br />
20101700810 30/05/2011<br />
AKL<br />
10302605055 04/04/2011<br />
AKL<br />
10302704282 25/02/2011<br />
SENSATEST Urinalysis Reagent<br />
Test Strip<br />
ACCU - CHECK Performa/Blood<br />
Glucose Meter<br />
BD Preset Arterial Blood Collection<br />
Syringe<br />
BD A-LINE Arterial Blood Collection<br />
Syringe<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA untuk NEOPHARMA BIOTECH<br />
ASIA SDN. BHD., MALAYSIA<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
BECTON DICKINSON & COMPANY, UNITED<br />
KINGDOM<br />
BECTON DICKINSON & COMPANY, UNITED<br />
KINGDOM<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
REMEL McFarland Equivalance<br />
Turbidity Standard REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
OXOID Sulphamandelate<br />
Supplement 1 x 10 Vials OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21106700942 24/10/2011 CCM 30 VITROLIFE AB., SWEDEN<br />
AKL<br />
20101604775 07/02/2011<br />
AKL<br />
20101113340 01/12/2011<br />
AKL<br />
20305700931 30/03/2011<br />
SIEMENS CAL Calibrator Z Advia<br />
Centaur System, ACS:180 System<br />
VITROS Immunodiagnostic<br />
Products Total B-hCG II Reagent<br />
Pack<br />
PreciControl HBsAg II Elecsys cobas<br />
e analyzer<br />
AKL<br />
20305700928 30/03/2011 HBsAg II Elecsys cobas e analyzer<br />
AKL<br />
20303110227 07/02/2011<br />
AKL<br />
20101705693 21/11/2011<br />
AKL<br />
20306705054 21/11/2011<br />
AKL<br />
20306704877 24/10/2011<br />
Rubella IgG Elecsys and cobas e<br />
analyzer<br />
Insulin Calset Elecsys and cobas e<br />
analyzers<br />
CA 72-4 Elecsys and cobas e<br />
analyzers<br />
CA 19-9 CALSET ELECSYS and<br />
cobas e analyzers<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT.ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305013129 04/01/2011<br />
COBAS TaqMan Analyzer and<br />
Accessories<br />
ROCHE DIAGNOSTICS LTD,Switzerland<br />
untuk ROCHE DIAGNOSTICS GmbH,Germany PT.ROCHE INDONESIA,<br />
AKL<br />
10209703406 26/01/2011 ElectricTubeSealerSE-250 CENTRON TECHNOLOGIES INC.,USA<br />
AKL<br />
10203111114 27/04/2011<br />
SHANDON Histocentre 3 and<br />
Accessories<br />
AKL<br />
20205110219 07/02/2011 CellaVision DM96<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
CELLAVISION AB., SWEDEN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
AKL<br />
21106110449 25/02/2011 IVF VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
10202604322 07/02/2011 BD BBL Horse Blood, Defribinated<br />
AKL<br />
10207110396 09/02/2011 BM Test HbA1c Diluent<br />
AKL<br />
20207110395 09/02/2011<br />
BM Test HbA1c Control (High dan<br />
Low)<br />
AKL<br />
20206110394 09/02/2011 BM Test HbA1c Reagent 2<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
PT.FRISMED HOSLAB<br />
INDONESIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. SYSMEX INDONESIA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20206110393 09/02/2011 BM Test HbA1c Reagent 1<br />
AKL<br />
20207110397 09/02/2011 BM Test HbA1c Calibrator (1 dan 2)<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20209110417 25/02/2011 SPAN Bovine Albumin SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20209110415 25/02/2011<br />
SPAN Anti-Human Serum (Poly<br />
specific) coombs Serum SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20303110416 25/02/2011<br />
SIGNAL-MF Flow Through Filarial<br />
Antibody Spot/Immunodot Test Kit SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20101110158 31/01/2011 IMMULITE Turbo NT-ProBNP NTP<br />
AKL<br />
20305110156 31/01/2011<br />
IMMULITE Thyroid Autoantibody<br />
(TAD) ControlTurbo NT-ProBNP<br />
NTP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
AKL<br />
20101110319 08/02/2011 AMS ALT (GPT) IFCC AMS U.K. LTD., UNITED KINGDOM<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
AKL<br />
20305110318 08/02/2011<br />
AKL<br />
10203110184 07/02/2011<br />
ABBOTT ReaTime HBV Calibrator<br />
Kit<br />
ABBOTT multi-Collect Speciment<br />
Collection Kit<br />
AKL<br />
20101110176 01/02/2011 i-STAT G3+ Cartridge<br />
PT. ABBOTT MOLECULAR INC., USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED, CANADA untuk ABBOTT POINT OF<br />
CARE, USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. TRANSMEDIC INDONESIA<br />
AKL<br />
10101110175 01/02/2011 GESAN URIC ACID LR GESAN PRODUCTION S.R.L., ITALY PT. LABTAMA ADISINDO<br />
AKL<br />
20101110213 07/02/2011 GESAN GLUCOSE LR GESAN PRODUCTION S.R.L., ITALY PT. LABTAMA ADISINDO<br />
AKL<br />
20101110171 01/02/2011 ADVIA CAL CO2 calibrator/Diluent<br />
AKL<br />
20101110235 07/02/2011 COBAS C1 Calibrator Solution 1<br />
AKL<br />
20101110234 07/02/2011 COBAS C2 Calibration Solution 2<br />
AKL<br />
20207110209 07/02/2011 i-STAT PT/INR Cartridge<br />
AKL<br />
20102110281 08/02/2011 VersaCell System<br />
AKL<br />
20305110157 31/01/2011<br />
IMMULITE Thyroglobulin (TG)<br />
Control<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTIC INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTIC INTL. LTD.,<br />
SWITZERLAND<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED, CANADA untuk ABBOTT POINT OF<br />
CARE, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
AKL<br />
20101110320 08/02/2011 AMS Glucose GOD-PAP AMS U.K. LTD., UNITED KINGDOM<br />
AKL<br />
20101110321 08/02/2011 AMS AST (GOT) IFCC AMS U.K. LTD., UNITED KINGDOM<br />
AKL<br />
20305110317 08/02/2011 ABBOTT RealTime HCV Control Kit<br />
AKL<br />
20101110278 08/02/2011<br />
MEIZHOU Calibration Standard<br />
Solution<br />
AKL<br />
20101110279 08/02/2011 MEIZHOU Refill Solution<br />
AKL<br />
20103110190 07/02/2011<br />
AKL<br />
20303110030 25/01/2011 ADVIA Centaur Toxo M<br />
PDF Creator - PDF4Free v3.0<br />
ABBOTT MOLECULAR INC., USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
MEIZHOU CORNLEY HI-TECH CO. LTD.,<br />
CHINA<br />
MEIZHOU CORNLEY HI-TECH CO. LTD.,<br />
CHINA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
ARCHITECT iGentamicin Reagent<br />
Kit ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA
AKL<br />
20303110189 07/02/2011 ADVIA Centaur Rub G QC<br />
AKL<br />
20101110187 07/02/2011<br />
DIMENSION TACR Flex Reagent<br />
Cartridge<br />
AKL<br />
20101110186 07/02/2011 DIMENSION TACR CAL<br />
AKL<br />
10101110151 31/01/2011 ADVIA Centaur CpS Ready Pack<br />
AKL<br />
10208110150 31/01/2011 SIEMENS T3 DIL<br />
AKL<br />
10201110149 31/01/2011<br />
ADVIA Autoslide Wright-Giemsa<br />
Buffer<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
FISHER DIAGNOSTICS, USA untuk<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101110173 01/02/2011 GESAN Cholesterol LR GESAN PRODUCTION S.R.L., ITALY PT. LABTAMA ADISINDO<br />
AKL<br />
10101110172 01/02/2011 GESAN SERACONTROL N GESAN PRODUCTION S.R.L., ITALY PT. LABTAMA ADISINDO<br />
AKL<br />
10101110174 01/02/2011 GESAN Triglycerides LR GESAN PRODUCTION S.R.L., ITALY PT. LABTAMA ADISINDO<br />
AKL<br />
10101110153 31/01/2011<br />
AKL<br />
10101110152 31/01/2011<br />
AKL<br />
20305110170 01/02/2011<br />
AKL<br />
20103110169 01/02/2011<br />
SIEMENS Rapid QC Complete Level<br />
3<br />
SIEMENS Rapid QC Complete Level<br />
2<br />
ADVIA Chemistry Rheumatoid<br />
Factor Reagent<br />
ADVIA Chemistry Phenobarbital 2<br />
Raegent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101110231 07/02/2011<br />
MISSION Expert Urinalysis Reagent<br />
Strip 10 S (BLO, BIL, URO, KET,<br />
GLU, PRO, NIT, LEU, pH, SG)<br />
AKL<br />
20103110232 07/02/2011 MISSION Breath Alcohol Detector<br />
ACON LABORATORIES INC, USA melalui<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON LABORATORIES INC, USA melalui<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10204110212 07/02/2011<br />
DIAGON DiaExtract Bacterial Cell<br />
Wash Solution (DiaExtract BCWS) DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10303110211 07/02/2011 DIAGON DiaMyco Kit DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101110193 07/02/2011 COMBITROL TS+ Level 1<br />
AKL<br />
10101110192 07/02/2011 COMBITROL TS+ Level 3<br />
AKL<br />
10101110194 07/02/2011 COMBITROL TS+ Level 2<br />
AKL<br />
20305110154 31/01/2011 IMMULITE IGF Control<br />
BIONOSTICS INC., USA untuk ROCHE<br />
DIAGNOSTICS GMBH., GERMANY melalui<br />
ROCHE DIAGNOSTICS INTL. LTD., SW<br />
BIONOSTICS INC., USA untuk ROCHE<br />
DIAGNOSTICS GMBH., GERMANY melalui<br />
ROCHE DIAGNOSTICS INTL. LTD., SW<br />
BIONOSTICS INC., USA untuk ROCHE<br />
DIAGNOSTICS GMBH., GERMANY melalui<br />
ROCHE DIAGNOSTICS INTL. LTD., SW<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305110155 31/01/2011 IMMULITE LBP Control<br />
AKL<br />
20103110191 07/02/2011<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LTD.,<br />
HONGKO<br />
PT. SIEMENS INDONESIA<br />
ARCHITECT iGentamicin<br />
Calibrators ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305110183 07/02/2011 ABBOTT m24sp Instrument ABBOTT MOLECULAR INC., USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
10302110182 07/02/2011 UROTEST AB MERCK KgaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20101110185 07/02/2011 i-STAT Crea Cartridge<br />
AKL<br />
20101110178 01/02/2011 i-STAT cTni Cartridge<br />
AKL<br />
20101110177 01/02/2011 i-STAT CHEM8+ Cartridge<br />
AKL<br />
20101110180 01/02/2011 i-STAT CK-MB Cartridge<br />
AKL<br />
10204110220 07/02/2011 RAYTO Concentrated Cleanser<br />
AKL<br />
20205110221 07/02/2011<br />
AKL<br />
10208701870 14/03/2011<br />
AKL<br />
10208701874 14/03/2011<br />
AKL<br />
20209806046 14/03/2011<br />
AKL<br />
10201110029 25/01/2011<br />
AKL<br />
10204110371 09/02/2011<br />
RAYTO 7600S Auto Hematology<br />
Analyzer<br />
DIAMED ID Wash Solution<br />
Concentrate B for DiaMed ID<br />
Automates<br />
DIAMED ID Wash Solution<br />
Concentrate A for DiaMed ID<br />
Automates<br />
DIAMED ID-DiaClon ABO/D +<br />
Revers Grouping for Patients<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED, CANADA untuk ABBOTT POINT OF<br />
CARE, USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED, CANADA untuk ABBOTT POINT OF<br />
CARE, USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED, CANADA untuk ABBOTT POINT OF<br />
CARE, USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED, CANADA untuk ABBOTT POINT OF<br />
CARE, USA<br />
RAYTO LIFE ANALYTICAL SCIENCES CO.<br />
LTD., P.R. CHINA<br />
RAYTO LIFE ANALYTICAL SCIENCES CO.<br />
LTD., P.R. CHINA<br />
DIAMED GmbH.,Switzerland<br />
DIAMED GmbH.,Switzerland<br />
DIAMED GmbH.,Switzerland<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT FRISMED HOSLAB<br />
INDONESIA.,<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
MERCK Gram-Color Modified<br />
(Phenol-Free) Staining Kit MERCK KgaA., GERMANY PT. MERCK Tbk<br />
SysWash Elecsys and cobas e<br />
analyzers<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20208110038 25/01/2011 IMMULITE Systems EPO Control<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LIMITED., HO<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101110032 25/01/2011 ADVIA Centaur Insulin IRI<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LIMITED., HO<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101110039 25/01/2011 ADVIA Centaur Insulin Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LIMITED., HO<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20303110031 25/01/2011 ADVIA Centaur Rub M QC<br />
AKL<br />
10204110373 09/02/2011<br />
ProCell Elecsys and cobas e<br />
analyzer<br />
AKL<br />
10204110275 08/02/2011 Multiclean cobas c systems<br />
AKL<br />
10204110276 08/02/2011 Hemolyzing Reagent Roche/Hitachi<br />
akl<br />
20303110226 07/02/2011<br />
AKL<br />
10204110274 08/02/2011<br />
AKL<br />
10101110485 02/03/2011<br />
PDF Creator - PDF4Free v3.0<br />
Rubella IgM Elecsys and cobas e<br />
analyzer<br />
CleanCell Elecsys and cobas e<br />
analyzer<br />
BeneCheck Uric Acid Monitoring<br />
System<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTHCARE DIAGNOSTICS LIMITED., HO<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN - ROC<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. INDOCORE PERKASA
AKL<br />
20101110486 02/03/2011<br />
AKL<br />
20101110379 09/02/2011<br />
AKL<br />
20101110378 09/02/2011<br />
AKL<br />
20103113541 28/12/2011<br />
AKL<br />
20103110480 02/03/2011<br />
AKL<br />
20103110481 02/03/2011<br />
AKL<br />
20103110482 02/03/2011<br />
AKL<br />
20103110536 07/03/2011<br />
AKL<br />
20103110535 07/03/2011<br />
AKL<br />
20103110478 02/03/2011<br />
AKL<br />
20103110479 02/03/2011<br />
AKL<br />
20103110389 09/02/2011<br />
AKL<br />
20103110388 09/02/2011<br />
AKL<br />
20205110533 07/03/2011<br />
AKL<br />
20205110532 07/03/2011<br />
BeneCheck Dual Monitoring System<br />
Total Cholesterol & Blood Glucose<br />
ISE Standard High Roche/Hitachi<br />
Cobas Systems<br />
ISE Standard Low Roche/Hitachi<br />
Cobas Systems<br />
ABON THC One Step Marijuana<br />
Test Device (Urine)<br />
ARCHITECT / AEROSET OpiM<br />
Opiates<br />
ARCHITECT / AEROSET CocM<br />
Cocaine<br />
ARCHITECT / AEROSET THCM<br />
Cannabinoids<br />
ARCHITECT / AEROSET AmphM<br />
Amphetamine/Methamphetamine<br />
ARCHITECT / AEROSET Opiates<br />
Calibrator<br />
ARCHITECT / AEROSET MethM<br />
Methadone<br />
ARCHITECT/AEROSET BenzM<br />
Benzodiazepines<br />
ARCHITECT iPhenobarbital<br />
calibrator<br />
ARCHITECT iPhenobarbital Reagent<br />
Kit<br />
SHENZHEN KT6280 Fully Auto<br />
Hematology Analyzer and<br />
Accessories<br />
SHENZHEN KT6180 Fully Auto<br />
Hematology Analyzer and<br />
Accessories<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN - ROC<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA untuk ALERE SAN DIEGO INC., USA<br />
melalui INVERNESS MEDICAL I<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
MICROGENICS CO., USA melalui ABBOTT<br />
LABORATORIES., USA<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT LABORATORIES., USA<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT LABORATORIES., USA<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
PT. INDOCORE PERKASA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ALIZAH<br />
PT. ALIZAH<br />
AKL<br />
10102110529 07/03/2011<br />
SHENZHEN BA600-1 Urine<br />
Analyzer Analyzer and Accessories<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
PT. ALIZAH<br />
AKL<br />
10102110530 07/03/2011<br />
SHENZHEN WP21B Semi-auto<br />
Chemistry Analyzer and Accessories<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
PT. ALIZAH<br />
AKL<br />
20102110531 07/03/2011<br />
AKL<br />
10205110376 09/02/2011<br />
SHENZHEN GS300 Fully Auto<br />
Chemistry Analyzer and Accessories<br />
IRIA ESR Auto Analyzer and<br />
accessories<br />
SHENZHEN GENIUS ELECTRONICS CO.<br />
LTD., CHINA<br />
LINEAR CHEMICALS S.L., SPAIN<br />
AKL<br />
10302110453 25/02/2011 FEMLAB Vaginitis Test Kit AMERITEK INC., USA<br />
PT. ALIZAH<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
10101110523 07/03/2011 LABKIT CHOLESTEROL LS CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
10101110526 07/03/2011 LABKIT GPT (ALT) LQ CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
10101110524 07/03/2011 LABKIT URIC ACID LS CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
10101110525 07/03/2011 LABKIT TRIGLYCERIDES LS CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
20101110232 11/02/2011 LABKIT UREA / LQ CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
10102110044 25/01/2011 ERBA Chem 5 V3 and Accessories<br />
AKL<br />
10102110043 25/01/2011 ERBA Chem 7 and Accessories<br />
ERBA DIAGNOSTICS MANNHEIM GMBH.,<br />
GERMANY<br />
ERBA DIAGNOSTICS MANNHEIM GMBH.,<br />
GERMANY<br />
PT. GENTA BUANA ASTADECA<br />
PT. GENTA BUANA ASTADECA<br />
AKL<br />
20101110233 11/02/2011 LABKIT GOT (AST) / LQ CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
20101110236 11/02/2011 LABKIT CREATININE J CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
20101110237 11/02/2011 LABKIT TOTAL PROTEIN CHEMELEX S.A., SPAIN PT. GENTA BUANA ASTADECA<br />
AKL<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
20101110483 02/03/2011 BeneCheck Blood Glucose Test Strip LTD., TAIWAN - ROC<br />
PT. INDOCORE PERKASA<br />
AKL<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
10101110484 02/03/2011 BeneCheck Uric Acid Test Strip LTD., TAIWAN - ROC<br />
PT. INDOCORE PERKASA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101110487 02/03/2011<br />
BeneCheck Total Cholesterol Test<br />
Strip<br />
AKL<br />
21603110316 08/02/2011 On Cell Plus Lancets<br />
AKL<br />
21603110315 08/02/2011<br />
On Cell Plus auto-Lancet Soft<br />
Lancing Device<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN - ROC<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PT. INDOCORE PERKASA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10101110454 25/02/2011 SmartLAB Control Solution HMM DIAGNOSTICS GMBH., GERMANY PT. MITRAASA PRATAMA<br />
AKL<br />
20101110352 09/02/2011<br />
AKL<br />
20101110353 09/02/2011<br />
AKL<br />
20101110354 09/02/2011<br />
AKL<br />
10102110156 09/02/2011<br />
AKL<br />
20205110158 09/02/2011<br />
AKL<br />
10102110157 09/02/2011<br />
AKL<br />
20303110537 07/03/2011<br />
SmartLAB mini Self Monitoring Blood<br />
Glucose Meter HMM DIAGNOSTICS GMBH., GERMANY PT. MITRAASA PRATAMA<br />
SmartLAB genie Self Monitoring<br />
Blood Glucose Meter HMM DIAGNOSTICS GMBH., GERMANY PT. MITRAASA PRATAMA<br />
SmartLAB pro Blood Glucose Test<br />
Strips HMM DIAGNOSTICS GMBH., GERMANY PT. MITRAASA PRATAMA<br />
DIRUI CS-T240 Auto Chemistry<br />
Analyzer<br />
DIRUI BF-5180 Automatic<br />
Hematology Analyzer<br />
DIRUI CS-300 Auto Chemistry<br />
Analyzer<br />
SUNNYPROTEK Malaria P.f/Pan<br />
One Step Malaria Test<br />
AKL<br />
20101110694 25/03/2011 DIASYS CK-MB FS<br />
DIRUI INDUSTRIAL CO. LTD., PR. CHINA<br />
DIRUI INDUSTRIAL CO. LTD., PR. CHINA<br />
DIRUI INDUSTRIAL CO. LTD., PR. CHINA<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., CHINA<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
AKL<br />
10101110520 07/03/2011 AMS Cholesterol CHOO-PAP AMS U.K. LTD., UNITED KINGDOM<br />
AKL<br />
10101110519 07/03/2011 AMS Trigliceride GPO-PAP AMS U.K. LTD., UNITED KINGDOM<br />
AKL<br />
10102110538 08/03/2011<br />
AKL<br />
10204110542 08/03/2011<br />
AKL<br />
10204110541 08/03/2011<br />
AKL<br />
10204110540 08/03/2011<br />
AKL<br />
10102110539 08/03/2011<br />
HAMILTON Microlab STAR<br />
Intrument and Accessories<br />
PreClean M Modular Analytic E170<br />
and cobas e 601 Analyzers<br />
ProCell M Modular Analytic E170 and<br />
cobas e 601 Analyzers<br />
ProCell M Modular Analytic E170 and<br />
cobas e 601 Analyzers<br />
HAMILTON Microlab STARlet<br />
Instrument and accessories<br />
AKL<br />
10101110527 07/03/2011 ROCHE Cardiac Control Myoglobin<br />
AKL<br />
10203110236 07/02/2011 BenchMark GX and Accessories<br />
AKL<br />
10101110372 09/02/2011<br />
INSTC Instrument Check cobas c<br />
501<br />
AKL<br />
10208110222 07/02/2011 RAYTO Lyse<br />
AKL<br />
10204110224 07/02/2011 RAYTO Cleanser<br />
AKL<br />
10208110223 07/02/2011 RAYTO Diluent<br />
AKL<br />
10101110233 07/02/2011 MEIZHOU Electrolyte Quality Control<br />
AKL<br />
20303110188 07/02/2011 ADVIA Centaur Toxo M QC<br />
AKL<br />
20102013624 14/03/2011<br />
AKL<br />
10103110231 11/02/2011<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS I<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
RAYTO LIFE ANALYTICAL SCIENCES CO.<br />
LTD., P.R. CHINA<br />
RAYTO LIFE ANALYTICAL SCIENCES CO.<br />
LTD., P.R. CHINA<br />
RAYTO LIFE ANALYTICAL SCIENCES CO.<br />
LTD., P.R. CHINA<br />
MEIZHOU CORNLEY HI-TECH CO. LTD.,<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
Automatic Clinical Analyzer & HIROSE ELECTRONIC SYSTEMS CO. LTD.,<br />
Accessories Model TMS 24i Premium JAPAN untuk TOKYO BOEKI MACHINERY<br />
and accessories<br />
LTD., JAPAN<br />
JEOL Clinical Chemistry Analyzer<br />
JCA-BM 6010/C<br />
AKL<br />
10101110392 09/02/2011 i-STAT CONTROL LEVEL 3<br />
AKL<br />
10101110390 09/02/2011 i-STAT CONTROL LEVEL 1<br />
PDF Creator - PDF4Free v3.0<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
BIONOSTICS INC., USA untuk ABBOTT<br />
POINT OF CARE., USA<br />
BIONOSTICS INC., USA untuk ABBOTT<br />
POINT OF CARE., USA<br />
http://www.pdf4free.com<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MULTI MITRA BIOTECH<br />
PT. MULTIREDJEKI KITA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SIEMENS INDONESIA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SYSMEX INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA
AKL<br />
10101110391 09/02/2011 i-STAT CONTROL LEVEL 2<br />
AKL<br />
20101110348 09/02/2011 i-STAT CG8+ Cartridge<br />
AKL<br />
20207110351 09/02/2011 i-STAT Celite ACT Cartridge<br />
AKL<br />
20101110349 09/02/2011 i-STAT 6+ Cartridge<br />
AKL<br />
20101110350 09/02/2011 i-STAT EG7+ Cartridge<br />
AKL<br />
20101110375 09/02/2011 i-STAT CG4+ Cartridge<br />
BIONOSTICS INC., USA untuk ABBOTT<br />
POINT OF CARE., USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED., CANADA untuk ABBOTT POINT<br />
OF CARE., USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED., CANADA untuk ABBOTT POINT<br />
OF CARE., USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED., CANADA untuk ABBOTT POINT<br />
OF CARE., USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED., CANADA untuk ABBOTT POINT<br />
OF CARE., USA<br />
ABBOTT POINT OF CARE CANADA<br />
LIMITED., CANADA untuk ABBOTT POINT<br />
OF CARE., USA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
PT. TRANSMEDIC INDONESIA<br />
AKL<br />
20205110280 08/02/2011 CELL-DYN Ruby System ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20207110697 25/03/2011<br />
AKL<br />
20303110229 07/02/2011<br />
AKL<br />
20102110556 09/03/2011<br />
AKL<br />
20102110568 10/03/2011<br />
AKL<br />
20102110570 10/03/2011<br />
AKL<br />
20102110567 10/03/2011<br />
AKL<br />
20102110569 10/03/2011<br />
AKL<br />
20205110572 10/03/2011<br />
AKL<br />
10102110566 10/03/2011<br />
SIEMENS Innovance D-Dimer<br />
Sample Diluent<br />
ProciControl Rubella IgM Elecsys<br />
and cobas e analyzer<br />
MINDRAY BS-300 Chemistry<br />
Analyzer and Accessories<br />
MINDRAY BS-200 Chemistry<br />
Analyzer and Accessories<br />
MINDRAY BS-380 Chemistry<br />
Analyzer and Accessories<br />
MINDRAY BS-120 Chemistry<br />
Analyzer and Accessories<br />
MINDRAY BS-400 Chemistry<br />
Analyzer and Accessories<br />
MINDRAY BC-5300 Auto<br />
Hematology Analyzer and and<br />
Accessories<br />
MINDRAY BA-88A Semi-Auto<br />
Chemistry nalyzer and Accessories<br />
AKL<br />
20305110814 13/04/2011 ADVIA Centaur aHBs2<br />
AKL<br />
20305110813 13/04/2011 ADVIA Centaur HBeAg<br />
AKL<br />
20305110812 13/04/2011 ADVIA Centaur Systems HBeAg QC<br />
AKL<br />
20305110811 13/04/2011 ADVIA Centaur Systems aHBe QC<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPO<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
PT. SYSMEX INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101110801 12/04/2011 BIOLABO Cholesterol CHOD PAP BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
10101110802 12/04/2011<br />
AKL<br />
10101110798 12/04/2011<br />
AKL<br />
10101110800 12/04/2011<br />
AKL<br />
10101110799 12/04/2011<br />
AKL<br />
20305110810 13/04/2011 ADVIA Centaur aHBe<br />
AKL<br />
10208110803 12/04/2011<br />
BIOLABO Triglycerides GPO<br />
Method BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIOLABO HDL-Cholesterol (PTA)<br />
Precipitant BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIOLABO GAMMA-GT Soluble<br />
GPNA BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIOLABO HDL-Cholesterol Direct<br />
Method BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
Q-MAY Factor Auto Common Diluent<br />
Type K<br />
AKL<br />
20208110807 12/04/2011 Q-MAY Multiple Sera A Type K<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LIMITED., HONGKONG<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT SUMBERMITRA AGUNGJAYA<br />
PT SUMBERMITRA AGUNGJAYA
AKL<br />
20208110806 12/04/2011 Q-MAY Multiple Sera N Type K<br />
AKL<br />
20207110804 12/04/2011 Q-MAY Factor Auto D-Dimer Type K<br />
AKL<br />
20208110808 12/04/2011<br />
AKL<br />
20208110809 12/04/2011<br />
Q-MAY Factor Auto P-FDP<br />
Calibrator Type K<br />
Q-MAY Factor D-dimer Calibrator<br />
Type K<br />
AKL<br />
20207110805 12/04/2011 Q-MAY Factor Auto P-FDP Type K<br />
AKL<br />
10101110795 12/04/2011 AMNIOQUICK<br />
AKL<br />
20205110571 10/03/2011<br />
AKL<br />
20205110574 10/03/2011<br />
AKL<br />
20103113540 28/12/2011<br />
MINDRAY BC-5500 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
MINDRAY BC-5800 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
ABON PCP One Step Phencyclidine<br />
Test Strip (Urine)<br />
AKL<br />
10101700392 30/03/2011 Combiscreen Control<br />
AKL<br />
20205110555 09/03/2011<br />
AKL<br />
20205110553 09/03/2011<br />
AKL<br />
20306110699 25/03/2011<br />
AKL<br />
20205110947 25/04/2011<br />
MINDRAY BC-3200 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
MINDRAY BC-1800 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
Q-MAY LABORATORY CORPORATION,<br />
JAPAN melalui SEIKEN SANGYOU CO. LTD.,<br />
JAPAN<br />
BIOSYNEX IMMUNODIAGNOSTICS,<br />
FRANCE<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA untuk ALERE SAN DIEGO INC., USA<br />
melalui INVERNESS MEDICAL I<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONIC CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONIC CO. LTD., P.R. CHINA<br />
PT SUMBERMITRA AGUNGJAYA<br />
PT SUMBERMITRA AGUNGJAYA<br />
PT SUMBERMITRA AGUNGJAYA<br />
PT SUMBERMITRA AGUNGJAYA<br />
PT SUMBERMITRA AGUNGJAYA<br />
PT. NELTA MULTI GRACIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. BAVARIA COMBININDO<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
SCHEBO Tumor M2-PK ELISA<br />
Stool Test SCHEBO BIOTECH AG, Germany PT. ALAM PUTRA SAKTI, jakarta<br />
VerifyNow P2Y12 ( Platelet Function<br />
Assay ) ACCUMETRICS INC, USA PT. HARMONI PRIMA MEDIKA<br />
AKL<br />
20101110988 26/04/2011 ARKRAY Aution Screen ARKRAY GLOBAL BUSSINES INC., JAPAN<br />
AKL<br />
10204110561 10/03/2011 S1 Rinse Solution<br />
AKL<br />
10101110620 16/03/2011 Combitrol Plus B Level 3<br />
AKL<br />
10101110619 16/03/2011 Combitrol Plus B Level 2<br />
AKL<br />
10101110618 16/03/2011 Combitrol Plus B Level 1<br />
AKL<br />
10204110610 14/03/2011<br />
COBAS 4N NaOH/System Cleaning<br />
Solution Roche/Hitachi<br />
AKL<br />
10204110608 14/03/2011 ROCHE SMS Roche/Hitachi 911<br />
AKL<br />
10204110609 14/03/2011 COBAS NaOH-D Roche/Hitachi 912<br />
AKL<br />
10204110611 14/03/2011 COBAS Multiclean Roche/Hitachi<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20205111012 26/04/2011 Pima Analyser and Accessories ALERE TECHNOLOGIES GmbH., gERMANY PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303110673 23/03/2011<br />
AKL<br />
10103110745 07/04/2011<br />
AKL<br />
20103110534 07/03/2011<br />
PDF Creator - PDF4Free v3.0<br />
ADVANTAGE Dengue NS1 Ag & Ab<br />
Combi Card J. MITRA & CO. PVT. LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
ARCHITECT/AEROSET Opiates<br />
Low Control Set<br />
ARCHITECT/AEROSET<br />
Cannabinoids Calibrator<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
http://www.pdf4free.com<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA
AKL<br />
20208110950 25/04/2011<br />
AKL<br />
20205110948 25/04/2011<br />
VerifyNow Assay Wet Quality Control<br />
(WQC) Level 1 dan 2 ACCUMETRICS INC., USA PT. HARMONI PRIMA MEDIKA<br />
VerifyNow IIb/IIIa Test (platelet<br />
function assay) ACCUMETRICS INC., USA PT. HARMONI PRIMA MEDIKA<br />
AKL<br />
20101111033 26/04/2011<br />
DUS 10M (Leukocytes, Nitrite,<br />
Urobilinogen, Protein, pH, Blood,<br />
Spesific Gravity, Ketones, Bilirubin DFI CO. LTD., KOREA PT. INDOCORE PERKASA<br />
AKL<br />
20101111032 26/04/2011<br />
DUS 11M (Leukocytes, Nitrite,<br />
Urobilinogen, Protein, pH, Blood,<br />
Spesific Gravity, Ketones, Bilirubin DFI CO. LTD., KOREA PT. INDOCORE PERKASA<br />
AKL<br />
20207111029 26/04/2011 MISSION Hb Hemoglobin Test Strip<br />
AKL<br />
10101111189 28/04/2011<br />
AKL<br />
20101111180 28/04/2011<br />
AKL<br />
20101111179 28/04/2011<br />
AKL<br />
20101111181 28/04/2011<br />
VITROS Chemistry Products URIC<br />
Slides - URIC DT Slides<br />
VITROS Chemistry Products K<br />
Slides / K DT Slides<br />
VITROS Chemistri Products ALB<br />
Slides / ALB DT Slides<br />
VITROS Chemistry Products ALKP<br />
DT Slides<br />
AKL<br />
20305110616 14/03/2011 ARCHITECT CRP Calibrator WR<br />
AKL<br />
20103110613 14/03/2011<br />
AKL<br />
10103110605 14/03/2011<br />
AKL<br />
10103110603 14/03/2011<br />
AKL<br />
10103110604 14/03/2011<br />
AKL<br />
20103110615 14/03/2011<br />
AKL<br />
20103110614 14/03/2011<br />
AKL<br />
20103110612 14/03/2011<br />
AKL<br />
10103110746 07/04/2011<br />
ARCHITECT/AEROSET DOA MC I<br />
Calibrator<br />
ARCHITECT/AEROSET DOA MC II<br />
Control Set<br />
ARCHITECT/AEROSET DOA MC<br />
III Control Set<br />
ARCHITECT/AEROSET DOA MC I<br />
Control Set<br />
ARCHITECT/AEROSET EcstM<br />
Ecstasy<br />
ARCHITECT/AEROSET Ecstasy<br />
Calibrator<br />
ARCHITECT/AEROSET DOA MC<br />
Neg Cal<br />
ARCHITECT/AEROSET<br />
Cannabinoid Control<br />
AKL<br />
20306111041 27/04/2011 ARCHITECT Cyfra 21-1 Calibrators<br />
AKL<br />
20306111042 27/04/2011 ARCHITECT Cyfra 21-1 Reagent Kit<br />
AKL<br />
20101111352 23/05/2011 U-RIGHT Blood Glucose Test Strip<br />
AKL<br />
20101111351 23/05/2011<br />
U-RIGHT Blood Glucose Monitoring<br />
System<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
SENTINEL CH, SpA, ITALY melalui ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTIC INC., USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
FUJIREBIO DIAGNOSTIC INC., USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
TAIDOC TECHNOLOGY CORPORATION,<br />
TAIWAN - ROC melalui JCL HEALTHCARE<br />
SDN. BHD., MALAYSIA<br />
TAIDOC TECHNOLOGY CORPORATION,<br />
TAIWAN - ROC melalui JCL HEALTHCARE<br />
SDN. BHD., MALAYSIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. BIO AXION HEALTHINDO<br />
PT. BIO AXION HEALTHINDO<br />
AKL<br />
20101111251 29/04/2011 SYSMEX Albumin Reagent ( ALB )<br />
SYSMEX WUXI CO, LTD., China Melalui<br />
SYSMEX ASIA PASIFIC PTE. LTD., Singapore<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10101111188 28/04/2011<br />
AKL<br />
20103110543 07/03/2011<br />
SYSMEX Alanine Aminotransferase<br />
Reagent R1-R2<br />
ARCHITECT/AEROSET<br />
Cannabinoids Calibrator<br />
SYSMEX WUXI CO, LTD., China Melalui<br />
SYSMEX ASIA PASIFIC PTE. LTD., Singapore<br />
MICROGENICS CO, USA melalui ABBOTT<br />
LABORATORIES, USA<br />
AKL<br />
20101110559 10/03/2011 AMS ALP (AMP) IFCC AMS U.K. LTD, United Kingdom<br />
AKL<br />
20101110672 23/03/2011<br />
AKL<br />
20101110671 23/02/2011<br />
SOLITEST One Step Urine<br />
Pregnancy Test Card<br />
SOLITEST One Step Urine<br />
Pregnancy Test Strip<br />
GUILIN ZHONGHUI TECHNOLOGY CO,<br />
LTD, China<br />
GUILIN ZHONGHUI TECHNOLOGY CO,<br />
LTD, China<br />
PT. SYSMEX INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. D & V INTERNATIONAL<br />
MAKMUR GEMILANG<br />
PT. D & V INTERNATIONAL<br />
MAKMUR GEMILANG<br />
AKL<br />
20303110557 10/03/2011 IMMUNOQUICK Malaria +4 BIOSYSNEX, France PT. NELTA MULTI GRACIA<br />
AKL<br />
10205110676 22/08/2011<br />
AKL<br />
10101110563 10/03/2011<br />
AKL<br />
10101110564 10/03/2011<br />
RADIOMETER ABL 80-FLEX<br />
BASIC and Accessories<br />
RADIOMETER MADICAL ApS, Denmark<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
WOMAN CHOICE - Ovulation Test<br />
Midstream IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
WOMAN CHOICE - Ovulation Test<br />
Strip IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101110565 10/03/2011<br />
SPEEDY TEST - Ovulation Test<br />
Cassette IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
AKL<br />
10101110677 23/03/2011 RANDOX HUM ASY CONTROL 2<br />
AKL<br />
20307110679 23/03/2011 RANDOX RX SERIES HbA1c<br />
AKL<br />
10101110678 23/03/2011 RANDOX HUM ASY CONTROL 3<br />
AKL<br />
20101110680 23/03/2011 RANDOX RX SERIES CYSC<br />
AKL<br />
10101110452 25/02/2011 RANDOX SLDL-C<br />
AKL<br />
10101110562 10/03/2011 ISETROL Level 1, 2, 3<br />
AKL<br />
10204110522 07/03/2011 PCR Cleanup Kit<br />
AKL<br />
10101110617 16/03/2011 SnapPak<br />
AKL<br />
20101010041 25/01/2011<br />
AKL<br />
20101010042 25/01/2011<br />
K-Lite 3 AU Electrolyte Analyzer and<br />
Accessories<br />
K-Lite 5 AU Electrolyte Analyzer and<br />
Accessories<br />
RANDOX LABORATORIES, LTD, United<br />
Kongdom<br />
RANDOX LABORATORIES, LTD, United<br />
Kongdom<br />
RANDOX LABORATORIES, LTD, United<br />
Kongdom<br />
RANDOX LABORATORIES, LTD, United<br />
Kongdom<br />
RANDOX LABORATORIES, LTD, United<br />
Kongdom<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
malalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
CELERA CO, USA melalui ABOTT<br />
MOLECULAR INC, USA<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
malalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
MEIZHOU CORNLEY HI-TECH Co., LTD,<br />
China<br />
MEIZHOU CORNLEY HI-TECH Co., LTD,<br />
China<br />
AKL<br />
10101110521 07/03/2011 AMS GGT ( Carboxy) AMS U.K. LTD, United Kingdom<br />
AKL<br />
20101110558 10/03/2011<br />
AKL<br />
20101110560 10/03/2011<br />
AKL<br />
20102110242 11/02/2011<br />
AMS UREA GLDH Kinetic &<br />
Endpoint<br />
AMS Bilirubin (Jendrassik) Total &<br />
Direct<br />
CDx 90 Fully Automated Clinical<br />
Chemistry<br />
AKL<br />
20305110670 23/03/2011 INDEC, IB Virolisa EBV EA IgA<br />
AKL<br />
20305110704 28/03/2011 INDEC, IB Virolisa EBV VCA IgA<br />
AKL<br />
20305110703 28/03/2011 INDEC, IB Virolisa EBV EBNA-1 IgA<br />
AMS U.K. LTD, United Kingdom<br />
AMS U.K. LTD, United Kingdom<br />
THERMO FISCHER SCIENTIFIC CLINICAL<br />
DIAGNOSTIC DIVISION, USA<br />
IBL INTERNATIONAL GmbH., Germany<br />
dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, Jakarta<br />
IBL INTERNATIONAL GmbH., Germany<br />
dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, Jakarta<br />
IBL INTERNATIONAL GmbH., Germany<br />
dikemas ulang oleh PT. INDEC<br />
DIAGNOSTICS, Jakarta<br />
PT. RAFA TOPAZ UTAMA<br />
PT. RAFA TOPAZ UTAMA<br />
PT. RAFA TOPAZ UTAMA<br />
PT. RAFA TOPAZ UTAMA<br />
PT. RAFA TOPAZ UTAMA<br />
PT. ROCHE INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. ROCHE INDONESIA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. GRESIK SARANA TIRTA<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
AKL<br />
20306110698 25/03/2011 SCHEBO Tumor M2-PK Quick-Prep SCHEBO BIOTECH AG, Germany PT. ALAM PUTRA SAKTI<br />
AKL<br />
203061106987 25/02/2011 SCHOBE Tumor M2-PK Quick-Prep SCHEBO BIOTECH AG, Germany PT. ALAM PUTRA SAKTI<br />
AKL<br />
20205110159 10/02/2011 Mission HB Meter<br />
ACON BIOTECH (HANGZHOU) Co. Ltd,<br />
China<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20101110374 09/02/2011 i-STAT 1 Analyzer + Accessories ABBOTT POINT OF CARE, USA PT. TRANSMEDIC INDONESIA<br />
AKL<br />
10101111048 27/04/2011 KONELAB Lipoprotein (a) Control<br />
AKL<br />
20305111046 27/04/2011<br />
AKL<br />
20101111177 28/04/2011<br />
AKL<br />
10101111182 28/04/2011<br />
KONELAB CRP High Sensitivity<br />
Cintrol<br />
THERMO FISHER SCIENTIFIC OY, Finland<br />
melalui THERMO FISHER SCIENTIFIC INC,<br />
Australia<br />
THERMO FISHER SCIENTIFIC OY, Finland<br />
melalui THERMO FISHER SCIENTIFIC INC,<br />
Australia<br />
VITROS Chemistry Products 250 /<br />
350 Reference Fluid ORTHO - CLINICAL DIAGNOSTIC, Inc., USA<br />
ONE TOUCH Select Control<br />
Solution<br />
AKL<br />
20305110456 25/02/2011 GENESIS CagA IgG<br />
AKL<br />
10303110458 25/02/2011 GENESIS H. Pylori IgM<br />
AKL<br />
10303110457 25/02/2011 GENESIS Hp-G Screen<br />
AKL<br />
20101111178 28/04/2011<br />
AKL<br />
20303702110 26/05/2011<br />
AKL<br />
20205110976 26/04/2011<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Chemestry Products K<br />
slides K DT Slides<br />
ACON Syphilis Ultra Rapid Test<br />
Device (Whole Blood / Serum /<br />
Plasma)<br />
QUINTUS 5 - Part Hematology<br />
BIONOSTICS, Inc., USA untuk LIFESCAN<br />
EUROPE., Switzerland<br />
GENESIS DIAGNOSTICS LTD., United<br />
Kingdom<br />
GENESIS DIAGNOSTICS LTD., United<br />
Kingdom<br />
GENESIS DIAGNOSTICS LTD., United<br />
Kingdom<br />
ORTHO CLINICAL DIAGNOSTIC,inc., USA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA untuk INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
SER<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
Analyser BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
http://www.pdf4free.com
AKL<br />
10102110377 09/02/2011<br />
AKL<br />
20101111390 30/05/2011<br />
HERA Clinical Chemistry Photometer<br />
and Accessories<br />
AKL<br />
10101111006 26/04/2011 DIALINE Triglycerides<br />
AKL<br />
20101111003 26/04/2011 DIALINE Albumin<br />
AKL<br />
10101111004 26/04/2011 DIALINE Lipase<br />
AKL<br />
10101111005 26/04/2011 DIALINE Cholesterol<br />
AKL<br />
20101111080 27/04/2011 DIALINE ASAT / AST (GOT)<br />
LINEAR CHEMICALS S.L., SPAIN<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
BIOLABO AST/TGO Colorimetric<br />
Method BIOLABO SA, FRANCE PT. C.V. KHARISMA UTAMA<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE LTD., SINGAPORE<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
AKL<br />
20101111205 28/04/2011 ABX Pentra Creatinine 120 CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305111203 28/04/2011 ABX Pentra IgG CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305111204 28/04/2011 ABX Pentra IgA CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305111206 28/04/2011 ABX Pentra IgM CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305111201 28/04/2011 ABX Pentra Transferin CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101111202 28/04/2011 ABX Pentra Total Protein 100 CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101111047 27/04/2011 KONELAB Utrol<br />
AKL<br />
20305111050 27/04/2011 KONELAB Apoliprotein B<br />
AKL<br />
20305111049 27/04/2011 KONELAB CRP High Sensitivy<br />
AKL<br />
10101110886 20/04/2011 KONELAB - HBDH<br />
AKL<br />
20101110887 20/04/2011 KONELAB U/ CSF PROTEIN<br />
AKL<br />
10103110884 20/04/2011<br />
KONELAB Cholinesterase with<br />
Debucaine<br />
AKL<br />
10101110993 26/04/2011 KONELAB Lipase (Colorimetric)<br />
AKL<br />
20101110992 26/04/2011 KONELAB CK (IFCC)<br />
AKL<br />
10101110994 26/04/2011 KONELAB LDH (IFCC)<br />
AKL<br />
20101110995 26/04/2011 KONELAB CK - MB<br />
AKL<br />
20101110996 26/04/2011 KONELAB Creatinine (Enzymatic)<br />
AKL<br />
20103110885 20/04/2011 KONELAB Calcium<br />
AKL<br />
20101110888 20/04/2011 KONELAB AMYLASE (IFCC)<br />
AKL<br />
10203111131 27/04/2011<br />
PDF Creator - PDF4Free v3.0<br />
THERMO SCIENTIFIC Histostar<br />
and Accessories<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO SHANDON LTD, TRADING AS<br />
THERMO FISHER SCIENTIFIC., UK<br />
http://www.pdf4free.com<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA
AKL<br />
20101111133 27/04/2011 KONELAB Acid Phosphatase<br />
AKL<br />
20305111132 27/04/2011<br />
AKL<br />
10203111134 27/04/2011<br />
AKL<br />
20101111176 28/04/2011<br />
AKL<br />
20101111175 28/04/2011<br />
AKL<br />
10101111207 28/04/2011<br />
AKL<br />
10204111208 28/04/2011<br />
AKL<br />
20101111174 28/04/2011<br />
KONELAB Rheumatoid Factors 2<br />
(RF 2)<br />
THERMO SCIENTIFIC Autostainer<br />
480S and Accessories<br />
VITROS Chemistry Products<br />
Calibrator Kit 4<br />
VITROS Chemistry Products<br />
Calibrator Kit 3<br />
VITROS Chemistry Products dHDL<br />
Slides<br />
VITROS Chemistry Products 7%<br />
BSA<br />
ONE TOUCH SELECT SIMPLE<br />
Blood Glucose Monitoring System<br />
and Accessories<br />
THERMO SHANDON LTD, TRADING AS<br />
THERMO FISHER SCIENTIFIC., UK<br />
PT. ENSEVAL MEDIKA PRIMA<br />
THERMO SHANDON LTD, TRADING AS<br />
THERMO FISHER SCIENTIFIC., UK<br />
PT. ENSEVAL MEDIKA PRIMA<br />
THERMO SHANDON LTD, TRADING AS<br />
THERMO FISHER SCIENTIFIC., UK<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA INDONESIA<br />
FLEXTRONIC IND (SHENZHEN) CO. LTD.,<br />
CHINA untuk LIFESCAN EUROPE,<br />
SWITZERLAND<br />
AKL<br />
20305110160 10/02/2011 CTK On Site Dengue Ag Rapid Test CTK BIOTECH INC, USA<br />
AKL<br />
20205110573 10/03/2011<br />
MINDRAY BC-5380 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
AKL<br />
10303110459 25/02/2011 GENESIS H. Pylori IgA<br />
AKL<br />
20305111218 29/04/2011<br />
AKL<br />
20303111484 09/06/2011<br />
GENARRAYT Microarray Hardware<br />
GD250<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
GENESIS DIAGNOSTICS LTD., UNITED<br />
KINGDOM<br />
GENESIS DIAGNOSTICS LTD., UNITED<br />
KINGDOM<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
DIAQUICK Chikungunya IgM<br />
Cassette DIALAB GMBH., AUSTRIA PT. NELTA MULTI GRACIA<br />
AKL<br />
20303111382 30/05/2011 LIAISON Rubella IgG DIASORIN S.p.A., ITALY<br />
AKL<br />
20303110380 30/05/2011 LIAISON Toxo IgM DIASORIN S.p.A., ITALY<br />
AKL<br />
20303111381 30/05/2011 LIAISON Toxo IgG II DIASORIN S.p.A., ITALY<br />
AKL<br />
20303111384 30/05/2011 LIAISON CMV IgM DIASORIN S.p.A., ITALY<br />
AKL<br />
20303111383 30/05/2011 LIAISON CMV IgG DIASORIN S.p.A., ITALY<br />
AKL<br />
20102111002 26/04/2011<br />
AKL<br />
20101110944 25/04/2011<br />
AKL<br />
20101110945 25/04/2011<br />
AKL<br />
20101110946 25/04/2011<br />
AKL<br />
10204110943 25/04/2011<br />
AKL<br />
10204110941 25/04/2011<br />
AKL<br />
20208110941 25/04/2011<br />
AKL<br />
10101111256 29/04/2011<br />
AKL<br />
20101111230 29/04/2011<br />
AKL<br />
20101111196 28/04/2011<br />
AKL<br />
20101111199 28/04/2011<br />
AKL<br />
20101111232 29/04/2011<br />
AKL<br />
20101111200 28/04/2011<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
BIOTECNICA BT 1500 Chemistry<br />
Analyzer BIOTECNICA INSTRUMENTS SpA., ITALY PT. RAJA ERBA INDOCHEM<br />
TOKYO BOEKI MACHINERY<br />
calibrator 1<br />
TOKYO BOEKI MACHINERY<br />
calibrator 2<br />
TOKYO BOEKI MACHINERY Urine<br />
Calibrator Set<br />
TOKYO BOEKI MACHINERY<br />
Diluent for Urine<br />
TOKYO BOEKI MACHINERY<br />
Cleaning Solution<br />
TOKYO BOEKI MACHINERY<br />
Standard Solution for Hemo - Dialysis<br />
Solution<br />
SYSMEX Uric Acid Reagent (UA-R1<br />
/ UA-R2)<br />
SYSMEX Aspartate<br />
Aminotransferase Reagent (AST - R1<br />
/ AST - R2)<br />
SYSMEX Amylase Reagent (AMY-<br />
R1 / AMY-R2)<br />
SYSMEX Bilirubin Total Reagent (T-<br />
BIL-R1 / T-BIL-R2)<br />
SYSMEX Creatinine Reagent (CRE -<br />
R1 / CRE - R2)<br />
SYSMEX Alkaline Phosphate<br />
Reagent (ALP-R1 / ALP-R2)<br />
JOKOH CO. LTD., JAPAN untik TOKYO<br />
BOEKI MACHINERY LTD., JAPAN<br />
JOKOH CO. LTD., JAPAN untik TOKYO<br />
BOEKI MACHINERY LTD., JAPAN<br />
JOKOH CO. LTD., JAPAN untik TOKYO<br />
BOEKI MACHINERY LTD., JAPAN<br />
JOKOH CO. LTD., JAPAN untik TOKYO<br />
BOEKI MACHINERY LTD., JAPAN<br />
JOKOH CO. LTD., JAPAN untik TOKYO<br />
BOEKI MACHINERY LTD., JAPAN<br />
JOKOH CO. LTD., JAPAN untik TOKYO<br />
BOEKI MACHINERY LTD., JAPAN<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101111229 29/04/2011<br />
AKL<br />
20101111231 29/04/2011<br />
AKL<br />
10101111255 29/04/2011<br />
AKL<br />
20101111198 28/04/2011<br />
SYSMEX Glucose Reagent (GLU -<br />
R1 / GLU - R2)<br />
SYSMEX Urea Nitrogen Reagent<br />
(BUN - R1 / BUN - R2)<br />
SYSMEX Cholesterol Total Reagent<br />
(T-CHO-R1 / T-CHO-R2)<br />
SYSMEX Bilirubin Direct Reagent (D-<br />
BIL-R1 / D-BIL-R2)<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10203011141 27/04/2011<br />
AKL<br />
10203111115 27/04/2011<br />
AKL<br />
10203111116 27/04/2011<br />
AKL<br />
20102111036 27/04/2011<br />
AKL<br />
20205110974 26/04/2011<br />
SHANDON Citadel 2000 Automated<br />
Tissue Processor and Accessories<br />
SHANDON Varistain 24-4 and<br />
Accessories<br />
SHANDON Cytospin 4<br />
Cytocentrifuge Accessories<br />
KONELAB 20XT Analyzer and<br />
Accessories<br />
EPOC Blood Analysis System and<br />
Accessories<br />
AKL<br />
10302111028 26/04/2011 INFINITE F50 and Accessories<br />
AKL<br />
10204111086 27/04/2011 DIALINE Acid Washing Solution<br />
AKL<br />
10204111087 27/04/2011 DIALINE Alkaline Washing Solution<br />
AKL<br />
20101110876 20/04/2011<br />
DIALINE Creatinine JAFFE-<br />
METHOD<br />
AKL<br />
20101111081 27/04/2011 DIALINE Urea<br />
AKL<br />
20101111082 27/04/2011 DIALINE Bilirubin Direct<br />
AKL<br />
20101111083 27/04/2011 DIALINE Glucose Hexokinase<br />
AKL<br />
10101111084 27/04/2011 DIALINE Lipoprotein (a)<br />
AKL<br />
10101110870 20/04/2011<br />
DIALINE HDL CHOL Direct-<br />
Selective<br />
AKL<br />
20101110871 20/04/2011 DIALINE CK-NAC IFCC/DGKC<br />
AKL<br />
20101110873 20/04/2011 DIALINE CK-MB IFCC/DGKC<br />
AKL<br />
10101110874 20/04/2011 DIALINE a-HBDH DGKC<br />
AKL<br />
20101110875 20/04/2011 DIALINE Total Bilirubin<br />
AKL<br />
20101110877 20/04/2011 DIALINE ALK Phosphatase<br />
AKL<br />
10101110869 20/04/2011 DIALINE ALAT/ALT (GPT)<br />
AKL<br />
10101110880 20/04/2011 DIALINE LDL/Chol<br />
PDF Creator - PDF4Free v3.0<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
EPOCAL INC., CANADA melalui ALERE INC.,<br />
CANADA<br />
TECAN AUSTRIA GMBH., AUSTRIA melalui<br />
TECAN SALES INTERNATIONAL GMBH.,<br />
AUSTRIA<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA
AKL<br />
10101110879 20/04/2011 DIALINE GGT<br />
AKL<br />
10101110878 20/04/2011 DIALINE Uric Acid<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
AKL<br />
10101111217 29/04/2011 SYSMEX Iron Reagent Fe R1 - R2<br />
AKL<br />
10101111216 29/04/2011<br />
SYSMEX y-Glutamyl Transferase<br />
Reagent (y-GT-R1 dan y-GT-R2)<br />
AKL<br />
10102111109 16/06/2011 URYXXON 500<br />
AKL<br />
20101111551 14/06/2011<br />
AKL<br />
10101111555 14/06/2011<br />
AKL<br />
20101111553 14/06/2011<br />
AKL<br />
20103111487 09/06/2011<br />
AKL<br />
20103111486 09/06/2011<br />
ARCHITECT 25-OH Vitamin D<br />
Reagent Kit<br />
ARCHITECT 25-OH Vitamin D<br />
Controls<br />
ARCHITECT 25-OH Vitamin D<br />
Calibrators<br />
SYSMEX WUXI CO. LTD., melalui SYSMEX<br />
ASIA PASIFIC PTE. LTD., SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
MACHEREY - NAGEL GMBH & CO. KG.,<br />
GERMANY<br />
BIOKIT S.A., SPAIN untuk ABBOTT GMBH &<br />
CO. KG., GERMANY<br />
BIOKIT S.A., SPAIN untuk ABBOTT GMBH &<br />
CO. KG., GERMANY<br />
BIOKIT S.A., SPAIN untuk ABBOTT GMBH &<br />
CO. KG., GERMANY<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. MRK DIAGNOSTICS<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
ARCHITECT I Carbamazepine<br />
Calibrators ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ARCHITECT I Carbamazepine<br />
Reagent Kit ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20101111552 14/06/2011 ARCHITECT LH Calibrators<br />
AKL<br />
20306111044 27/04/2011 ARCHITECT Cyfra 21-1 Controls<br />
AKL<br />
20102110230 11/02/2011<br />
ARCHITECT i1000 System and<br />
Accessories<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, LONGFORD-IRELAND<br />
FUJIREBIO DIAGNOSTIC INC, USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
FLEXTRONICS MANUFACTURING<br />
(SINGAPORE) PTE. LTD., SINGAPORE untuk<br />
ABBOTT LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20306111416 06/06/2011 NucliSENS Easyq HPV v1-1 BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101111554 14/06/2011 ARCHITECT LH Reagent Kit<br />
AKL<br />
20103701503 01/07/2011<br />
C.f.a.s DAT Qualitative Plus Roche<br />
Systems<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
LONGFORD-IRELAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305604613 27/04/2011<br />
SENSATEST Anti HCV One Step<br />
Diagnostic Rapid Test<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD. P.R., CHINA melalui NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., MALAY<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20305604604 27/04/2011<br />
SENSATEST One-Step HCV Test<br />
system<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD. P.R., CHINA melalui NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., MALAY<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303111112 27/04/2011<br />
SENSATEST Malaria P.F / P.V<br />
Antibody Test Cassette<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD. P.R., CHINA melalui NEOPHARMA<br />
BIOTECH ASIA SDN. BHD., MALAY<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303110696 25/03/2011 SD BIOLINE Malaria Ag P.f/Pan STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303702946 25/02/2011<br />
AKL<br />
20303702945 25/02/2011<br />
AKL<br />
20102111569 14/06/2011<br />
AKL<br />
20101111569 14/06/2011<br />
REMEL Shigella boydii Polyvalent 3<br />
(12-15) REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
REMEL Shigella boydii Polyvalent 2<br />
(7-11) REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
ROCHE Cobas h 232 and<br />
Accessories<br />
ROCHE Cobas h 232 and<br />
Accessories<br />
AKL<br />
10208111145 27/04/2011 ADVIA Centaur Multi - Diluent 10<br />
AKL<br />
10208111146 27/04/2011 ADVIA Centaur Multi - Diluent 11<br />
AKL<br />
20305111254 29/04/2011 ADVIA Chemistry wr CRP Cal<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305111213 29/04/2011 ADVIA Chemistry wr CRP Control<br />
AKL<br />
10101111212 29/04/2011<br />
ADVIA Centaur eE2 + 30 Ready<br />
Pack<br />
AKL<br />
10204111210 29/04/2011 ADVIA Centaur eE2 Dil Ready Pack<br />
AKL<br />
20101111211 29/04/2011<br />
AKL<br />
20103701474 25/04/2011<br />
ADVIA Centaur Systems Calibrator<br />
30<br />
ACETA Acetaminophen Cobas<br />
Integra cobas c systems<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LTD., HONGKONG<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20102111043 25/04/2011 ABBOTT Thin Lancets ABBOTT DIABETES CARE INC., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10203013644 30/05/2011<br />
AKL<br />
10203013645 30/05/2011<br />
AKL<br />
10203013643 30/05/2011<br />
AKL<br />
10203013642 30/05/2011<br />
AKL<br />
10203111113 27/04/2011<br />
MICROM STP 120 Tissue<br />
Processor dan Accessories<br />
MICROM HM 550 CRYOSTAT and<br />
Accessories<br />
MICROM HM 355S Rotary<br />
Microtome and Accessories<br />
MICROM STP 420 ES Tissue<br />
Processor and Accessories<br />
SHANDON Finesse - 325 and<br />
Accessories<br />
MICROM INTERNATIONAL GMBH.,<br />
GERMANY part of THERMO FISHER<br />
SCIENTIFIC., GERMANY<br />
MICROM INTERNATIONAL GMBH.,<br />
GERMANY part of THERMO FISHER<br />
SCIENTIFIC., GERMANY<br />
MICROM INTERNATIONAL GMBH.,<br />
GERMANY part of THERMO FISHER<br />
SCIENTIFIC., GERMANY<br />
MICROM INTERNATIONAL GMBH.,<br />
GERMANY part of THERMO FISHER<br />
SCIENTIFIC., GERMANY<br />
THERMO FISHER SCIENTIFIC INC., USA<br />
melalui THERMO FISHER SCIENTIFIC., UK<br />
AKL<br />
20303111209 28/04/2011 FOKUS Malaria Pv/Pf Cassette CORE DIAGNOSTICS LTD., UK<br />
AKL<br />
20303111329 01/07/2011 RESZON BRUGIA Rapid<br />
RESZON DIAGNOSTICS INTERNATIONAL<br />
SDN BHD,Malaysia<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT PRIMATANDU<br />
MOTAROPRATAMA<br />
AKL<br />
20101110990 26/04/2011 BIOLABO Glucose GOD - PAP BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20207110991 26/04/2011 BIOLABO Bio - Fibri BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20101110989 26/04/2011<br />
BIOLABO Phosphatase Alkaline<br />
(DEA) BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20205111208 20/06/2011 ABACUS 3 Hematology Analyzer DIATRON MI ZRT., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
20205111209 20/06/2011 ABACUS 5 Hematology Analyzer DIATRON MI ZRT., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10102111391 07/07/2011<br />
AKL<br />
10102111390 07/07/2011<br />
EPPENDORF centrifuge 5418 and<br />
Acessories EPPENDORF AG., GERMANY PT. ENSEVAL MEDIKA PRIMA<br />
EPPENDORF centrifuge 5430R and<br />
Acessories EPPENDORF AG., GERMANY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101111583 14/06/2011 DANA Blood Glucose Dtrip SOOIL DEVELOPMENT CO. LTD., KOREA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10102111395 07/07/2011<br />
AKL<br />
10102111394 07/07/2011<br />
AKL<br />
10102111393 07/07/2011<br />
AKL<br />
10102111392 07/07/2011<br />
EPPENDORF Centrifuge 5418R and<br />
Accessories EPPENDORF AG., GERMANY PT. ENSEVAL MEDIKA PRIMA<br />
EPPENDORF Centrifuge 5430 and<br />
Accessories EPPENDORF AG., GERMANY PT. ENSEVAL MEDIKA PRIMA<br />
EPPENDORF Centrifuge 5424 and<br />
Accessories EPPENDORF AG., GERMANY PT. ENSEVAL MEDIKA PRIMA<br />
EPPENDORF Centrifuge 5424R and<br />
Accessories EPPENDORF AG., GERMANY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305111330 01/07/2011 INDOSEHAT HBsAg ELISA<br />
AKL<br />
10101111562 14/06/2011<br />
AKL<br />
10101111560 14/06/2011<br />
AKL<br />
10101111561 14/06/2011<br />
AKL<br />
10208111111 17/06/2011<br />
AKL<br />
20101111113 17/06/2011<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Chemistry Products PHOS<br />
Slides / PHOS DT Slides<br />
VITROS Chemistry Products UPRO<br />
Performance Verifier II<br />
VITROS Chemistry Products<br />
Isoenzymes Performance Verifier I<br />
VITROS Chemistry Products<br />
Speciality Diluent<br />
EZ SMART 608 Blood Glucose<br />
Monitoring System<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO. LTD., CHINA<br />
PT. INDONESIA SEHAT<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
PT. MANDIRI NUGRAHA<br />
TYSON BIORESEARCH INC., TAIWAN AJITUNNGAL<br />
http://www.pdf4free.com
AKL<br />
20101111112 17/06/2011<br />
EZ SMART 608 Blood Glucose Test<br />
Strip<br />
AKL<br />
20305111329 01/07/2011 BRUGIA Rapid Test<br />
AKL<br />
10204111114 17/06/2011 SMS/Acid Wash Roche / Hitachi<br />
TYSON BIORESEARCH INC., TAIWAN<br />
RESZON DIAGNOSTICS INTERNATIONAL<br />
SDN. BHD., MALAYSIA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
PT. RIMATANDU MOTARO<br />
PRATAMA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305111485 09/06/2011 RIDA AllergyScreen Panel I IR R-BIOPHARM AG., GERMANY PT. SEMERU PERKASA PERMAI<br />
AKL<br />
20304111581 14/06/2011 ANEUVYSION DNA Probe Kit ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20101111578 14/06/2011<br />
AKL<br />
20101111576 14/06/2011<br />
SYSMEX Creatine Kinase-MB<br />
Reagent (CK-MB) R1-R2<br />
SYSMEX reatine Kinase Reagent<br />
(CK-R1/CK-R2)<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
AKL<br />
10101110987 26/04/2011 POCKETCHEM BA ARKRAY GLOBAL BUSSINES INC., JAPAN<br />
AKL<br />
10102110986 26/04/2011<br />
AKL<br />
21106111378 30/05/2011<br />
AKL<br />
20305111379 30/05/2011<br />
AKL<br />
10303111303 18/05/2011<br />
Acura Manual 855 Digital Reading<br />
Micropipette<br />
EPPENDORF Micromanipular<br />
Transferman NK2 and Accessories<br />
EPPENDORF RealTime PCR<br />
Mastercycler ep Realplex and<br />
Accessories<br />
LEICA ST4020 - Linear Stainer and<br />
Accessories<br />
SOCOREX ISBA S.A., SWITZERLAND<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MYCROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
20101111035 26/04/2011 BIOLABO AST / TGO (IFCC) BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
10101111037 27/04/2011 BIOLABO ALT / TGP (IFCC) BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
10101111038 27/04/2011 BIOLABO Uric Acid Uricase Method BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20101111482 09/06/2011<br />
KONELAB Specical Protein<br />
Calibrator<br />
AKL<br />
20101111479 09/06/2011 KONELAB Reference Electrode Kit<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101110890 20/04/2011 KEYLAB Eurocontrol P BPC BIOSED S.r.l., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20101110892 20/04/2011 KEYLAB Total Bilirubin BPC BIOSED S.r.l., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101110889 20/04/2011 KEYLAB Eurocontrol N BPC BIOSED S.r.l., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101110891 20/04/2011 KEYLAB LDL Cholesterol BPC BIOSED S.r.l., ITALY PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303111414 06/06/2011<br />
AKL<br />
10101111135 27/04/2011 KONELAB Iron<br />
AKL<br />
10101111136 27/04/2011 KONELAB Magnesium<br />
AKL<br />
10101111137 27/04/2011<br />
NucliSENS EasyQ HSV 1/2<br />
Reagents BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
KONELAB Lipoprotein (A) Control<br />
High<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC., AUSTRALIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303111415 06/06/2011 VIDAS TOXO Competition BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20103110975 26/04/2011<br />
AKL<br />
20102111376 30/05/2011<br />
PULSE SCIENTIFIC Amphetamine<br />
Strip<br />
ERBA XL200 Automatic Chemistry<br />
Analyzer and Accessories<br />
PULSE SCIENTIFIC INC., CANADA<br />
ERBA DIAGNOSTICS MANNHEIM GMBH.,<br />
GERMANY<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. GENTA BUANA ASTADECA<br />
AKL<br />
20205110951 25/04/2011 VerifyNow Aspirin Assay ACCUMETRICS INC., USA PT. HARMONI PRIMA MEDIKA<br />
AKL<br />
20101111025 26/04/2011<br />
AKL<br />
20101111026 26/04/2011<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Chemistry Products<br />
Calibrator Kit 1<br />
VITROS Chemistry Products<br />
Calibrator Kit 2<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
http://www.pdf4free.com<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA
AKL<br />
20101111173 28/04/2011<br />
VITROS Chemistry Products CI<br />
Slides - CT DT Slides<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10101111108 27/04/2011<br />
AKL<br />
20101111110 27/04/2011<br />
AKL<br />
20101111172 28/04/2011<br />
AKL<br />
10101111109 27/04/2011<br />
AKL<br />
10101111024 26/04/2011<br />
VITROS Chemistry Products ALT<br />
Slides / ALT / SGPT DT Slides<br />
VITROS Chemistry Products CREA<br />
Slides / CREA DT Slides<br />
VITROS Chemistry Products<br />
BUN/UREA Slides / BUN/UREA DT<br />
Slides<br />
VITROS Chemistry Products TRIG<br />
Slides / TRIG DT Slides<br />
VITROS Chemistry Products CHOL<br />
Slides / CHOL DT Slides<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTIC INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
30305111308 19/05/2011<br />
AKL<br />
20101110977 26/04/2011<br />
SPEEDYTEST Anti HIV (1+2) Test<br />
(Whole Blood/Serum/Plasma) IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
STANBIO Creatinine Liquicolor<br />
(Endopoint)<br />
STANBIO LABORATORY, USA<br />
AKL<br />
20101110978 26/04/2011 STANBIO Glycohemoglobin Pre - Fil STANBIO LABORATORY, USA<br />
AKL<br />
20305110981 26/04/2011 AXIOM HBsAg ELISA AXIOM GMBH., GERMANY<br />
AKL<br />
20101110973 26/04/2011 STANBIO Total Protein Liquicolor STANBIO LABORATORY, USA<br />
AKL<br />
20305110980 26/04/2011 AXIOM HBsAb ELISA AXIOM GMBH., GERMANY<br />
AKL<br />
20305110979 26/04/2011<br />
AKL<br />
20205110868 20/04/2011<br />
AKL<br />
20306111580 14/06/2011<br />
AKL<br />
20102111027 26/04/2011<br />
AKL<br />
20101111579 14/06/2011<br />
AKL<br />
20101111577 14/06/2011<br />
AKL<br />
20305111195 28/04/2011<br />
AXIOM HCV ELISA - Version 4<br />
(SAV)<br />
AXIOM GMBH., GERMANY<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
FAST BCC - 3000 B and<br />
Accessories DIRUI INDUSTRIAL CO. LTD., CHINA PT. SAPTA LARONA MUDA<br />
PATHVYSION HER-2 DNA Probe<br />
Kit<br />
ZN - 588 Full - auto Chemistry<br />
Analyzer<br />
SYSMEX Lactate Dehidrogenese<br />
Reagent (LDH-R1/LDH-R2)<br />
SYSMEX Total Bile Acid Reagent<br />
(TBA)<br />
SYSMEX C - Reactive Protein<br />
Reagent (CRP)<br />
ABBOTT MOLECULAR INC., USA<br />
RAYTO LIFE and ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
AKL<br />
20101111252 29/04/2011<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SYSMEX Cystatin C Reagent (CysC) SINGAPORE<br />
AKL<br />
20208111183 28/04/2011 BERICHROM Heparin UF calibrator<br />
SIEMENS HEALTHCARE PRODUCTS<br />
GMBH., GERMANY melalui SYSMEX ASIA<br />
PASIFIC PTE. LTD., SINGAPORE<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20101111377 30/05/2011 VACUETTE K2 EDTA GREINER BIO-ONE GMBH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
10101111477 09/06/2011 KONELAB Select Ion High<br />
AKL<br />
10101111474 09/06/2011 KONELAB Select Ion Low<br />
AKL<br />
10101111476 09/06/2011 KONELAB Select Ion Normal<br />
AKL<br />
20101111481 09/06/2011 KONELAB ISE Calibrator 1<br />
AKL<br />
20101111480 09/06/2011 KONELAB Albumin U Calibrator<br />
AKL<br />
10101111475 09/06/2011 KONELAB Albumin U Control<br />
AKL<br />
20205111582 14/06/2011 CELL-DYN Immuno PLT (CD 61)<br />
AKL<br />
20208111575 14/06/2011 CELL-DYN Retic Plus Control<br />
AKL<br />
20208111574 14/06/2011<br />
CELL-DYN 29 Plus Control (with<br />
Retic)<br />
AKL<br />
20306110041 27/04/2011 ARCHITECT Cyfra 21-1 Calibrator<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND<br />
BD BIOSCIENCES, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
STRECK, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
STRECK, USA untuk ABBOTT<br />
LABORATORIES, USA<br />
FUJIREBIO DIAGNOSTIC INC, USA untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101111147 28/04/2011<br />
AKL<br />
20303110789 12/04/2011<br />
AKL<br />
20303110790 12/04/2011<br />
AKL<br />
20102111110 16/06/2011<br />
BLUE CROSS One Step HCG<br />
Pregnancy Test Strip 4mm<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
PT. ABHIMATA MANUNGGAL<br />
INNOGENETICS INNO-LiPA<br />
Genotyping Extra Amp. INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
INNOGENETICS INNO-LiPA<br />
Genotyping Extra INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
SAPPHIRE 300 Automated Clinical<br />
Chemistry Analyser AUDIT DIAGNOSTICS., IRELAND PT. BUMI INDAH PUTRA<br />
AKL<br />
20101111386 30/05/2011 BIOLABO Bilirubin total and Direct BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20207111388 30/05/2011<br />
AKL<br />
10101111039 27/04/2011<br />
AKL<br />
20207111387 30/05/2011<br />
BIOLABO BIO-TP Prpthrombin Time<br />
(PT) BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIOLABO LDL - Cholesterol Direct<br />
Method BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
BIOLABO BIO-CK Activated Partial<br />
Thromboplastin Time (APTT) BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20101111395 01/06/2011 BIOLABO Urea Colorometric Method BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20101111389 30/05/2011 BIOLABO Albumin BCG Method BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
20305111023 26/04/2011<br />
KONELAB APO A1 / APO B<br />
Calibrator<br />
AKL<br />
20101111020 26/04/2011 KONELAB Lipoprotein (a) Calibrator<br />
AKL<br />
20305111021 26/04/2011<br />
KONELAB Rheumatoid Factors<br />
Control<br />
AKL<br />
10101111152 27/04/2011 KONELAB Specitrol Control Serum<br />
AKL<br />
20305111022 26/04/2011 KONELAB Apolipoprotein A1<br />
AKL<br />
10203111018 26/04/2011<br />
THERMO SCIENTIFIC MICROM<br />
CTM 6 Coversliper and Accessories<br />
AKL<br />
10101111130 27/04/2011 KONELAB Lipoprotein (a)<br />
AKL<br />
20207111138 27/04/2011 KONELAB HbA1c Control Normal<br />
AKL<br />
20207111141 27/04/2011<br />
KONELAB HbA1c Pretreatment<br />
Liquid<br />
AKL<br />
10101111140 27/04/2011 KONELAB Phosphorus<br />
AKL<br />
20207111139 27/04/2011 KONELAB HbA1c<br />
AKL<br />
10203111019 26/04/2011<br />
AKL<br />
20205110543 08/03/2011<br />
AKL<br />
20205110243 11/02/2011<br />
AKL<br />
10102110241 11/02/2011<br />
AKL<br />
20205110949 25/04/2011<br />
AKL<br />
10102111233 29/04/2011<br />
AKL<br />
10102111234 29/04/2011<br />
THERMO SCIENTIFIC MICROM<br />
HM 560 Cryostat and Accessories<br />
CompoLab HB system and<br />
Accessories<br />
RAYTO ST - 7600 Auto Hematology<br />
Analyzer<br />
RAYTO ST - 9000 Chemistry<br />
Analyzer<br />
VerifyNow Automated Platelet<br />
Function Assay System and<br />
Accessories<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
MICROM INTERNATIONAL GMBH part of<br />
THERMO FISHER SCIENTIFIC INC.,<br />
GERMANY<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC INC., AUSTRALIA<br />
MICROM INTERNATIONAL GMBH part of<br />
THERMO FISHER SCIENTIFIC INC.,<br />
GERMANY<br />
CELLMED AG., GERMANY untuk<br />
FRESENIUS KABI AG., GERMANY<br />
RAYTO LIFE AND ANALYZER SCIENTIFIC<br />
CO. LTD., CHINA<br />
RAYTO LIFE AND ANALYZER SCIENTIFIC<br />
CO. LTD., CHINA<br />
ACCUMETRICS INC., USA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. FRESENIUS KABI<br />
INDONESIA<br />
PT. GRESIK SARANA TIRTA<br />
PT. GRESIK SARANA TIRTA<br />
PT. HARMONI DINAMIK<br />
INDONESIA<br />
DFI DUS R - 720 Urine Chemistry<br />
Analyzer DFI CO. LTD., KOREA PT. INDOCORE PERKASA<br />
DFI DUS R - 300 Urine Chemistry<br />
Analyzer DFI CO. LTD., KOREA PT. INDOCORE PERKASA<br />
AKL<br />
10101111559 14/06/2011<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Chemistry Products<br />
Isoenzyme Performance Verifier II<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
http://www.pdf4free.com<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA
AKL<br />
10101111558 14/06/2011<br />
AKL<br />
10101111030 26/04/2011<br />
AKL<br />
10101111031 26/04/2011<br />
AKL<br />
10102110963 26/04/2011<br />
AKL<br />
20101111116 17/06/2011<br />
AKL<br />
20101111115 17/06/2011<br />
AKL<br />
10302111010 26/04/2011<br />
AKL<br />
10302111011 26/04/2011<br />
AKL<br />
10302111009 26/04/2011<br />
AKL<br />
10302111008 26/04/2011<br />
VITROS Chemistry Products UPRO<br />
Performance Verifier I<br />
VITROS hemistry Products<br />
Performance Verifier I<br />
VITROS Chemistry Products<br />
Performance Verifier II<br />
MISSION U120 Expert Urine<br />
Analyzer<br />
GUARDIAN Pregnancy Test<br />
Cassette<br />
GUARDIAN Pregnancy Test<br />
Midstream<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO - CLINICAL DIAGNOSTICS INC.,<br />
USA<br />
ORTHO - CLINICAL DIAGNOSTICS INC.,<br />
USA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
W.H.P.M BIORESEARCH AND<br />
TECHNOLOGY CO. LTD., CHINA<br />
W.H.P.M BIORESEARCH AND<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
MERCKOPLATE Agar for Fungsi<br />
acc to KIMMIG modified Merck KGaA., Germany PT. MERCK Tbk<br />
MERCKOPLATE Mueller - Hinton<br />
Agar with Blood Merck KGaA., Germany PT. MERCK Tbk<br />
MERCKOPLATE Standard I Nutrien<br />
Agar Merck KGaA., Germany PT. MERCK Tbk<br />
MERCKOPLATE Selective Agar for<br />
Pathogenic Fungsi Merck KGaA., Germany PT. MERCK Tbk<br />
AKL<br />
10302111007 26/04/2011 MERCKOPLATE BPLS Agar Merck KGaA., Germany PT. MERCK Tbk<br />
AKL<br />
20205110554 09/03/2011<br />
AKL<br />
20205110552 09/03/2011<br />
MINDRAY BC-3000 Plus Auto<br />
Hematology Analyzer and<br />
Accessories<br />
MINDRAY BC-2800 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
AKL<br />
20303110794 12/04/2011 IMMUNOQUICK Contact Malaria +4<br />
AKL<br />
20303110793 12/04/2011 IMMUNOQUICK ELIFA HSV IgM<br />
AKL<br />
20303110792 12/04/2011 IMMUNOQUICK ELIFA HSV IgG<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
BIOSYNEX IMMUNODIAGNOSTICS,<br />
FRANCE<br />
BIOSYNEX IMMUNODIAGNOSTICS,<br />
FRANCE<br />
BIOSYNEX IMMUNODIAGNOSTICS,<br />
FRANCE<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. NELTA MULTI GRACIA<br />
PT. NELTA MULTI GRACIA<br />
PT. NELTA MULTI GRACIA<br />
AKL<br />
20306110796 12/04/2011 UBC Rapid IDL BIOTECH AB, SWEDEN PT. NELTA MULTI GRACIA<br />
AKL<br />
10208111187 28/04/2011<br />
LIAISON 25 OH Vitamin D Total<br />
Assay Specimen Diluent<br />
AKL<br />
20305111235 29/04/2011 MATRITECH NMP22 BladderCheck<br />
AKL<br />
20101111142 27/04/2011 DELFIA NeonatalTSH Kit<br />
AKL<br />
20205111391 30/05/2011<br />
Automatic Hematology Analyzer MS4-<br />
S<br />
AKL<br />
10204110744 07/04/2011 Sample Cleaner 2 cobas c systems<br />
AKL<br />
10204110953 25/04/2011 COBAS Multiclean Roche / Hitachi<br />
akl<br />
10204110959 26/04/2011 ROCHE Ph. Reference Solution<br />
AKL<br />
10204110952 25/04/2011<br />
Cell wash Solution II / Acid Roche /<br />
Hitachi<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.P.A., ITALY<br />
BINAX INC., USA a subsidiary of INVERNESS<br />
MEDICAL INNOVATION., USA melalui<br />
INVERNESS MEDICAL INNOVAT<br />
WALLAC OY., FINLAND untuk<br />
PERKINELMER INC. CO., USA melalui<br />
PERKINELMER SINGAPORE PTE. LTD.,<br />
SINGAPO<br />
MELET SHLOESING LABORATORIES,<br />
FRANCE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. RAJA ERBA INDOCHEM<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101111015 26/04/2011 FAST Liquick - ASAT PZ CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111016 26/04/2011 FAST Liquick - Bil total PZ CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101111014 26/04/2011 FAST Liquick - ALAT PZ CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111013 26/04/2011 FAST Liquick - Albumin PZ CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111017 26/04/2011 FAST Liquick - Bil Direct PZ CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10102110882 20/04/2011 FAST DR 7000D and Accessories DIRUI INDUSTRIAL CO. LTD., CHINA PT. SAPTA LARONA MUDA<br />
AKL<br />
10102110881 20/04/2011<br />
FAST H - 300 B Urine Analyzer And<br />
Accessories DIRUI INDUSTRIAL CO. LTD., CHINA PT. SAPTA LARONA MUDA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20102110883 20/04/2011<br />
AKL<br />
20205111483 09/06/2011<br />
FAST CS - T240 Automatic<br />
Chemistry Analyzer and Accessories DIRUI INDUSTRIAL CO. LTD., CHINA PT. SAPTA LARONA MUDA<br />
ZENIX ZN-144 Full-auto Hematology<br />
Analyzer<br />
RAYTO LIFE AND ANALYZER SCIENTIFIC<br />
CO. LTD., CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
AKL<br />
20208111253 28/04/2011<br />
BERICHROM Heparin LMW<br />
Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SINGAP<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10101111184 28/04/2011<br />
BERICHROM Heparin LMW Control<br />
1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SINGAP<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10101111185 28/04/2011 BERICHROM Heparin UF Control 1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SINGAP<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10101111186 28/04/2011 BERICHROM Heparin UF Control 2<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SINGAP<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20208111197 28/04/2011<br />
AKL<br />
20305111194 28/04/2011<br />
AKL<br />
20305111192 28/04/2011<br />
AKL<br />
20101111193 28/04/2011<br />
BERICHROM Heparin LMW Control<br />
2<br />
SYSMEX Apolipoprotein A1 Reagent<br />
(APO A1)<br />
SYSMEX Apolipoprotein B reagent<br />
(ApoB)<br />
SYSMEX Inorganic Phosphate<br />
Reagent (IP)<br />
AKL<br />
20101111249 28/04/2011 SYSMEX Total Protein reagent (TP)<br />
AKL<br />
20101111250 29/04/2011 SYSMEX Calcium Reagent (Ca)<br />
AKL<br />
10101111215 29/04/2011<br />
SYSMEX Cholesterol HDL Reagent<br />
(HDL-C)<br />
AKL<br />
10101111489 18/07/2011 ADVIA Centaur SHBG<br />
AKL<br />
20305111236 29/04/2011 MATRITECH NMP22 BladderCheck<br />
AKL<br />
10102111643 29/07/2011<br />
SORVALL Evolution RC Superspeed<br />
Centrifuge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SINGAP<br />
SYSMEX WUXI, CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI, CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI, CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI, CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI, CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX WUXI, CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
BINAX INC., USA a subsidiary of INVERNESS<br />
MEDICAL INNOVATION, USA melalui<br />
INVERNESS MEDICAL INNOVATI<br />
THERMO ELECTRON LED GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20305111636 06/03/2011 ABX Pentra SP Control Low HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20303701648 19/07/2011 Foresight HCV Antibody EIA Test Kit<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20101111385 30/05/2011 BIOLABO Urea U.V HL BIOLABO SA., FRANCE PT. C.V. KHARISMA UTAMA<br />
AKL<br />
30305111451 11/07/2011 FOKUS Anti HIV 1/2 Check Device PULSE SCIENTIFIC INC., CANADA<br />
AKL<br />
20102111397 07/07/2011<br />
VITROS 350 Chemistry System and<br />
accessories<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20209111464 12/07/2011 DIAGAST Screen-Lys DIAGAST, FRANCE PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
30305111492 19/07/2011 SD BIOLINE HIV 1/2 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
30305111634 28/07/2011 MUREX HIV Ag/Ab Combination<br />
DIASORIN S.p.A., UK melalui DIASORIN<br />
S.p.a., ITALY<br />
AKL<br />
20306111583 22/07/2011 LIAISON CA 125 II DIASORIN S.p.A., ITALY<br />
AKL<br />
20306111584 22/07/2011 LIAISON CA 15-3 DIASORIN S.p.A., ITALY<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB
AKL<br />
20305111581 22/07/2011 LIAISON HBeAg DIASORIN S.p.A., ITALY<br />
AKL<br />
20305111582 22/07/2011 LIAISON Anti-HBc DIASORIN S.p.A., ITALY<br />
AKL<br />
20305111585 22/07/2011 LIAISON Anti-Hbe DIASORIN S.p.A., ITALY<br />
AKL<br />
10204111648 29/07/2011 ROCHE Cleaning Solution<br />
AKL<br />
10204111647 29/07/2011 ROCHE BUFFER Type 2<br />
AKL<br />
10204111646 29/07/2011 ROCHE BUFFER Type 1<br />
AKL<br />
10204111649 29/07/2011 ROCHE Deproteinizer<br />
AKL<br />
20305111389 07/07/2011<br />
HBsAg II Quant Elecsys and Cobas<br />
e analyzer<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10101111651 29/07/2011<br />
AKL<br />
10206111557 14/06/2011<br />
SYSMEX Lipoprotein (a) Reagent<br />
[LP (a)]<br />
VACUETTE Automated ESR<br />
System Sed Rate Screener 100/II<br />
(SRS100/II)<br />
SYSMEX WUXI CO. LTD., CHINA melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SYSMEX<br />
VITAL DIAGNOSTICS S.r.l., ITALY untuk<br />
GREINER BIO-ONE GMBH., AUSTRIA<br />
PT. SYSMEX INDONESIA<br />
PT. WIDYA MITRA PERSADA<br />
AKL<br />
20101111586 22/07/2011 Vacueete Visio Plus Needles GREINER BIO-ONE, AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
10102111568 18/07/2011<br />
AKL<br />
10102111486 18/07/2011<br />
AKL<br />
20102111450 11/07/2011<br />
AKL<br />
20205111449 11/07/2011<br />
HOSPITEX Biochemistry Analyzer<br />
Master T HOSPITEX LABORATORIES S.r.l., ITALY PT. BAVARIA COMBININDO<br />
HOSPITEX Flame Photometer<br />
Screen Lyte and Accessories HOSPITEX LABORATORIES S.r.l., ITALY PT. BAVARIA COMBININDO<br />
HOSPITEX Automatic Fully Random<br />
Clinical Chemistry Analyzer MEGA<br />
200 and Accessories HOSPITEX LABORATORIES S.r.l., ITALY PT. BAVARIA COMBININDO<br />
HOSPITEX Hematology Analyzer<br />
Hemascreen 18 HOSPITEX LABORATORIES S.r.l., ITALY PT. BAVARIA COMBININDO<br />
AKL<br />
20101111620 25/07/2011 INTHERMA VACUUM EDTA K3<br />
AKL<br />
20101111621 25/07/2011<br />
INTHERMA VACUUM No Additive<br />
(PLAIN)<br />
AKL<br />
20101111619 25/07/2011 Mini Tube INTHERMA No Additive<br />
AKL<br />
20101111617 25/07/2011 INTHERMA Blood Collection Needle<br />
AKL<br />
20101111618 25/07/2011<br />
AKL<br />
20102111398 07/07/2011<br />
AKL<br />
20102111400 07/07/2011<br />
AKL<br />
20102111399 07/07/2011<br />
INTHERMA VACUUM Gel & Clot<br />
Activator<br />
KONELAB Prime 30 ISE Analyzer<br />
and Accessories<br />
KONELAB Prime 60 ISE Analyzer<br />
and Accessories<br />
KONELAB 20 XT Analyzer and<br />
Accessories<br />
AKL<br />
20101111644 29/07/2011 ONE TOUCH Select Test Strips<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melaLUI THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melaLUI THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND melaLUI THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
LIFESCAN PRODUCTS LLC., USA dan<br />
LIFESCAN SCOTLAND LTD, UK untuk<br />
LIFESCAN EUROPE, SWITZERLAND<br />
AKL<br />
20101111396 07/07/2011 VITROS Chemistry Calibrator Kit 8 ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
AKL<br />
20101111387 07/07/2011<br />
AKL<br />
20101111548 20/07/2011<br />
AKL<br />
20101111549 20/07/2011<br />
AKL<br />
20101111547 20/07/2011<br />
AKL<br />
20101111546 20/07/2011<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Chemistry Products 950/FS<br />
Reference Fluid<br />
VITROS Chemistry Products<br />
Calibrator Kit 9<br />
VITROS Chemistry Products AMYL<br />
Slides / AMYL DT Slides<br />
VITROS Chemistry Products TBIL /<br />
TBIL DT Slides<br />
VITROS Chemistry Products<br />
Calibrator Kit 10<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
http://www.pdf4free.com<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA
AKL<br />
20103111551 20/07/2011<br />
AKL<br />
20101111545 20/07/2011<br />
AKL<br />
20101111529 20/07/2011<br />
AKL<br />
20101111530 20/07/2011<br />
AKL<br />
20103111553 20/07/2011<br />
AKL<br />
20103111552 20/07/2011<br />
AKL<br />
20303111543 13/06/2011<br />
VITROS Chemistry Products SALI<br />
Slides<br />
VITROS Chemistry Products<br />
Calibrator Kit 25<br />
VITROS Chemistry Products tp<br />
Slides / TP DT Slides<br />
VITROS Chemistry Products GLU<br />
Slides / GLU DT Slides<br />
VITROS Chemistry Products DGXN<br />
Slides<br />
VITROS Chemistry Products ACET<br />
Slides<br />
SENSATEST One-Step Dengue<br />
Test IgG/IgM<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ATLASLINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA melalui NEOPHARM BIOTECH<br />
ASIA SDN. BHD., MALYSIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20303111379 06/07/2011 SD BIOLINE Syphilis 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10302111504 18/07/2011<br />
MERCKOPLATE Boiled Blood Agar<br />
(Chocolate Agar) MERCK KgaA, GERMANY PT. MERCK Tbk<br />
AKL<br />
10201111503 18/07/2011 MERCKOFIX Spray Fixative MERCK KgaA, GERMANY PT. MERCK Tbk<br />
AKL<br />
10201111505 18/07/2011<br />
AKL<br />
10302111506 18/07/2011<br />
DNA Staining Kit According to<br />
Feulgen MERCK KgaA, GERMANY PT. MERCK Tbk<br />
MERCKOPLATE MacCONKEY<br />
Agar MERCK KgaA, GERMANY PT. MERCK Tbk<br />
AKL<br />
10302111507 18/07/2011 MERCKOPLATE Rambach Agar MERCK KgaA, GERMANY PT. MERCK Tbk<br />
AKL<br />
20101111554 20/07/2011 PATHFAST CK-MB<br />
AKL<br />
20207111555 20/07/2011 PATHFAST D-Dimer<br />
AKL<br />
20101111556 20/07/2011 PATHFAST c-Tnl<br />
AKL<br />
20305111540 20/07/2011 MUREX Anti-HBc (Total)<br />
AKL<br />
20305111541 20/07/2011 MUREX Anti-HCV (Version 4.0)<br />
AKL<br />
20305111542 20/07/2011 MUREX HCV Ag/Ab Combination<br />
AKL<br />
20303111426 07/07/2011 ICE Syphilis<br />
AKL<br />
20305111425 07/07/2011 MUREX HBsAg Version 3<br />
AKL<br />
10102111556 14/06/2011<br />
AKL<br />
20101111485 18/07/2011<br />
PATHFAST Compact<br />
Immunoassays Analyzer and<br />
Accessories<br />
LIAISON 25 OH Vitamin D Total<br />
Assay<br />
MITSUBISHI CHEMICAL MEDIENCE CORP,<br />
JAPAN untuk MITSUBISHI CHEMICAL<br />
EUROPE GMBH., GERMANY<br />
MITSUBISHI CHEMICAL MEDIENCE CORP,<br />
JAPAN untuk MITSUBISHI CHEMICAL<br />
EUROPE GMBH., GERMANY<br />
MITSUBISHI CHEMICAL MEDIENCE CORP,<br />
JAPAN untuk MITSUBISHI CHEMICAL<br />
EUROPE GMBH., GERMANY<br />
DIASORIN SpA, UK Branch, UK untuk<br />
DIASORIN SpA., ITALY<br />
DIASORIN SpA, UK Branch, UK untuk<br />
DIASORIN SpA., ITALY<br />
DIASORIN SpA, UK Branch, UK untuk<br />
DIASORIN SpA., ITALY<br />
DIASORIN SpA, UK Branch, UK untuk<br />
DIASORIN SpA., ITALY<br />
DIASORIN S.p.A. UK Branch, UK melalui<br />
DIASORIN S.p.A., ITALY<br />
MITSUBISHI CHEMICAL MEDIENCE CORP,<br />
JAPAN untuk MITSUBISHI CHEMICAL<br />
EUROPE GMBH., GERMANY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
AKL<br />
10102111539 20/07/2011 LIAISON Module DIASORIN S.p.A., ITALY<br />
AKL<br />
10204111537 20/07/2011 LIAISON Starter Kit DIASORIN S.p.A., ITALY<br />
AKL<br />
10101111536 20/07/2011 LIAISON Light Check DIASORIN S.p.A., ITALY<br />
AKL<br />
10101111481 18/07/2011<br />
AKL<br />
10208111488 18/07/2011<br />
AKL<br />
10208111487 18/07/2011<br />
LIAISON Control 25 OH Vitamin D<br />
total Assay<br />
LIAISON Specimen Diluent<br />
Thymidine Kinase<br />
LIAISON Specimen Diluent BAP<br />
OSTASE<br />
AKL<br />
20101111484 18/07/2011 LIAISON Osteocalcin<br />
AKL<br />
10101111480 18/07/2011 LIAISON Control BAP OSTASE<br />
AKL<br />
10101111482 18/07/2011 LIAISON Control Osteocalcin<br />
AKL<br />
10208111538 20/07/2011<br />
LIAISON Specimen Diluent<br />
Osteocalcin<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10102111483 18/07/2011 1420 VICTOR D Multilabel Counter<br />
AKL<br />
20101111528 20/07/2011 LIAISON BAP Ostase<br />
AKL<br />
20306111527 20/07/2011 LIAISON Control Thymidine Kinase<br />
AKL<br />
20306111526 20/07/2011 LIAISON Thymidine Kinase<br />
AKL<br />
20103111543 20/07/2011 Amphetamines II Roche/Hitachi<br />
AKL<br />
20101111550 20/07/2011 ROCHE Rinse<br />
AKL<br />
20101111544 20/07/2011<br />
AKL<br />
20102111565 21/07/2011<br />
AKL<br />
20102111566 21/07/2011<br />
ISE Calibrator Indirect/Urine cobas c<br />
111<br />
Modular P 800 Module )EVO) and<br />
Accessories<br />
ISE 900 (EVO) for MODULAR<br />
ANALYTICS and Accessories<br />
PERKINELMER LIFE AND ANALYTICAL<br />
SCIENCES, FINLAND untuk PERKINELMER,<br />
USA melalui PERKINELMER SINGAPO<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., ITALY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20301111502 18/07/2011 ParaHIT f Dipstick SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20303111501 18/07/2011 ParaHIT f Device SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20303111500 18/07/2011 ParaHIT Total Dipstick SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20303111499 18/07/2011 ParaHIT Total Device SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20305111491 18/07/2011<br />
Immulite System CRP C-Reactive<br />
Protein<br />
AKL<br />
20305111492 18/07/2011 ADVIA Chemistry CRP-2 Calibrator<br />
AKL<br />
20305111493 18/07/2011<br />
ADVIA Chemistry Wide Range C-<br />
Reactive Protein (wr CRP)<br />
AKL<br />
20305111490 18/07/2011 ADVIA Centaur System HBcT QC<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS HEALTHCARE<br />
DIAGNOSTICS LTD., HONGKONG<br />
AKL<br />
20306111645 29/07/2011 UROVYSION Bladder Cancer Kit ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20305112282 05/09/2011<br />
AKL<br />
20305112283 05/09/2011<br />
AKL<br />
20305112284 05/09/2011<br />
AKL<br />
20305112286 05/09/2011<br />
AKL<br />
20305112285 05/09/2011<br />
AKL<br />
20305112287 05/09/2011<br />
AKL<br />
10101111985 09/08/2011<br />
AKL<br />
10101111986 09/08/2011<br />
AKL<br />
10101111984 09/08/2011<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
ExiPrep 16 Plus Fully Automated<br />
Nucleic Acid Extraction System BIONEER CORPORATION, KOREA PT GREEN MEDICA<br />
Exicycler 96 Real-Time Quantitative<br />
Thermal Block BIONEER CORPORATION, KOREA PT GREEN MEDICA<br />
ExiPrep 16 Pro Automated Nucleic<br />
Acid Extraction System BIONEER CORPORATION, KOREA PT GREEN MEDICA<br />
ACCUPOWER HCV Quantitative RT-<br />
PCR Kit BIONEER CORPORATION, KOREA PT GREEN MEDICA<br />
ACCUPOWER HBV Quantitative<br />
PCR Kit BIONEER CORPORATION, KOREA PT GREEN MEDICA<br />
ACCUPOWER MTB Real Time<br />
PCR Kit BIONEER CORPORATION, KOREA PT GREEN MEDICA<br />
IMMULITE 2000 Systems Growth SIEMENS HEALTHCARE DIAGNOSTIC<br />
Hormone GRH ( Recombinant 98/574 PRODUCTS LTD.,UK melalui SIEMENS<br />
)<br />
HEALTCARE DIAGNOSTICS.,Hongkong<br />
IMMULITE FBC Free Beta HCG<br />
Control<br />
IMMULITE UE3 Unconjugated<br />
Estriol<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD.,UK melalui SIEMENS<br />
HEALTCARE DIAGNOSTICS.,Hongkong<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD.,UK melalui SIEMENS<br />
HEALTCARE DIAGNOSTICS.,Hongkong<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20303112210 26/08/2011 HUMAN Hexagon Malaria Combi HUMAN GmbH., Germany PT SALI POLAPA BERSAMA<br />
AKL<br />
10203111858 08/08/2011 KOS Rapid Tissue Processor MILESTONES S.R.I., ITALY PT. INDO CLARO SEJAHTERA<br />
AKL<br />
20209701782 07/07/2011 DIAGAST ABD-Lys DIAGAST, FRANCE PT. MEDQUEST JAYA GLOBAL<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20209112096 15/08/2011 HUMATYPE Anti-D (IgM/IgG) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
21106111929 09/08/2011<br />
ORIGIO Syn Vitro Flush with<br />
Heparin<br />
AKL<br />
21106111928 09/08/2011 ORIGIO UNIVERSAL IVF Medium<br />
AKL<br />
21106111927 09/08/2011 ORIGIO UTM Transfer Medium<br />
ORIGIO A/S. DENMARK melalui MEDIQUEST<br />
PTE. LTD., SINGAPORE<br />
ORIGIO A/S. DENMARK melalui MEDIQUEST<br />
PTE. LTD., SINGAPORE<br />
ORIGIO A/S. DENMARK melalui MEDIQUEST<br />
PTE. LTD., SINGAPORE<br />
PT. FORTUNA HEALTHCARE<br />
PT. FORTUNA HEALTHCARE<br />
PT. FORTUNA HEALTHCARE<br />
AKL<br />
20305112120 16/08/2011<br />
BIO-RAD Autoimmune EIA ANA<br />
Screening Test<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES, SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20303112211 26/08/2011 HUMAN Hexagon Dengue HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101111808 08/08/2011<br />
AKL<br />
10204111807 08/08/2011<br />
AKL<br />
20205111863 08/08/2011<br />
VITROS Chemistry Products Liquid<br />
Performance Verifier I<br />
VITROS Chemistry Products Urine<br />
Electrolyte Diluent<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
DIRUI Hematology Analyzer BCC -<br />
3000B and Accessories DIRUI INDUSTRIAL CO. LTD., P.R., CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10102111862 08/08/2011<br />
AKL<br />
20101111917 09/08/2011<br />
AKL<br />
20101111916 09/08/2011<br />
DIRUI DR-7000D Semi-Automatic<br />
Chemistry Analyzer and Accessories DIRUI INDUSTRIAL CO. LTD., P.R., CHINA PT. SETIA ANUGERAH MEDIKA<br />
COBAS ISE Diluent Gen.2 cobas c<br />
System<br />
ROCHE CARDIAC Myoglobin Cobas<br />
h232<br />
AKL<br />
20101112001 09/08/2011 DIALINE Glucose GOD PAP<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY untuk DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DIATRON PROMEDIKA<br />
AKL<br />
10304111860 08/08/2011 INFINITE 200 PRO and Accessories TECAN AUSTRIA GMBH., AUSTRIA PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
10101111967 09/08/2011<br />
VITROS Chemistry Products GGT<br />
Slides / GGT DT Slides<br />
AKL<br />
20101112004 09/08/2011 DIALINE BioCAL Lp(a)<br />
AKL<br />
10101112003 09/08/2011 DIALINE Bionorm Lp(a)<br />
AKL<br />
20101111995 09/08/2011<br />
AKL<br />
20101111997 09/08/2011<br />
AKL<br />
20101111994 09/08/2011<br />
VITROS Chemistry Products Ca<br />
Slides / Ca DT Slides<br />
VITROS Chemistry Products Fe<br />
Slides / Fe DT Slides<br />
VITROS Chemistry Products AST<br />
Slides / AST DT Slides<br />
AKL<br />
20101112002 09/08/2011 DIALINE - Amylase<br />
AKL<br />
10101111923 09/08/2011 LIAISON Estradiol<br />
AKL<br />
10101111924 09/08/2011 LIAISON Progesterone<br />
AKL<br />
10101111925 09/08/2011 LIAISON Progesterone Control Set<br />
AKL<br />
10101111926 09/08/2011 LIAISON Estradiol Control Set<br />
AKL<br />
20305111650 29/07/2011<br />
SPEEDYTEST HBsAg Test<br />
Cassette<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY untuk DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. DIATRON PROMEDIKA<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY untuk DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
PT. DIATRON PROMEDIKA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY untuk DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASORIN INC, USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC, USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC, USA melalui DIASORIN<br />
S.p.A., ITALY<br />
DIASORIN INC, USA melalui DIASORIN<br />
S.p.A., ITALY<br />
AIDE DIAGNOSTIC CO. LTD., CHINA untuk<br />
IND DIAGNOSTIC INC., CANADA<br />
PT. DIATRON PROMEDIKA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. LANIROS DIAN PHARMA<br />
AKL<br />
20101111534 20/07/2011 FAST Liquick Cor - Glucose PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111533 20/07/2011 FAST Liquick Cor - Creatinine PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111531 20/07/2011<br />
FAST Liquick Cor - CK (Creatine<br />
Kinase) PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111532 20/07/2011 FAST Liquick Cor - CK-MB PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20208111739 03/08/2011 FAST Control Serum Human HN/Hp PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101111740 03/08/2011<br />
FAST MULTICALIBRATOR Level 1<br />
dan 2 PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101111535 20/07/2011 GUARDIAN Ovulation Test Strip<br />
W.H.P.B BIORESEARCH AND<br />
TECHNOLOGIES CO. LTD., CHINA<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
AKL<br />
10101111689 02/08/2011 FAST Cormay HDL - Direct PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101111690 02/08/2011 FAST Cormay LDL - Direct PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101111691 02/08/2011 FAST Liquick Cor-UREA PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101111692 02/08/2011 FAST Liquick Cor-Triglyceride PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101111693 02/08/2011 FAST Liquick Cor-CHOLESTEROL PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10204111920 09/08/2011<br />
ROCHE Sodium Electrode<br />
Conditioner<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101111667 02/08/2011 GilucoDr Auto test Strip ALLMEDICUS CO.,LTD Korea PT. INDOCORE PERKASA<br />
AKL<br />
20101111668 02/08/2011 GlucoDr Auto System ALLMEDICUS CO.,LTD Korea PT. INDOCORE PERKASA<br />
AKL<br />
10204111922 09/08/2011<br />
COBAS ISE Reference Solution<br />
Cobas c 111<br />
AKL<br />
10204111921 09/08/2011 COBAS SMS Roche/Hitachi<br />
AKL<br />
10204111919 09/08/2011<br />
COBAS Hitergent 33 Roche/Hitachi<br />
911<br />
AKL<br />
10101112020 10/08/2011 Cobas b 123 AutoQC Pack Tri-Level<br />
AKL<br />
20101112022 10/08/2011 ROCHE Calibration Gas 1<br />
AKL<br />
20101112021 10/08/2011 ROCHE Calibration Gas 2<br />
AKL<br />
20101111817 08/08/2011<br />
ACCU - CHECK Performa Blood<br />
Glucose Metter<br />
AKL<br />
20101111818 08/08/2011 ACCU-CHECK Performa Nano<br />
AKL<br />
10102702622 01/08/2011<br />
LABOTRON Microplate Reader<br />
Model LB-6200<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
AKL<br />
20102112100 15/08/2011 ETI-Max 3000 DIASORIN S.p.A. Italy<br />
AKL<br />
10102701462 16/08/2011<br />
AKL<br />
20102112158 16/08/2011<br />
AKL<br />
20305112024 10/08/2011<br />
SINNOWA Automatic Chemistry<br />
Analyzer BS-3000M<br />
SINNOW D280 Automatic<br />
Biochemistry Analyzer<br />
VITROS Chemistry Produts CRP<br />
Slides<br />
AKL<br />
20305111988 09/08/2011 LIASON Control CMV IgG DIASORIN S.p.A. Italy<br />
AKL<br />
20305111989 09/08/2011 LIAISON Toxo IgG Avidity II DIASORIN S.p.A. Italy<br />
AKL<br />
10101111983 09/08/2011 OVUTEST Strip Uji Kesuburan<br />
AKL<br />
10102112160 16/08/2011 INSIGHT U120 Urine Analyzer<br />
AKL<br />
10102112159 16/08/2011 INSIGHT U500 Urine Analyzer<br />
AKL<br />
20101112162 16/08/2011<br />
AKL<br />
20101112161 16/08/2011<br />
AKL<br />
20101112163 16/09/2011<br />
AKL<br />
INSIGHT Urinalysis Reagent Strip 1<br />
G ( GLU )<br />
INSIGHT Urinalysis Reagent Strip<br />
3P (PRO/pH/GLU )<br />
INSIGHT Urinalysis Reagent Strips<br />
10U (<br />
LEU/NIT/URO/PRO/pH/BLO/SG/KE<br />
T/BIL/GLU )<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
SINNOWA MEDICAL SCIENCE &<br />
PT. MITRA BAHAGIA CITRA<br />
TECHNOLOGY CO.,LTD., China<br />
MEDIKA<br />
SINNOWA MEDICAL SCIENCE &<br />
PT. MITRA BAHAGIA CITRA<br />
TECHNOLOGY CO.,LTD., China<br />
MEDIKA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
SHENZHEN GLD BIOTECHNOLOGY LTD.,<br />
China<br />
ACON BIOTECH ( HANGZHOU )<br />
CO.,LTD.,china<br />
ACON BIOTECH ( HENGZHOU ) CO., LTD.,<br />
China<br />
ACON BIOTECH ( HENGZHOU ) CO., LTD.,<br />
China<br />
ACON BIOTECH ( HENGZHOU ) CO., LTD.,<br />
China<br />
ACON BIOTECH ( HENGZHOU ) CO., LTD.,<br />
China<br />
20305112012 10/08/2011 LIAISON Control Toxo IgM DIASORIN S.p.A. Italy<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com<br />
PT. DANPAC PHARMA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB
AKL<br />
20305112009 10/08/2011 LIAISON HBsAg DIASORIN S.p.A. Italy<br />
AKL<br />
20305112010 10/08/2011 LIAISON Control HBsAg DIASORIN S.p.A. Italy<br />
AKL<br />
20305112011 10/08/2011 LIAISON Control Toxo IgG II DIASORIN S.p.A. Italy<br />
AKL<br />
20305112008 10/08/2011 LIAISON HBsAg Confirmatory Test DIASORIN S.p.A. Italy<br />
AKL<br />
20305112013 10/08/2011 LIAISON HBc IgM DIASORIN S.p.A. Italy<br />
AKL<br />
20305112017 10/08/2011 LIAISON Control Anti-HBs DIASORIN S.p.A. Italy<br />
AKL<br />
20306112014 10/08/2011 LIAISON Control PSA<br />
AKL<br />
201011111815 08/08/2011<br />
VITROS Chemistry Produts<br />
Calibrator Kit 7<br />
DIASORIN GmbH ., Germany melalui<br />
DIASORIN S.p.A., Italy<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA<br />
AKL<br />
20305112015 10/08/2011 LIAISON Control Anti-HBe DIASORIN S.p.A. Italy PT. PACIFIQ<br />
AKL<br />
20101111816 08/08/2011<br />
AKL<br />
20101111811 08/08/2011<br />
VITROS Chemistry Produts<br />
Calibrator Kit 6<br />
VITROS Chemistry products LDH<br />
Slides / LDH DT Slides<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10204112475 30/09/2011 ABX Pentara HDL Direct 100 CP HARIBA ABX,France PT. DOS NI ROHA<br />
AKL<br />
30305112534 06/10/2011 New Virolisa HIV 1+2 Ab/Ag<br />
DIALAB GMBH., AUSTRIA dikemas ulang oleh<br />
PT. INDEC DIAGNOSTICS, JAKARTA<br />
AKL<br />
20801112436 27/09/2011 Pentax Video Gastroscope HOYA CORPORATION, Japan<br />
AKL<br />
10304112478 30/09/2011<br />
AKL<br />
10304112479 30/09/2011<br />
PT. INDEC DIAGNOSTICS<br />
PT. DHARMA BHAKTI MEDIKA<br />
SEJATI<br />
AIA-900 Automated Enzyme<br />
Immunoassay Analyzer and<br />
Accessories TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AIA-360 Automated Enzyme<br />
Immunoassay System and<br />
Accessories TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10204112416 26/09/2011 BioMajesty Reagent Probe Wash 1<br />
AKL<br />
21106111639 29/07/2011 ORIGIO Acidified Tyrodes Solution<br />
AKL<br />
21106111638 29/07/2011 ORIGIO SYNVITRO Hyadase<br />
AKL<br />
21106111640 29/07/2011<br />
AKL<br />
20102111688 08/08/2011<br />
AKL<br />
20102112108 15/08/2011<br />
AKL<br />
10101112113 15/08/2011<br />
AKL<br />
20101112101 15/08/2011<br />
AKL<br />
20101112101 15/08/2011<br />
ORIGIO ISM1 Culture Medium<br />
without phenol Red<br />
JOEL LTD, Japan Untuk SYSMEX<br />
CORPORATION, Japan Melalui SYSMEX ASIA<br />
PASIFIC PTE. LTD., Singapore<br />
ORIGIO A/S, DENMARK melalui MEDQUEST<br />
PTE., LTD., SINGAPORE<br />
ORIGIO A/S, DENMARK melalui MEDQUEST<br />
PTE., LTD., SINGAPORE<br />
ORIGIO A/S, DENMARK melalui MEDQUEST<br />
PTE., LTD., SINGAPORE<br />
PT. SYSMEX INDONESIA, Jakarta<br />
PT. FORTUNA HEALTHCARE<br />
PT. FORTUNA HEALTHCARE<br />
PT. FORTUNA HEALTHCARE<br />
FAST CS-800 Automatic Chemistry<br />
and Accessories DIRUI INDUSTRIAL CO.,LTD.,P.R.CHINA PT.SAPTA LARONA MUDA<br />
MINDRAY BS-800 Chemistry<br />
Analyzer and Accessories<br />
STANBIO Ser-T-FY Level 1 Control<br />
Serum<br />
Clever Chek Blood Glucose<br />
Monitoring System<br />
Clever Chek Blood Glucose<br />
Monitoring System<br />
AKL<br />
10204112418 26/09/2011 BioMajesty Cuvette Wash Solution 7<br />
AKL<br />
10204112419 26/09/2011 BioMajesty Cuvette Conditioner EX<br />
AKL<br />
10101112423 26/09/2011 MEDITAPE CHECK 1<br />
AKL<br />
10101112422 26/09/2011 MEDITAPE CHECK 2<br />
AKL<br />
10102112424 26/09/2011<br />
AKL<br />
20103112025 10/08/2011<br />
AKL<br />
20103112026 10/08/2011<br />
SYSMEX UX-2000 Fully Automated<br />
Integrated Urine Analyzer and<br />
Accessories<br />
VITROS Chemistry Products ALC<br />
Slides<br />
VITROS Chemistry Products AcP<br />
Slides<br />
SHENZEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO., LTD.,China<br />
STANBIO LABORATORY,USA<br />
TAIDOC TECHNOLOGY CORPORATION,<br />
Taiwan<br />
TAIDOC TECHNOLOGY CORPORATION,<br />
Taiwan<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
ARKRAY FACTORY INC, JAPAN untuk<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD., SIN<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT.MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303112030 10/08/2011 FAST Salmonella Paratyphi BH OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20303112031 10/08/2011 FAST Salmonella Paratyphi CO OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20305112029 10/08/2011<br />
VITROS Chemistry Products CRP<br />
Performance Verifier II<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101112018 10/08/2011 SENSITIF Compact DINONA INC., KOREA PT. DANPAC PHARMA<br />
AKL<br />
20101111979 09/08/2011<br />
AKL<br />
10102111999 09/08/2011<br />
DIRECTTEST lat Deteksi Kehamilan<br />
Pribadi<br />
SHANGHAI CHEMTRON BIOTECH CO.<br />
LTD., CHINA<br />
PT. PUSPA PHARMA<br />
FAST Urine Analyzer H-800 and<br />
Accessories DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SAPTA LARONA MUDA<br />
AKL<br />
20303112032 10/08/2011 FAST Salmonella Paratyphi CH OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20102111859 08/08/2011<br />
RADIOMETER AQT 90 Flex<br />
Analyzer and Accessories<br />
AKL<br />
21106111964 09/08/2011 ORIGIO Sperm Preparation Medium<br />
AKL<br />
21106111966 09/08/2011 ORIGIO SupraSperm<br />
AKL<br />
21106111965 09/08/2011<br />
AKL<br />
20101111996 09/08/2011<br />
MEDICULT IVM System with Phenol<br />
Red<br />
VITROS Chemistry Products BuBc<br />
Slides<br />
RADIOMETER MEDICAL APS., DENMARK<br />
ORIGIO A/S DENMARK melalui MEDIQUEST<br />
PTE. LTD., SINGAPORE<br />
ORIGIO A/S DENMARK melalui MEDIQUEST<br />
PTE. LTD., SINGAPORE<br />
ORIGIO A/S DENMARK melalui MEDIQUEST<br />
PTE. LTD., SINGAPORE<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. FORTUNA HEALTHCARE<br />
PT. FORTUNA HEALTHCARE<br />
PT. FORTUNA HEALTHCARE<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20306112125 16/08/2011 INNO-LiPA HLA-B Update Plus INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
20306112560 11/10/2011<br />
AKL<br />
20306112559 11/10/2011<br />
AKL<br />
20101112585 11/10/2011<br />
AKL<br />
20305112563 11/10/2011 Cobas Z 480<br />
AKL<br />
10204112581 11/10/2011<br />
HE4 CalSet Elecsys and cobas e<br />
Analyzers<br />
PreciControl HE4 Elecsys and cobas<br />
e Analyzers<br />
ROCHE Vitamin D Total CalSet<br />
Elecsys and cobas e Analyzers<br />
BIOMAJESTY ISE Detergent<br />
Solution<br />
AKL<br />
10204112582 11/10/2011 BIOMAJESTY ISE Buffer (IS)<br />
AKL<br />
20303112336 26/09/2011<br />
IMMULITE 2000 Systems TXP<br />
Toxoplasma Quantitative IgG<br />
AKL<br />
20305112337 26/09/2011 ADVIA Centaur Systems aHBs2 QC<br />
AKL<br />
10101112584 11/10/2011 SYSMEX BAC2 UX II Pack - BAC<br />
AKL<br />
10101112583 11/10/2011 SYSMEX SED2 UX II Pack - SED<br />
AKL<br />
20101112119 16/08/2011<br />
AKL<br />
20101112117 16/08/2011<br />
AKL<br />
20101112118 16/08/2011<br />
AKL<br />
20102112557 11/10/2011<br />
AKL<br />
20102112558 11/10/2011<br />
AKL<br />
20102112556 11/10/2011<br />
OMRON Blood Glucose Monitoring<br />
Kit HEA - 220<br />
OMRON Blood Glucose Monitoring<br />
Kit HEA - 221<br />
OMRON Blood Glucose Test Strip<br />
HEA-STP 20<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD.,UK melalui SIEMENS<br />
HEALTCARE DIAGNOSTICS.,Hongkong<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD.,UK melalui SIEMENS<br />
HEALTCARE DIAGNOSTICS.,Hongkong<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
ARKRAY FACTORY INC, JAPAN melalui<br />
OMRON HEALTHCARE SINGAPORE PTE.<br />
LTD., SINGAPORE<br />
ARKRAY FACTORY INC, JAPAN melalui<br />
OMRON HEALTHCARE SINGAPORE PTE.<br />
LTD., SINGAPORE<br />
ARKRAY FACTORY INC, JAPAN melalui<br />
OMRON HEALTHCARE SINGAPORE PTE.<br />
LTD., SINGAPORE<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. INDOCORE PERKASA<br />
PT. INDOCORE PERKASA<br />
PT. INDOCORE PERKASA<br />
MIURA One Clinical Chemistry<br />
Autoanalyzer I.S.E. S.r.l., ITALY PT. DKSH INDONESIA<br />
MIURA Clinical Chemistry<br />
Autoanalyzer I.S.E. S.r.l., ITALY PT. DKSH INDONESIA<br />
MIURA 200 Clinical Chemistry<br />
Autoanalyzer I.S.E. S.r.l., ITALY PT. DKSH INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10102112116 16/08/2011 EPPENDORF Research Plus 3121<br />
AKL<br />
10102112114 16/08/2011 EPPENDORF Research Plus 3120<br />
AKL<br />
10102112115 16/08/2011 EPPENDORF Research Plus 3122<br />
AKL<br />
21106111641 29/07/2011<br />
ORIGIO ISM2 Culture Medium<br />
Without Phenol Red<br />
AKL<br />
20101112340 26/09/2011 INDEC AgD GLUCOSE<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
ORIGIO A/S, DENMARK melalui MEDIQUEST<br />
PTE., LTD., SINGAPORE<br />
AGAPPE DIAGNOSTICS, INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. FORTUNA HEALTHCARE<br />
PT. INDEC DIAGNOSTICS<br />
AKL<br />
20101112359 26/09/2011<br />
VITROS Immunodiagnostic Products<br />
Free T3 (FT3) Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101112357 26/09/2011<br />
VITROS Immunodiagnostic Products<br />
TSH Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101112358 26/09/2011<br />
AKL<br />
20305112536 07/10/2011<br />
VITROS Immunodiagnostic Products<br />
Total T3 (TT3) Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
Anti-HBs Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
AKL<br />
10208112537 07/10/2011 ORTHO 0,8% Red Cell Diluent ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
AKL<br />
20103112543 07/10/2011<br />
VITROS Chemistry Products CRBM<br />
Slides<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305112601 17/10/2011<br />
AKL<br />
20305112602 17/10/2011<br />
AKL<br />
20101112544 07/10/2011<br />
AKL<br />
20103112549 07/10/2011<br />
AKL<br />
20101112548 07/10/2011<br />
AKL<br />
20103112547 07/10/2011<br />
AKL<br />
20101112546 07/10/2011<br />
VITROS Immunodiagnostic Products<br />
Anti-HCV Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
HBsAg Reagent Pack<br />
VITROS Chemistry Products CK<br />
Slides / CK DT Slides<br />
VITROS Chemistry Products PHBR<br />
Slides<br />
VITROS Chemistry Products UPRO<br />
Slides<br />
VITROS Chemistry Products THEO<br />
Slides / THEO DT Slides<br />
VITROS Chemistry Products LAC<br />
Slides<br />
AKL<br />
10102112342 26/09/2011 MISSION U500 Urine Analyzer<br />
AKL<br />
10102112341 26/09/2011<br />
MISSION Expert U500 Urine<br />
Analyzer<br />
AKL<br />
20209112577 11/10/2011 CARPER Anti IgM Monoclonal<br />
AKL<br />
20209112578 11/10/2011 CARPER Anti AB Monoclonal<br />
AKL<br />
20209112579 11/10/2011 CARPER Anti A Monoclonal<br />
AKL<br />
10204112365 26/09/2011 DELFIA Inducer<br />
AKL<br />
10204112366 26/09/2011 DELFIA Wash Concentrate<br />
AKL<br />
20101112362 26/09/2011 Neonatal G6PD Kit<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ACON BIOTECH (HANGZHOU) CO. LTD.,<br />
CHINA<br />
PLASMATEC LABORATORIES PRODUCTS.,<br />
UNITED KINGDOM<br />
PLASMATEC LABORATORIES PRODUCTS.,<br />
UNITED KINGDOM<br />
PLASMATEC LABORATORIES PRODUCTS.,<br />
UNITED KINGDOM<br />
WALLAC OY., FINLAND untuk PERKIN<br />
ELMER INC. CORPORATION, USA melalui<br />
PERKIN ELMER SINGAPORE PTE. LTD<br />
WALLAC OY., FINLAND untuk PERKIN<br />
ELMER INC. CORPORATION, USA melalui<br />
PERKIN ELMER SINGAPORE PTE. LTD<br />
WALLAC OY., FINLAND untuk PERKIN<br />
ELMER INC. CORPORATION, USA melalui<br />
PERKIN ELMER SINGAPORE PTE. LTD<br />
AKL<br />
20303112621 17/10/2011 LIAISON Control Rubella IgM DIASORIN S.p.A., ITALY<br />
AKL<br />
20101112620 17/10/2011 LIAISON TSH DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112623 17/10/2011 LIAISON Control HSV - 1/2 IgG DIASORIN S.p.A., ITALY<br />
AKL<br />
20306112624 17/10/2011 LIAISON AFP DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112622 17/10/2011 LIAISON Control HSV - 1/2 IgM DIASORIN S.p.A., ITALY<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303112626 17/10/2011 LIAISON CMV IgG Avidity DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112628 17/10/2011 LIAISON Control CMV IgG Avidity DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112629 17/10/2011 LIAISON Control CMV IgM DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112627 17/10/2011 LIAISON Control Rubella IgG DIASORIN S.p.A., ITALY<br />
AKL<br />
10208112651 19/10/2011<br />
LIAISON 1/84 PTH Specimen<br />
Diluent<br />
AKL<br />
10101112640 19/10/2011 ACCU-CHEK Active Control<br />
AKL<br />
20101112566 11/10/2011 COBAS b 123 Fluid Pack COOX<br />
AKL<br />
20101112565 11/10/2011 COBAS b 123 Fluid Pack<br />
AKL<br />
20102112641 19/10/2011<br />
AKL<br />
10204112643 19/10/2011<br />
AKL<br />
10102112603 17/10/2011 ZENIX-390 Microplate Washer<br />
AKL<br />
10102112604 17/10/2011 ZENIX-320 Microplate Reader<br />
DIASORIN INC., USA melalui DIASORIN<br />
S..p.A., ITALY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
FAST CS-240 Automatic Chemistry<br />
Analyzer DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SAPTA LARONA MUDA<br />
DIRUI Cleanser for BCC-3000<br />
Series Auto Hematology Analyzer DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10204112417 26/09/2011 BioMajesty Reagent Probe Wash K<br />
AKL<br />
20305112564 11/10/2011<br />
Cobas 4800 System and<br />
Accessories<br />
AKL<br />
10102112562 11/10/2011 Cobas X 480<br />
RAYTO LIFE AND ANALYTICAL SCIENCE<br />
CO. LTD., CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCE<br />
CO. LTD., CHINA<br />
JOEL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PASIFIC PTE. LTD., SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
AKL<br />
20101112576 11/10/2011 LABTEST Albumin LABTEST DIAGNOSTICA S.A., Brazil<br />
AKL<br />
20101112574 11/10/2011 LABTEST Total Proteins LABTEST DIAGNOSTICA S.A., Brazil<br />
AKL<br />
20303112415 26/09/2011<br />
HSV-1 IgG Elecsys and cobas e<br />
analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
AKL<br />
20101112575 11/10/2011 LABTEST CK-MB Liquiform LABTEST DIAGNOSTICA S.A., Brazil<br />
AKL<br />
20101112631 19/10/2011 WELLCO Welltest Test Kehamilan<br />
AKL<br />
20101112632 19/10/2011<br />
AKL<br />
20303112630 19/10/2011<br />
WELLCO Welltest Strip test<br />
Kehamilan<br />
WELLCO Malaria PF/Pan Whole<br />
Blood Test<br />
GUANGZHOU WONDFO BIOTECH CO,<br />
LTD., CHINA<br />
GUANGZHOU WONDFO BIOTECH CO,<br />
LTD., CHINA<br />
GUANGZHOU WONDFO BIOTECH CO,LTD,<br />
China<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SYSMEX INDONESIA<br />
PT.ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. ROCHE INDONESIA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. GUNUNG MAS JAYA<br />
SENTOSA<br />
PT. GUNUNG MAS JAYA<br />
SENTOSA<br />
PT. GUNUNG MAS JAYA<br />
SENTOSA<br />
AKL<br />
20205112428 26/09/2011 Mythic 22 AL Hematology Analyzer ORPHEE SA, Switzerland PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20101112427 26/09/2011<br />
VITROS Immunodiagnostic products<br />
free T4 ( FT4) Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10204112709 24/10/2011 DIATRO Clenz DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10208112708 24/10/2011 DIATRO Dil-5P DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10204112710 24/10/2011 DIATRO Cleaner DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10205112725 24/10/2011 DIATRO Diff 5P DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10204112711 24/10/2011 DIATRO Hypoclean DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10208112726 24/10/2011 DIATRO Lyse Diff DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10208112723 24/10/2011 DIATRO Dil Diff DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
10208112722 24/10/2011 DIATRO Lyse EO DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208112724 24/10/2011 DIATRO Lyse 5P DIATRON MI PLC., HUNGARY PT. DIATRON PROMEDIKA<br />
AKL<br />
20101112545 10/07/2011<br />
AKL<br />
10208112642 19/10/2011<br />
AKL<br />
10204112644 19/10/2011<br />
AKL<br />
10204112645 19/10/2011<br />
VITROS Chemistry Products ECO2<br />
Slides<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
DIRUI Diluent for BCC-3000 Series<br />
Auto Hematology Analyzer DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SETIA ANUGERAH MEDIKA<br />
DIRUI Cleanser BCC-3000 B-Z<br />
Cleanser DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SETIA ANUGERAH MEDIKA<br />
DIRUI Cleanser BCC-3000 Probe<br />
Cleanser DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10208112646 19/10/2011 DIRUI Lyse (BCC-3000) DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20205112542 07/10/2011<br />
ARTHO AUTOVUE Innova and<br />
Accessories<br />
AKL<br />
20303112650 19/10/2011 LIAISON HSV - 1/2 IgG Assays DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112649 19/10/2011 LIAISON HSV - 1/2 IgM Assays DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112648 19/10/2011 LIAISON Control Toxo IgG Avidity II DIASORIN S.p.A., ITALY<br />
AKL<br />
20303112647 19/10/2011 LIAISON Rubella IgM Assay DIASORIN S.p.A., ITALY<br />
AKL<br />
20101112465 26/09/2011 Dsi CK-MB FS<br />
AKL<br />
10103112420 26/09/2011 DSI Cholinesterase FS<br />
AKL<br />
20101112466 28/09/2011<br />
AKL<br />
10102112756 26/10/2011<br />
AKL<br />
10102112619 17/10/2011<br />
AKL<br />
10102112666 21/10/2011<br />
AKL<br />
10102112668 21/10/2011<br />
AKL<br />
10102112618 17/10/2011<br />
AKL<br />
10102112616 17/10/2011<br />
AKL<br />
10102112667 21/10/2011<br />
AKL<br />
10102112749 25/10/2011<br />
AKL<br />
10102112617 17/10/2011<br />
AKL<br />
10102112733 24/10/2011<br />
DSI Albumin in Urine / CFS FS<br />
(MicroAlbumin)<br />
SORVALL RC4 Centrifuge and<br />
Accessories<br />
SORVALL Legend XT Centrifuge<br />
and accessories<br />
SORVALL Legend XTR Centrifuge<br />
and accessories<br />
SORVALL RC 12 BP Plus Large<br />
Capacity Centrifuge Low-Speed<br />
Centrifuge and accessories<br />
SORVALL ST16 Centrifuge and<br />
accessories<br />
SORVALL ST40<br />
accessories<br />
SORVALL ST 40R<br />
accessories<br />
Centrifuge and<br />
Centrifuge and<br />
SORVALL RC 3BP+Low-speed<br />
Centrifuge and accessories<br />
SORVALL ST16R<br />
accessories<br />
Centrifuge and<br />
SORVALL Legend Micro 21R<br />
Centrifuge and accessories<br />
TECAN SCHWEIZ AG., SWITZERLAND PT. JOHNSON & JOHNSON<br />
untuk ORTHO CLINICAL DIAGNOSTICS, USA INDONESIA<br />
DIASYS DIAGNOSTIC SYSTEM GMBH,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10102112729 24/10/2011<br />
AKL<br />
10102112735 24/10/2011<br />
AKL<br />
10102112734 24/10/2011<br />
AKL<br />
10102112789 28/10/2011<br />
AKL<br />
10102112790 28/10/2011<br />
AKL<br />
10102112736 24/10/2011<br />
AKL<br />
10102112730 24/10/2011<br />
SORVALL Legend X1 Centrifuge<br />
and accessories<br />
SORVALL Legend Micro 17<br />
Centrifuge and accessories<br />
SORVALL Legend Micro 17R<br />
Centrifuge and accessories<br />
SORVALL Biofuge Primo R<br />
Centrifuge and accessories<br />
SORVALL Biofuge Primo Centrifuge<br />
and accessories<br />
SORVALL Legend Micro 21<br />
Centrifuge and accessories<br />
SORVALL Legend X1R Centrifuge<br />
and accessories<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
THERMO ELECTRON LED. GMBH.,<br />
GERMANY melalui THERMO FISHER<br />
SCIENTIFIC PTE. LTD., SINGAPORE a<br />
subsidia<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20101112468 26/09/2011<br />
Macherey-Nagel Medi-Test Uryxxon<br />
Stick 10 (Blood, Urobilmogen,<br />
Bilirubin, Protein, Nitrite, Ketones,<br />
MACHEREY NAGEL GMBH & CO. KG.,<br />
GERMANY<br />
PT. MRK DIAGNOSTICS<br />
AKL<br />
10101112798 28/10/2011 FAST Liquick-Cor HBDH PZ CORMAY SA., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101112799 28/10/2011 FAST Liquick-Cor Gamma GT PZ CORMAY SA., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20305112739 24/10/2011 BIO-TECH HBsAg ELISA Kit ZHONGSHAN BIO-TECH CO. LTD., CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20205112356 26/09/2011 INNOVASTAR and Accessories<br />
AKL<br />
20303112791 28/10/2011 RESZON PanLF Rapid<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY<br />
RESZON DIAGNOSTIC INTERNATIONAL<br />
SDN. BHD., MALAYSIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. RIMATANDU MOTARO<br />
PRATAMA<br />
AKL<br />
20208112797 28/10/2011 TULIP PLASMATROL H-I TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101112792 28/10/2011<br />
BENECHECK Plus Multi-Monitoring<br />
System (Total Cholesterol, Blood<br />
Glucose, Uric Acid)<br />
AKL<br />
10101112788 28/10/2011 DELFIA Xpress uE3<br />
AKL<br />
20305112539 05/10/2011<br />
AKL<br />
20305112538 05/10/2011<br />
AKL<br />
10101112793 28/10/2011<br />
AKL<br />
20305112541 07/10/2011<br />
AKL<br />
20305112540 07/10/2011<br />
AKL<br />
10101112728 24/10/2011<br />
AKL<br />
10101112727 24/10/2011<br />
AKL<br />
20101112731 24/10/2011<br />
AKL<br />
20101112732 24/10/2011<br />
VITROS Immunodiagnostic Products<br />
Anti-HIV 1+2 Controls<br />
VITROS Immunodiagnostic Products<br />
HBsAg Controls<br />
VITROS Immunodiagnostic Products<br />
LH Reagent Pack<br />
VITROS Immunodiagnostic<br />
Products Anti-HBs Calibrator<br />
VITROS Immunodiagnostic<br />
Products HBsAg Calibrator<br />
NESCO MultiCheck Uric Acid Test<br />
Strip<br />
NESCO MultiCheck Cholesterol Test<br />
Strip<br />
NESCO MultiCheck Glucose Test<br />
Strip<br />
NESCO MultiCheck Glucose /<br />
Cholesterol / Uric Acid Multi Function<br />
Monitoring System<br />
AKL<br />
20101112599 17/10/2011 BIOMAJESTY Internal Standard<br />
PDF Creator - PDF4Free v3.0<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN<br />
WALLAC OY, FINLAND untuk PERKIN<br />
ELMER, USA melalui PERKIN ELMER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
BIOPTIK TECHNOLOGY INC., TAIWAN<br />
melalui KERNEL INT'L CORP., TAIWAN<br />
BIOPTIK TECHNOLOGY INC., TAIWAN<br />
melalui KERNEL INT'L CORP., TAIWAN<br />
BIOPTIK TECHNOLOGY INC., TAIWAN<br />
melalui KERNEL INT'L CORP., TAIWAN<br />
BIOPTIK TECHNOLOGY INC., TAIWAN<br />
melalui KERNEL INT'L CORP., TAIWAN<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. MITRAASA PRATAMA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. DJUNIAR & DJUNIAR<br />
PT. DJUNIAR & DJUNIAR<br />
PT. DJUNIAR & DJUNIAR<br />
PT. DJUNIAR & DJUNIAR<br />
PT. SYSMEX INDONESIA
AKL<br />
20101112598 17/10/2011<br />
AKL<br />
20101112597 17/10/2011<br />
AKL<br />
10204112606 17/10/2011<br />
AKL<br />
10204112607 17/10/2011<br />
BIOMAJESTY ISE Urine Standard<br />
Set<br />
BIOMAJESTY ISE Serum Standard<br />
Set (NA)<br />
SYSMEX BAC1 UX II SEARCH -<br />
BAC<br />
SYSMEX SED1 UX II SEARCH -<br />
SED<br />
AKL<br />
10204112605 17/10/2011 SYSMEX UX II SHEATH<br />
AKL<br />
20102112355 26/09/2011 RESPONS 920 and Accessories<br />
AKL<br />
10102112354 26/09/2011 RESPONS 910 and Accessories<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
JEOL LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
20101112608 17/10/2011<br />
VITROS Immunodiagnostic Products<br />
Free T3 (FT3) Calibrator<br />
ORTHO CLINICAL DIAGNOSTIC INC.,<br />
UNITED KINDOM<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305112738 24/10/2011 BIO-TECH HCV IgG ELISA Kit ZHONGHAN BIO-TECH CO. LTD., CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20305112768 27/10/2011<br />
VITROS Immunodiagnostic Products<br />
HBsAg ES Reagent Pack<br />
ORTHO CLINICAL DIAGNOSTIC INC.,<br />
UNITED KINDOM<br />
AKL<br />
20101112571 11/10/2011 LABTEST Urea UV Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
20101112572 11/10/2011 LABTEST AST/GOT Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
10101112570 11/10/2011 LABTEST Uric Acid Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
10101112568 11/10/2011 LABTEST ALT/GPT Acid Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
10101112567 11/10/2011 LABTEST Triglycerides Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
10101112569 11/10/2011 LABTEST Cholesterol Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
20101112573 11/10/2011 LABTEST Glucose PAP Liquiform LABTEST DIAGNOSTICA S.A., BRAZIL<br />
AKL<br />
10204112720 24/10/2011 MINDRAY M-18E E-Z Cleanser<br />
AKL<br />
10204112718 24/10/2011 MINDRAY M-18P Probe Cleanser<br />
AKL<br />
10204112717 24/10/2011 MINDRAY M-18R Rinse<br />
AKL<br />
10208112721 24/10/2011 MINDRAY M-18D Diluent<br />
AKL<br />
10208112719 24/10/2011 MINDRAY M-18CFL Lyse<br />
AKL<br />
10208112715 24/10/2011 MINDRAY M-53 LEO (I) Lyse<br />
AKL<br />
10208112713 24/10/2011 MINDRAY M-53 LH Lyse<br />
AKL<br />
10204112712 24/10/2011 MINDRAY M-53 Cleanser<br />
AKL<br />
10208112716 24/10/2011 MINDRAY M-53D Diluent<br />
AKL<br />
10204111670 02/08/2011<br />
COBAS NAOHD Detergent 1 cobas<br />
c system<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10101111687 02/08/2011 FAST Liquick Cor-UA PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10204111669 02/08/2011<br />
AKL<br />
10208111671 02/08/2011<br />
AKL<br />
20102111567 21/07/2011<br />
PDF Creator - PDF4Free v3.0<br />
COBAS SMS Detergent 2 cobas c<br />
system<br />
COBAS NACL Diluent NaCi 9%<br />
cobas c system<br />
Modular E Module (EVO) and<br />
Accessories<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101112122 16/08/2011<br />
AKL<br />
20101112121 16/08/2011<br />
AKL<br />
20101112123 16/08/2011<br />
DALF Serum Tube, Red Cap 13x75<br />
Plastic<br />
DALF EDTA K3 Tube, Purple Cap<br />
13x75 Plastic<br />
DALF SST Tube, Yellow Cap 13x75<br />
Plastic<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., P.R. CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., P.R. CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., P.R. CHINA<br />
PT. JAFAREL MEDIATICS<br />
PT. JAFAREL MEDIATICS<br />
PT. JAFAREL MEDIATICS<br />
AKL<br />
10102112214 26/08/2011 SIRIO S Elisa Analyzer SEAC S.r.l., ITALY PT. RAJA ERBA INDOCHEM<br />
AKL<br />
10102112213 26/08/2011 DROP Elisa Analyzer SEAC S.r.l., ITALY PT. RAJA ERBA INDOCHEM<br />
AKL<br />
10102112212 26/08/2011<br />
SLIM Semi-Automatic Chemistry<br />
Analyzer SEAC S.r.l., ITALY PT. RAJA ERBA INDOCHEM<br />
AKL<br />
20101111804 08/08/2011 FAST Liquick-Cor Calcium (ARZ III) PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10304113017 22/11/2011 i-CHROMA Reader BODITECH MED INC., KOREA PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20101113033 22/11/2011 INTHERMA Vacuum EDTA (K3)<br />
AKL<br />
20101113034 22/11/2011<br />
AKL<br />
20101113035 22/11/2011<br />
INTHERMA Vacuum Gel & Clot<br />
Activator<br />
INTHERMA Vacuum 3.2% Sodium<br />
Citrate<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., P.R. CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., P.R. CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., P.R. CHINA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20101111803 08/08/2011 FAST Liquick Cor-Total Protein PZ. CORMAY SA., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10207112439 26/09/2011<br />
AKL<br />
20101112338 26/09/2011<br />
AKL<br />
20305112414 26/09/2011<br />
AKL<br />
20305112413 26/09/2011<br />
A1CD2 cobas c111 Hemolyzing<br />
Reagent Gen.2<br />
ACCU-CHECK Advantage / Sensor<br />
Comfort<br />
Anti-Tg CalSet Elecsys and cobas e<br />
analyzers<br />
Anti-TPO CalSet Elecsys and cobas<br />
e analyzers<br />
AKL<br />
10204112412 26/09/2011 ROCHE Cleaning Solution<br />
AKL<br />
10204112411 26/09/2011 ROCHE Uterine Diluent<br />
AKL<br />
10101111918 09/08/2011<br />
AKL<br />
20102112462 28/09/2011<br />
AKL<br />
20102112464 28/09/2011<br />
AKL<br />
20102112463 28/09/2011<br />
AKL<br />
10204112444 27/09/2011<br />
AKL<br />
10201112443 27/09/2011<br />
ROCHE CONFITEST III Level 1, 2,<br />
3<br />
COBAS b 123 (2) System and<br />
Accessories<br />
COBAS b 123 (3) System and<br />
Accessories<br />
COBAS b 123 (4) System and<br />
Accessories<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GRAZ, GMBH,<br />
AUSTRIA untuk ROCHE DIAGNOSTICS<br />
GMBH., GERMANY melalui ROCHE<br />
DIAGNOSTI<br />
ROCHE DIAGNOSTICS GRAZ, GMBH,<br />
AUSTRIA untuk ROCHE DIAGNOSTICS<br />
GMBH., GERMANY melalui ROCHE<br />
DIAGNOSTI<br />
ROCHE DIAGNOSTICS GRAZ, GMBH,<br />
AUSTRIA untuk ROCHE DIAGNOSTICS<br />
GMBH., GERMANY melalui ROCHE<br />
DIAGNOSTI<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PREVI Color Nozzle Cleaning<br />
Solution BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
PREVI Color Gram Acetone Fuschin<br />
Solution BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101112482 30/09/2011 ST AIA-PACK LH II TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101112483 30/09/2011 ST AIA-PACK FSH TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101112484 30/09/2011 ST AIA-PACK E2 TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101112480 30/09/2011 ST AIA-PACK PROG TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101112360 26/09/2011 KONELAB ISE Calibrator 4<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305112361 26/09/2011 VIDAS B2 Microglobulin BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303111856 08/08/2011<br />
AKL<br />
20101111857 08/08/2011<br />
ONCOPROBE Malaria Combo<br />
Antigen Rapid Test<br />
ONCOPROBE Troponin I Rapid<br />
Test<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary company of CDI/BIOCHEM CORP.,<br />
USA<br />
PT. ONCOPROBE UTAMA<br />
PT. ONCOPROBE UTAMA<br />
AKL<br />
20303112281 05/09/2011 B.R.A.H.M.S PCT-Q (Procalcitonin) B.R.A.H.M.S GMBH., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20303112270 26/08/2011 B.R.A.H.M.S PCT LIA B.R.A.H.M.S GMBH., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
10204112269 26/08/2011 B.R.A.H.M.S Basiskit LIA 1000 Det B.R.A.H.M.S GMBH., GERMANY PT. MULTI SARANA MEDIKA<br />
AKL<br />
10208701901 26/08/2011 DIAGON Diafix-EO Diff DIAGON KFT, HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10208701897 26/08/2011 DIAGON Diadifton Argos/Pentra DIAGON KFT, HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10208701896 26/08/2011 DIAGON Dialyse Argos/Pentra DIAGON KFT, HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10208701900 26/08/2011 DIAGON Dialyse-Baso II DIAGON KFT, HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20305112770 27/10/2011<br />
AKL<br />
20305112769 27/10/2011<br />
AKL<br />
10102112898 14/11/2011<br />
AKL<br />
10102112899 14/11/2011<br />
AKL<br />
20101112963 21/11/2011<br />
VITROS Immunodiagnostic Products<br />
Anti-HCV Calibrator<br />
VITROS Immunodiagnostic Products<br />
Anti-HIV 1+2 Calibrator<br />
URISED Fully Automated Urine<br />
Sediment Analyzer<br />
LABUMAT Fully Automated Urine<br />
Chemistry Analyzer<br />
ABSOLUT One Step HCG Urine<br />
Pregnancy Test<br />
AKL<br />
20101112982 21/11/2011 DSI Bicarbonate FS<br />
AKL<br />
20303112981 21/11/2011<br />
AKL<br />
20101112983 21/11/2011<br />
AKL<br />
20101113005 21/11/2011<br />
AKL<br />
10208113013 22/11/2011<br />
AKL<br />
20303113016 22/11/2011<br />
PreciControl CMV IgG Avidity<br />
Elecsys and cobas e analyzer<br />
CKL Creatine Kinase COBAS<br />
INTEGRA cobas c system<br />
CORTISOL Elecsys and cobas e<br />
analyzers<br />
Diluent Estradiol / Progesterone<br />
Elecsys Elecsys and cobas e<br />
analyzers<br />
CMV IgG Avidity Elecsys and cobas<br />
e analizers<br />
AKL<br />
10204112960 21/11/2011 ISE Etcher Cobas Integra<br />
AKL<br />
20101112961 21/11/2011<br />
AKL<br />
10208112979 21/11/2011<br />
AKL<br />
20303112980 21/11/2011<br />
Vitamin D Total Elecsys and cobas e<br />
analyzers<br />
Diluent NSE Elecsys and cobas e<br />
analyzers<br />
Anti-HAV IgM Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20305112955 21/11/2011 DALF WT HBsAg DIP Test<br />
ORTHO-CLINICAL DIAGNOSTICS., UNITED<br />
KINGDOM<br />
ORTHO-CLINICAL DIAGNOSTICS., UNITED<br />
KINGDOM<br />
77 ELEKTRONIKA MUSZERIPARI K.f.t.,<br />
HUNGARY<br />
77 ELEKTRONIKA MUSZERIPARI K.f.t.,<br />
HUNGARY<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., CHINA<br />
DIASYS DIAGNOSTICS SYSTEMS GMBH.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., P.R. CHINA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. RAFA TOPAZ UTAMA<br />
PT. RAFA TOPAZ UTAMA<br />
PT. SELES GLOBAL FARMA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JAFAREL MEDIATICS<br />
AKL<br />
20102112904 14/11/2011<br />
AKL<br />
20101112594 17/10/2011 MEDITAPE II 10K<br />
AKL<br />
20101112593 17/10/2011 MEDITAPE II 7U<br />
FAST CS-400 Automatic Chemistry<br />
Analyzer and Accessories DIRUI INDUSTRIAL CO. LTD., P.R. CHINA PT. SAPTA LARONA MUDA<br />
ARKRAY INC., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPOR<br />
ARKRAY INC., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPOR<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101112596 17/10/2011 MEDITAPE II 9U<br />
AKL<br />
20101112595 17/10/2011 MEDITAPE II 10U<br />
AKL<br />
10204112425 26/09/2011 SYSMEX UX Clean -C<br />
AKL<br />
20205112949 21/11/2011<br />
AKL<br />
20303112938 21/11/2011<br />
ARKRAY INC., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPOR<br />
ARKRAY INC., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPOR<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
HUMACOUNT 30 TS and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
CARPER Salmonella H Paratyphi H<br />
Antigen<br />
AKL<br />
10208112947 21/11/2011 MINDRAY M-30CFL Lyse<br />
AKL<br />
10208112946 21/11/2011 MINDRAY M-30D Diluent<br />
AKL<br />
10204112945 21/11/2011 MINDRAY M-30E E-Z Cleanser<br />
AKL<br />
10204112944 21/11/2011 MINDRAY M-30P Probe Cleanser<br />
AKL<br />
10204112943 21/11/2011 MINDRAY M-30R Rinse<br />
AKL<br />
20101113032 22/11/2011<br />
AKL<br />
20304112897 14/11/2011<br />
INTHERMA Vacuum 3.8% Sodium<br />
Citrate<br />
XMATRX Auto FISH System and<br />
Accessories<br />
AKL<br />
10204112985 21/11/2011 ADVIA Centaur Probe Wash 3<br />
AKL<br />
20303112959 21/11/2011 PLATELIA Dengue NS1 Ag<br />
AKL<br />
20306112901 14/11/2011<br />
ROCHE HE4 Elecsys and cobas e<br />
analyzers<br />
PLASMATEC LABORATORY PRODUCT, UK<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
WEIHA HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
BIOGENEX LABORATORIES INC., USA<br />
melalui abbott molecular inc., usa<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
AKL<br />
10101112903 14/11/2011 LIAISON LH DIASORIN SpA., ITALY<br />
AKL<br />
10101112902 14/11/2011 LIAISON FSH DIASORIN SpA., ITALY<br />
AKL<br />
20205112957 21/11/2011<br />
AKL<br />
20305113007 21/11/2011<br />
AKL<br />
20305113008 21/11/2011<br />
AKL<br />
20303113006 21/11/2011<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SIEMENS INDONESIA<br />
PT. DOS NI ROHA<br />
PT. ROCHE INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
HOSPITEX Hemascreen 22<br />
Automated Hematology Analyzer and<br />
Accessories HOSPITEX DIAGNOSTICS S.r>l., ITALY PT. BAVARIA COMBININDO<br />
Anti-Tg Elecsys and cobas e<br />
analyzers<br />
Anti-TPO Elecsys and cobas e<br />
analyzers<br />
HSV-2 IgG Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20101112954 21/11/2011 DELFIA Xpress hCG Kit<br />
AKL<br />
20303112940 21/11/2011<br />
AKL<br />
20303112939 21/11/2011<br />
AKL<br />
20303112952 21/11/2011<br />
AKL<br />
20303112951 21/11/2011<br />
CARPER Salmonella H Paratyphi B<br />
Antigen<br />
CARPER Salmonella H Paratyphi A<br />
Antigen<br />
PreciControl Anti-HAV Elecsys and<br />
cobas e analyzers<br />
PreciControl Anti-HAV IgM Elecsys<br />
and cobas e analyzers<br />
AKL<br />
20303112958 21/11/2011 BIO-RAD Dengue NS1 Ag<br />
AKL<br />
20101112935 21/11/2011<br />
AKL<br />
20306112936 21/11/2011<br />
AKL<br />
20306112937 21/11/2011<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Immunodiagnostic Products<br />
LH calibrator<br />
VITROS Immunodiagnostic Products<br />
AFP calibrator<br />
VITROS Immunodiagnostic Products<br />
AFP Reagent Pack<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
WALLAC OY., FINLAND untuk PERKIN<br />
ELMER INC. CO., USA melalui PERKIN<br />
ELMER SINGAPORE PTE. LTD., SINGA<br />
PLASMATEC LABORATORY PRODUCT, UK<br />
PLASMATEC LABORATORY PRODUCT, UK<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DOS NI ROHA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA
AKL<br />
20306112942 21/11/2011<br />
VITROS Immunodiagnostic Products<br />
CEA Reagent Pack<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20306112941 21/11/2011<br />
VITROS Immunodiagnostic Products<br />
CEA Calibrators<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10304112956 21/11/2011<br />
Automated Enzyme Immunoassay<br />
Analyzer AIA-2000 and Accessories<br />
TOSOH CORPORATION-BIOSCIENCE<br />
DIVISION, JAPAN<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20102112911 14/11/2011<br />
ERBA XL-300 Fully Automated<br />
Random Access Clinical Chemistry<br />
Analyzer and Accessories<br />
TRANSASIA BIO-MEDICAL LTD., INDIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
20102112913 14/11/2011<br />
ERBA XL-640 Automated Random<br />
Access Clinical Chemistry Analyzer<br />
and Accessories<br />
TRANSASIA BIO-MEDICAL LTD., INDIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
20303112612 17/10/2011 FAST Salmonella Typhi O OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20303112613 17/10/2011 FAST Salmonella Typhi AO OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20303112614 17/10/2011 FAST Salmonella Typhi AH OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20303112611 17/10/2011 FAST Salmonella Typhi BO OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
20303112615 17/10/2011 FAST Salmonella Typhi H OMEGA DIAGNOSTICS LTD., UK PT. SAPTA LARONA MUDA<br />
AKL<br />
10203112748 25/10/2011<br />
AKL<br />
20305112794 28/10/2011<br />
AKL<br />
20103112795 28/10/2011<br />
AKL<br />
20103112796 28/10/2011<br />
AKL<br />
20101112332 26/09/2011<br />
AKL<br />
20101112331 26/09/2011<br />
AKL<br />
20101112330 26/09/2011<br />
ABBOTT VP 2000 System and<br />
Accessories<br />
ANTI-TPO Elecsys and cobas e<br />
analyzers<br />
DIGITOXIN CalSet Elecsys and<br />
cobas e analyzers<br />
DIGOXIN CalSet Elecsys and cobas<br />
e analyzers<br />
KONELAB Potassium (K) Micro<br />
Volume Electrode<br />
KONELAB Chloride (CI) Micro<br />
Volume Electrode<br />
KONELAB Sodium (Na) Micro<br />
Volume Electrode<br />
AKL<br />
20303112912 14/11/2011 PANBIO Dengue Early Rapid<br />
AKL<br />
20101704885 21/11/2011<br />
B-CROSSLAPS CalSet Elecsys and<br />
cobas e analyzers<br />
AKL<br />
20303112953 21/11/2011 Anti-HAV and cobas e analyzers<br />
ABBOTT MOLECULAR INC., USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
THERMO FISHER SCIENTIFIC OY,<br />
FINLAND melalui THERMO FISHER<br />
SCIENTIFIC, AUSTRALIA<br />
INVERNESS MEDICAL INNOVATIONS<br />
AUSTRALIA PTY. LTD., AUSTRALIA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20207113328 01/12/2011 BOULE QUINTUS 5-Part Stopper BOULE MEDICAL AB, SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
10208113329 01/12/2011 BOULE QUINTUS 5-Part Lyse BOULE MEDICAL AB, SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
10208113330 01/12/2011 BOULE QUINTUS 5-Part Diluent BOULE MEDICAL AB, SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20306113284 01/12/2011 TOSOH ST AIA-PACK PSA II TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306112706 24/10/2011<br />
CA 125 II Calset Elecsys and cobas<br />
e analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10102112707 24/10/2011<br />
AKL<br />
10208113014 22/11/2011<br />
MODULAR PRE ANALYTICS<br />
(MPA) and Accessories<br />
Diluent Hepatitis A Elecsys and<br />
cobas e analyzers<br />
HITACHI HIGH TECHNOLOGIES CO.,<br />
JAPAN untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTIC<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101111564 21/07/2011<br />
AKL<br />
20101112426 26/09/2011<br />
SI2 Serum Index Gen.2 COBAS<br />
INTEGRA cobas c system<br />
VITROS Immunodiagnostics<br />
Products Total T4 (TT4) Reagent<br />
Pack<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101112099 15/08/2011<br />
AKL<br />
20101112098 15/08/2011<br />
VITROS Immunodiagnostics<br />
Products Total T3 (TT3) Calibrator<br />
VITROS Immunodiagnostics<br />
Products TSH Calibrator<br />
AKL<br />
20101112339 26/09/2011 INDEC Agd UREA<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
AGAPPE DIAGNOSTICS, INDIA dikemas<br />
ulang oleh PT. INDEC DIAGNOSTICS,<br />
JAKARTA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. INDEC DIAGNOSTICS<br />
AKL<br />
20101112097 15/08/2011<br />
VITROS Immunodiagnostics<br />
Products Total T4 (TT4) Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20207113297 01/12/2011 NYCOCard HbA1C AXIS SHIELD PoC. AS, NORWEGIA PT. ALAM PUTRA SAKTI<br />
AKL<br />
20101808525 30/11/2011<br />
AKL<br />
20101113087 28/11/2011<br />
AKL<br />
20205113082 28/11/2011<br />
RADIOMETER ABL 5 Blood Gas<br />
Analysis (pH,cH,pCo2,pO2,B) and<br />
Accessories<br />
CREP2 Creatinine Plus ver.2<br />
COBAS INTEGRA cobas c systems<br />
NOVA BIOMEDICAL STAT<br />
PROFILE Critical Care Xpress<br />
AKL<br />
20101113299 01/12/2011 DIESSE Ves Tec Cuvette L<br />
AKL<br />
20101113301 01/12/2011 DIESSE Ves Tec<br />
AKL<br />
20101113300 01/12/2011 DIESSE Vacu Tec<br />
AKL<br />
20102705427 01/12/2011 ARCHITECT i-Theopylline Calibrator<br />
AKL<br />
20101113298 01/12/2011 DIESSE Vacu Tec Cuvette L<br />
AKL<br />
20102705734 29/11/2011<br />
COBAS INTEGRA 400 Plus +<br />
Accessories<br />
AKL<br />
20101113105 29/11/2011 DSI Total Protein FS<br />
AKL<br />
1010111986 09/08/2011<br />
AKL<br />
20101111815 08/08/2011<br />
IMMULITE FBC Free Beta HCG<br />
Control<br />
VITROS Chemistry Products<br />
Calibrator Kit 7<br />
RADIOMETER MEDICAL APS., DENMARK<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
NOVA BIOMEDICAL CORPORATION, USA<br />
DIESSE DIAGNOSTICS SENESE S.p.A.,<br />
ITALY<br />
DIESSE DIAGNOSTICS SENESE S.p.A.,<br />
ITALY<br />
DIESSE DIAGNOSTICS SENESE S.p.A.,<br />
ITALY<br />
BIOKIT, S.A., SPAIN untuk ABBOTT GMBH &<br />
CO. KG., GERMANY<br />
DIESSE DIAGNOSTICS SENESE S.p.A.,<br />
ITALY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ROCHE INDONESIA<br />
PT. TAMARA OVERSEAS<br />
CORPORATION<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABBOTT INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ROCHE INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS<br />
HEALTCARE DIAGNOSTICS LTD., Hongkon PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
AKL<br />
10204221709 24/10/2011 DIATRO Clenz DIATRON MI PLC., Hungary PT. DIATRON PROMEDIKA<br />
AKL<br />
20102112625 17/10/2011<br />
AKL<br />
10103112023 10/08/2011<br />
AKL<br />
20101112609 17/10/2011<br />
AKL<br />
20102112610 17/10/2011<br />
COBAS b 123 (1) System and<br />
Accessories<br />
VITROS Chemistry Products CHE<br />
Slides / CHE DT Slides<br />
VITROS Immunodiagnostic Products<br />
Free T4 (FT4) Calibrator<br />
GESAN Chem 200 Clinical<br />
Chemistry Automalyzer<br />
ROCHE DIAGNOSTICS GmbH., Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., Switzerland<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO CLINICAL DIAGNOSTICS INC., USA INDONESIA<br />
ORTHO CLINICAL DIAGNOSTICS INC., UK<br />
GESAN PRODUSTION S.r.I., Italy<br />
BIONOSTICS INC, USA untuk LIFESCAN<br />
AKL<br />
10101112477 30/09/2011<br />
EUROPE, A DIVISON OF CILANG GmbH,<br />
ONETOUCH VERIOControl Solution Switzerland<br />
AKL<br />
10101112421 26/09/2011 DSi Phosphatase FS<br />
DIASYS DOAGNOSTIC SYSTEMS GmbH,<br />
Germany<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SUNRISE INTERMEDICA<br />
INDO<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
10204112474 30/09/2011 ABX Pentra Clean Chem CP HORIBA ABX, France PT. DOS NI ROHA<br />
AKL<br />
10101112481 30/09/2011 ST AIA-PACK PRL TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101112669 21/10/2011<br />
AKL<br />
10101112670 21/10/2011<br />
PDF Creator - PDF4Free v3.0<br />
WELLCO Ovulasi Test Uji<br />
Kesuburan<br />
WELLCO Ovulasi Test Strip Uji<br />
Kesuburan<br />
GUANGZHOU WONDFO BIOTECH CO,<br />
LTD, China<br />
GUANGZHOU WONDFO BIOTECH CO,<br />
LTD, China<br />
http://www.pdf4free.com<br />
PT. GUNUNG MAS JAYA<br />
SENTOSA<br />
PT. GUNUNG MAS JAYA<br />
SENTOSA
AKL<br />
20305112016 10/08/2011 LIAISON Control HBeAg DIASORIN S.p.A., Italy<br />
AKL<br />
20205112019 10/08/2011<br />
AKL<br />
10101112027 10/08/2011<br />
AKL<br />
20305112028 10/08/2011<br />
MELET SCHLOESING MS9-S and<br />
Accessories<br />
VITROS Chemistry Products TDM<br />
Performance Verifier III<br />
VITROS Chemistry Products CRP<br />
Performance Verifier I<br />
MELET SCHLOESING LABORATPRIES,<br />
France<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. RAJA ERBA INDOCHEM<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10102111990 09/08/2011<br />
AKL<br />
10102111991 09/08/2011<br />
AKL<br />
10102111993 09/08/2011<br />
AKL<br />
10102111992 09/08/2011<br />
RAYTO RT-1904C Semi-Auto<br />
Chemistry Analyzer and Accessories<br />
RAYTO RT-2100C Microplate<br />
Raeder and Accessories<br />
RAYTO RT-150 Urine Analyzer and<br />
Accessories<br />
RAYTO RT-2600C Microplate<br />
Washer and Accessories<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD. China<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD. China<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD. China<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD. China<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
AKL<br />
20103111980 09/08/2011 BRIO 2+ Elisa Automated Analyzer SEAC S.r.I., Italy PT. RAJA ERBA INDOCHEM<br />
AKL<br />
21106111642 29/07/2011 ORIGIO Liquid Paraffin<br />
AKL<br />
10101111867 08/08/2011<br />
AKL<br />
10101111865 08/08/2011<br />
AKL<br />
1010111866 08/08/2011<br />
AKL<br />
10101111813 08/08/2011<br />
AKL<br />
10101111812 08/08/2011<br />
AKL<br />
20305111861 08/08/2011 RHEUMACHEC<br />
AKL<br />
2010311805 08/08/2011<br />
AKL<br />
20101111810 08/08/2011<br />
AKL<br />
10204111864 08/08/2011<br />
AKL<br />
20101111806 08/08/2011<br />
AKL<br />
10101111814 08/08/2011<br />
VITROS Chemistry Products TDM<br />
Performance Verifier II<br />
VITROS Chemistry Products AMON<br />
Slides<br />
VITROS Chemistry Products TDM<br />
Performance Verifier I<br />
VITROS Chemisrty Products PROT<br />
Slides<br />
VITROS Chemistry Products Mg<br />
Slides dan Mg DT Slides<br />
VITROS Chemistry Products Li<br />
Slides/ Li DT Slides<br />
VITROS Chemistry Products CK-MB<br />
Slides / CK-MB DT Slides<br />
VITROS Chemistry Products<br />
Immuno - Wash Fluid<br />
VITROS Chemistry Products<br />
Calibrator Kit 5<br />
VITROS Chemistry Products LIPA<br />
Slides / LIPA DT Slides<br />
AKL<br />
20102111982 09/08/2011 LIASON Analyzer and Accessories<br />
AKL<br />
20101112279 05/09/2011 MEDIGLOBE HCG Urine Test Strip<br />
AKL<br />
20101112280 05/09/2011<br />
AKL<br />
20102111981 09/08/2011<br />
MEDIGLOBE Urinalysis Reagent<br />
Strip 2 Parameter (Glucose and<br />
Protein)<br />
RAYTO CHEMRAY 240 Automated<br />
Chemistry Analyzer and Accessories<br />
AKL<br />
10204112325 26/09/2011 LAISON Wash / System Liquid<br />
AKL<br />
20306112329 26/09/2011 LAISON Control Fpsa<br />
ORIGIO A/S DENMARK melalui MEDIQUEST<br />
PTE., LTD., Singapore<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORGENTEC DIAGNOSTIKA GmbH.,<br />
Germany a OCD GROUP GmbH & CO.,<br />
Germany<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
DIASORIN DEUTSCHLAND GmbH, Germany<br />
melalui DIASORIN S.p. A., Italy<br />
GUANGZHOU WONDFO BIOTECH CO,<br />
LTD, China<br />
GUANGZHOU WONDFO BIOTECH CO,<br />
LTD, China<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD. China<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., Italy<br />
DIASORIN INC., USA melalui DIASORIN<br />
S.p.A., Italy<br />
AKL<br />
20209112335 26/09/2011 EPICLONE Anti-A CSL LIMITED, Australia<br />
AKL<br />
20209112334 26/09/2011 EPICLONE Anti-B CSL LIMITED, Australia<br />
AKL<br />
20209112333 26/09/2011 EPICLONE-2 Anti-D (IgM/ IgG) CSL LIMITED, Australia<br />
AKL<br />
20306112326 26/09/2011 LAISON CEA DIASORIN S.p.A., Italy<br />
AKL<br />
20306112328 26/09/2011 LAISON Fpsa<br />
DIASORIN GmbH., Fermany melalui<br />
DIASORIN S. P. A., Italy<br />
AKL<br />
20306112327 20/09/2011 LAISON CA 19-9 DIASORIN S.p.A., Italy<br />
AKL<br />
20306112363 26/09/2011 DELFIA Xpress Hafp Kit<br />
PDF Creator - PDF4Free v3.0<br />
WALLAC OY., Finland untuk PERKIN ELMER<br />
INC. CORPORATION, USA melalui PERKIN<br />
ELMER SINGAPORE PTE. LTD<br />
http://www.pdf4free.com<br />
PT. FORTUNA HEALTHCARE<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. NELTA MULTI GRACIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PHARMINDO RIMPANG<br />
KOKOH<br />
PT. PHARMINDO RIMPANG<br />
KOKOH<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB
AKL<br />
20101112364 26/09/2011 DELFIA Xpress PAPP-A Kit<br />
AKL<br />
10102111998 09/08/2011<br />
AKL<br />
20103111987 09/08/2011<br />
AKL<br />
10102112000 09/08/2011<br />
AKL<br />
10101112127 16/08/2011<br />
AKL<br />
20101112128 16/08/2011<br />
AKL<br />
20101112129 16/08/2011<br />
WALLAC OY., Finland untuk PERKIN ELMER<br />
INC. CORPORATION, USA melalui PERKIN<br />
ELMER SINGAPORE PTE. LTD<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
FAST Urine Analyzer H-50 and<br />
Accessories DIRUI INDUSTRIAL CO., LTD., P.R. China PT. SAPTA LARONA MUDA<br />
VITROS Chemistry Products PHTY<br />
Slides<br />
FAST Urine Analyzer H-500 and<br />
Accessories<br />
ABON LH One Step Ovulation Test<br />
Device ( Urine )<br />
ABON hCG One Step Pregnancy<br />
Test Strip ( Urine )<br />
ABON hCG One Step Pregnancy<br />
Test Device ( urine )<br />
AKL<br />
10102112105 15/08/2011 AFT-500 Electrolyte Analyzer<br />
AKL<br />
10102112106 15/08/2011 NB-201 Chemistry Analyzer<br />
AKL<br />
10101112107 15/08/2011<br />
AKL<br />
20206112130 16/08/2011<br />
STANBIO Ser-T-FY Level 2 Control<br />
Serum<br />
ABON FOB One Step Fecal Occult<br />
Blood Test Device ( Feces )<br />
AKL<br />
10102112104 15/08/2011 AFT-300 Electrolyte Analyzer<br />
AKL<br />
10102112102 15/08/2011<br />
AKL<br />
10102112103 15/08/2011<br />
ROBONIK Readwell Touch and<br />
accessories<br />
ROBONIK Washwell Plate and<br />
accessories<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
MEIZHOU CORNLEY HI-TECH CO LTD.,<br />
China<br />
CARETIUM MEDICAL INSTRUMENTS<br />
CO.,LTD .,China<br />
STABIO LABORATORY, USA<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
MEIZHOU CORNLEY HI-TECH CO LTD.,<br />
China<br />
ROBONIK ( INDIA ) PVT. LTD., India<br />
ROBONIK ( INDIA ) PVT. LTD., India<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20101113487 07/12/2011 i-CHROMA TSH BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20206113486 07/12/2011 i-CHROMA D-Dimer BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20305113483 07/12/2011 i-CHROMA Anti HBS BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20103113471 07/12/2011<br />
AKL<br />
20103113474 07/12/2011<br />
ABON MOP One Step Morphine<br />
Test Device ( Urine )<br />
ABON MTD One Step Methadone<br />
Test Strip ( Urine )<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20306113484 06/12/2011 i-CHROMA CEA BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20305113485 07/12/2011 i-CHROMA HBsAg BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20101113488 07/12/2011 i-CHROMA Tn I BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20101113489 07/12/2011 i-CHROMA T4 BODITECH MED INC., Korea PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
20103113472 07/12/2011<br />
AKL<br />
20103113473 07/12/2011<br />
ABON BZO One Step<br />
Benzodiazepine Test Device<br />
ABON MET One Step<br />
Methamphetamine Test Device (<br />
Urine )<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305113517 12/12/2011<br />
ABON HBsAg Hepatitis B Surface<br />
Antigen Rapid Test Device (Whole<br />
Blood / Serum/Plasma)<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20103113515 12/12/2011<br />
AKL<br />
20103113513 12/12/2011<br />
AKL<br />
20103113514 12/12/2011<br />
PDF Creator - PDF4Free v3.0<br />
ABON Multi-Drug One Step 4 Drug (<br />
COC,AMP, THC, MOP, BAR,BZO )<br />
Screen Test Panel ( Urine )<br />
ABON Multi-Drug One Step 6 Drug (<br />
COC, MET, THC, OPI,PCP ) Screen<br />
Test Panel ( Urine )<br />
ABON Multi - Drug One Step 5 Drug<br />
( COC,AMP,MET,THC) Screen Test<br />
Panel ( Urine )<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
http://www.pdf4free.com
AKL<br />
20103113516 12/12/2011<br />
ABON Multi - Drug One Step 3 Drug<br />
( MET,THC,MOP ) Screen Test<br />
Panel ( Urine )<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305113518 12/12/2011<br />
AKL<br />
20305113112 29/11/2011<br />
AKL<br />
10203113030 22/11/2011<br />
ABON HBsAg Hepatitis B Surface<br />
Antigen Rapid Test Device (Whole<br />
Blood / Serum/Plasma)<br />
ARCHITECH HBsAg Qualitative II<br />
Calibrators<br />
ABON BIOPHARM ( HANGZHOU) CO. LTD.,<br />
China melalui INVERNES MEDICAL<br />
INNOVATION ( HANGZHOU ) CONSULTIN<br />
ABBOTT IRELAND DIAGNOSTICS DIVISON,<br />
Ireland<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ABBOTT INDONESIA<br />
MAGNUS MLXi with LED and<br />
Battery Backup Microscope and<br />
Accessories MAGNUS ANALYTICS, India PT. C.V. KHARISMA UTAMA<br />
AKL<br />
10203113028 22/11/2011<br />
AKL<br />
10203113027 22/11/2011<br />
MAGNUS MLXi Trinocular with LED<br />
Microscope and Accessories MAGNUS ANALYTICS, India PT. C.V. KHARISMA UTAMA<br />
MAGNUS INVI-Inveeted Microscope<br />
for Tissue Culture Applicatons and<br />
Accessories MAGNUS ANALYTICS, India PT. C.V. KHARISMA UTAMA<br />
AKL<br />
10203113025 22/11/2011<br />
AKL<br />
10203113020 22/11/2011<br />
AKL<br />
20303112976 21/11/2011<br />
AKL<br />
20303112978 21/11/2011<br />
AKL<br />
20303112977 21/11/2011<br />
AKL<br />
20303112975 21/11/2011<br />
AKL<br />
20303112974 21/11/2011<br />
MAGNUS MSV Binocular Stereo<br />
Zoom Microscope and Accessories MAGNUS ANALYTICS, India PT. C.V. KHARISMA UTAMA<br />
MAGNUS MLX-B Microscope with<br />
LED and Baterry Backup Plus<br />
Accessories MAGNUS ANALYTICS, India PT. C.V. KHARISMA UTAMA<br />
CARPER Salmonella Typhi O<br />
Antigen<br />
CARPER Salmonella H Paratyphi C<br />
Antigen<br />
CARPER Salmonella O Paratyphi A<br />
Antigen<br />
CARPER Salmonella O Paratyphi C<br />
Antigen<br />
CARPER Salmonella O Paratyphi B<br />
Antigen<br />
PLASMATEC LABORATORY PRODUCKTS,<br />
UK<br />
PLASMATEC LABORATORY PRODUCKTS,<br />
UK<br />
PLASMATEC LABORATORY PRODUCKTS,<br />
UK<br />
PLASMATEC LABORATORY PRODUCKTS,<br />
UK<br />
PLASMATEC LABORATORY PRODUCKTS,<br />
UK<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
10303113015 22/11/2011<br />
AKL<br />
20102112680 24/10/2011<br />
AKL<br />
20101702711 28/10/2011<br />
AKL<br />
10203113054 28/11/2011<br />
AKL<br />
10203113055 28/11/2011<br />
JD BIOTECH Mycobacterium<br />
Tuberculosisi Antigen rapid Test Kit JEI DANIEL BIOTECH CORP, China PT. NELTA MULTI GRACIA<br />
SAPPHIRE 600 Plus Automated<br />
Clinical Chemistry Analyzer AUDIT DIAGNOSTICS, Ireland PT. BUMI INDAH PUTRA<br />
SMART CHEK® Blood Glucose<br />
Monitoring System<br />
HESTION Tissue Stainer and<br />
Accessories<br />
HESTION Embedding Center and<br />
Accessories<br />
AKL<br />
10204113104 29/11/2011 SYSMEX Reagent Probe Wash 2<br />
AKL<br />
10204113102 29/11/2011 SALINE Roche / Hitachi<br />
AKL<br />
20101113200 30/11/2011<br />
AKL<br />
20101113119 30/11/2011<br />
AKL<br />
20206113201 30/11/2011<br />
AKL<br />
20101113203 30/11/2011<br />
AKL<br />
20101113204 30/11/2011<br />
AKL<br />
20305113227 30/11/2011<br />
AKL<br />
20101113226 30/11/2011<br />
AKL<br />
20101113210 30/11/2011<br />
MAJOR BIOSYSYREM, Taiwan<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD. P.R. China untuk<br />
DIANTREE SCIENTIFIC, Australia<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD. P.R. China untuk<br />
DIANTREE SCIENTIFIC, Australia<br />
JOEL LTD., Japan untuk SYSMEX<br />
CORPORATION, Japan melalui SYSMEX ASIA<br />
PECIFIC PTE., LTD., Singapore<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. NUSANTARA BINA<br />
DIAGNOSTIKA<br />
PT. SARANA MEDICA OPTINDO<br />
PT. SARANA MEDICA OPTINDO<br />
PT. SYSMEX INDONESIA<br />
PT. ROCHE INDONESIA<br />
FREESYLE Optium H Blood<br />
Glucose Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
FREESYLE Optium Blood Glucose<br />
Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
SIEMENS HEMASTIX® Reagent<br />
Strips for Urinalysis<br />
KIMBALL ELECTRONICS POLAND Sp.<br />
Z.o.o., Poland untuk SIEMENS HEALTHCARE<br />
DIAGNOSTICS INC., USA<br />
PT. SIEMENS INDONESIA<br />
FREESTYLE Optium Blood Glucose<br />
Monotoring System ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
FREESTYLE Optium H Blood<br />
Glucose Monotoring System ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
ADVIA Centaur Systems HBc IgM<br />
QC<br />
ADVIA Chemistry GLUH_3 Glucose<br />
Hexokinase_3 Reagent<br />
ADVIA Centaur Systems Vitamin D<br />
Calibrator<br />
AKL<br />
20208113224 30/11/2011 ADVIA Chemistry HbA1c Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20306113223 30/11/2011<br />
AKL<br />
20305113116 29/11/2011<br />
AKL<br />
20305113114 29/11/2011<br />
AKL<br />
20305113115 29/11/2011<br />
AKL<br />
20305113113 29/11/2011<br />
AKL<br />
20101113202 30/11/2011<br />
AKL<br />
10203113053 28/11/2011<br />
AKL<br />
20103113237 30/11/2011<br />
AKL<br />
20101113309 01/12/2011<br />
AKL<br />
20306113343 01/12/2011<br />
IMMULITE® 2000 Systems BRM BR-<br />
MA (CA15-3)<br />
ARCHITECH HBsAg Qualitative II<br />
Reagent Kit<br />
ARCHITECH HBsAg Qualitative II<br />
Confirmatory Reagent Kit<br />
ARCHITECH HBsAg Qualitative II<br />
Contirmatory Manual Diluent<br />
ARCHITECH HBsAg Qualitative II<br />
Controls<br />
SIEMENS MULTISTIX®<br />
(Leukocytes, Nitrite, Urobilinogen,<br />
Protein, Ph, Blood, Specific Gravity,<br />
Ketone,<br />
HESTION Tissue Processor and<br />
Accessories<br />
TETRAMED Tes MDMA Ekstasi<br />
Strip - Urine<br />
VITROS® Immunodiagnostic<br />
Products CK-MB Calibrator<br />
VITROS® Immunodiagnostic<br />
Products PSA Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, Ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, Ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, Ireland<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, Ireland<br />
KIMBALL ELECTRONICS POLAND Sp.<br />
Z.o.o., Poland untuk SIEMENS HEALTHCARE<br />
DIAGNOSTICS INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SIEMENS INDONESIA<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD. P.R. China untuk<br />
DIANTREE SCIENTIFIC, Australia<br />
PT. SARANA MEDICA OPTINDO<br />
GUANGZHOU WONDFO BIOTECH CO.,<br />
LTD., P.R. China<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS, INC., UK INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS, INC., UK INDONESIA<br />
AKL<br />
20102113307 01/12/2011<br />
AKL<br />
20101113056 28/11/2011<br />
VITROS® EciQ Immunodiagnostic<br />
System and Accessories<br />
HGH CalSet Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20305113225 30/11/2011 ADVIA Centaur Systems HBcT QC<br />
ORTHO-CLINICAL DIAGNOSTICS, INC., UK<br />
ROCHE DIAGNOSTOCS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL, LTD., Switzerland<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
KIMBALL ELECTRONICS POLAND Sp.<br />
AKL<br />
20101113209 30/11/2011<br />
Z.o.o., Poland untuk SIEMENS HEALTHCARE<br />
SIEMENS CLINITEK Microalbumin 2 DIAGNOSTICS INC., USA<br />
AKL<br />
20101113308 01/12/2011<br />
AKL<br />
20103113207 30/11/2011<br />
AKL<br />
20501113196 29/11/2011<br />
AKL<br />
20504113195 29/11/2011<br />
VITROS® Immunodiagnostic<br />
Products CK-MB Reagent Pack<br />
TETRAMED Amphetamine Rapid<br />
Test<br />
ORTHO-CLINICAL DIAGNOSTICS, INC., UK<br />
GUANGZHOU WONDFO BIOTECH CO.,<br />
LTD., P.R. China<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
WELCH ALLYN Connex® Digital<br />
Blood Pressure and Accessories WELCH ALLYN., INC., USA PT. ENSEVAL MEDIKA PRIMA<br />
BOSTON SCIENTIFIC Rotablator<br />
Rotational Angioplasty System<br />
Console and Accessories and<br />
Dynaglide Foo<br />
BOSTON SCIENTIFIC CORPORATION.,<br />
USA<br />
AKL<br />
20101113292 01/12/2011 LAISON® HCG DIASORIN S.p.A., Italy<br />
AKL<br />
20305113239 30/11/2011 ADVIA Centaur HBsll Ready Pack<br />
AKL<br />
10102113091 29/11/2011<br />
AKL<br />
20101113205 30/11/2011<br />
AKL<br />
20101113206 30/11/2011<br />
SORVALL RC-6 Plus Centrifuge and<br />
Accessories<br />
TETRAMED HCG Preg Mids Rapid<br />
Test<br />
TETRAMED HCG Preg Strip Rapid<br />
Test<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
THERMO ELECTRON LED GmbH, Germany<br />
melalui THERMO FISHER SCIENTIFIC PTE.,<br />
LTD, Singapore a subsidiary<br />
GUANGZHOU WONDFO BIOTECH CO.,<br />
LTD., P.R. China<br />
GUANGZHOU WONDFO BIOTECH CO.,<br />
LTD., P.R. China<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. SIEMENS INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
AKL<br />
20101113347 01/12/2011 TOSOH ST AIA-PACK TSH TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113345 01/12/2011 TOSOH ST AIA-PACK TT3 TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113344 01/12/2011 TOSOH AIA-PACK Folate TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113333 01/12/2011<br />
AKL<br />
10103113295 01/12/2011 CHE2 Roche / Hitachi<br />
TOSOH AIA-PACK FSH Calibrator<br />
Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
ROCHE DIAGNOSTOCS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL, LTD., Switzerland<br />
ROCHE DIAGNOSTOCS GmbH, Germany<br />
AKL<br />
10204113296 01/12/2011<br />
melalui ROCHE DIAGNOSTICS<br />
ISE Deproteinizer COBAS INTERGA INTERNATIONAL, LTD., Switzerland<br />
AKL<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
20101113346 01/12/2011 TOSOH ST AIA-PACK T4 TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10805113423 19/12/2011 CONFIDENCE Adult Diapers Basic GT. PAPER CO. LTD., CHINA PT. SOFTEX INDONESIA<br />
AKL<br />
10805113424 19/12/2011 CONFIDENCE Adult Diapers Basic<br />
AKL<br />
11303113538 15/12/2011<br />
QUALITY HERO CORPORATION SDN.<br />
BHD., MALAYSIA<br />
PT. SOFTEX INDONESIA<br />
INTEGRA JARIT Minor Orthopaedic<br />
Surgical Instrument Set INTEGRA YORK PA. INC., USA PT. SURGIKA <strong>ALKES</strong>INDO<br />
AKL<br />
21302113403 16/12/2011 SYNTHES HCS 6.5 mm SYNTHES GMBH., SWITZERLAND PT. TRANSMEDIC INDONESIA<br />
AKL<br />
21302113404 16/12/2011 SYNTHES HCS 1.5 mm SYNTHES GMBH., SWITZERLAND PT. TRANSMEDIC INDONESIA<br />
AKL<br />
10903113545 16/12/2011 SERENITY Steel Wheelchair<br />
AKL<br />
10903113542 16/12/2011 SERENITY Commode<br />
AKL<br />
10903113544 16/12/2011 SERENITY Commode<br />
AKL<br />
10903113543 16/12/2011 SERENITY Commode<br />
AKL<br />
10703113537 15/12/2011<br />
AKL<br />
11603113540 15/12/2011<br />
AKL<br />
11104113536 15/12/2011<br />
AKL<br />
11603113539 15/12/2011<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
PT. SERENITY INDONESIA<br />
PT. SERENITY INDONESIA<br />
PT. SERENITY INDONESIA<br />
PT. SERENITY INDONESIA<br />
INTEGRA JARIT Nasal Basic Tray<br />
Instrument Set INTEGRA YORK PA. INC., USA PT. SURGIKA <strong>ALKES</strong>INDO<br />
INTEGRA JARIT Abdominal<br />
Hysterectomy Instrument Set INTEGRA YORK PA. INC., USA PT. SURGIKA <strong>ALKES</strong>INDO<br />
INTEGRA JARIT IUD Instrument<br />
Set INTEGRA YORK PA. INC., USA PT. SURGIKA <strong>ALKES</strong>INDO<br />
INTEGRA JARIT Amputation<br />
Surgical Instrument Set INTEGRA YORK PA. INC., USA PT. SURGIKA <strong>ALKES</strong>INDO<br />
AKL<br />
20505113529 15/12/2011 NC QUANTUM APEX MONORAIL BOSTON SCIENTIFIC CORPORATION, USA PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
11603113386 16/12/2011 YAMACO Dissecting Set PREXA INDUSTRIES, PAKISTAN<br />
AKL<br />
10703113388 16/12/2011 YAMACO Oral Instruments PREXA INDUSTRIES, PAKISTAN<br />
AKL<br />
11402113408 19/12/2011 SERENITY Walking Aid Rollator<br />
AKL<br />
11402113407 19/12/2011 SERENITY Walking Stick<br />
AKL<br />
10903113406 19/12/2011 SERENITY Commode<br />
AKL<br />
11402113410 19/12/2011 SERENITY Steel Wheelchair<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
FOSHAN DONGFANG MEDICAL<br />
EQUIPMENT MANUFACTORY LTD., CHINA<br />
for SERENITY GLOBAL LTD., UNITED<br />
KINGDOM<br />
PT. PANCA USAHA MEDIKA<br />
SARANA<br />
PT. PANCA USAHA MEDIKA<br />
SARANA<br />
PT. SERENITY INDONESIA<br />
PT. SERENITY INDONESIA<br />
PT. SERENITY INDONESIA<br />
PT. SERENITY INDONESIA<br />
AKL<br />
20101113348 01/12/2011 TOSOH ST AIA-PACK FT4 TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101113305 01/12/2011 TOSOH AIA-PACK Testosterone TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306113304 01/12/2011 TOSOH ST AIA-PACK CEA TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305113306 01/12/2011 TOSOH ST AIA-PACK IgE II TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113240 30/11/2011<br />
ADVIA Centaur Vitamin D total<br />
Ready Pack<br />
AKL<br />
20101113208 30/11/2011 BILD2 Roche / Hitachi<br />
AKL<br />
10208112714 24/10/2011 MINDRAY M-53 LEO (II) Lyse<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA
AKL<br />
10204113103 29/11/2011 SYSMEX Reagent Probe Wash S<br />
AKL<br />
20305113338 01/12/2011<br />
AKL<br />
20305113339 01/12/2011<br />
AKL<br />
20101113221 30/11/2011<br />
AKL<br />
20102113327 01/12/2011<br />
VITROS Immunodiagnostic<br />
Products Anti- HVC Controls<br />
VITROS Immunodiagnostic Anti-Hba<br />
Controls<br />
ONE TOUCH Verio Blood Glucose<br />
Test Strip<br />
JOEL LTD, Japan Untuk SYSMEX<br />
CORPORATION, Japan Melalui SYSMEX ASIA<br />
PASIFIC PTE. LTD., Singapore<br />
PT. SYSMEX INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK INDONESIA<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
UNIVERSAL BIOSENSORS PTY. LTD.,<br />
Australia untuk LIFESCAN<br />
EUROPE,Switzerland<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
Automatic Blochemistry Analyzer<br />
BOITECNICA BT-3500 and<br />
Accesories BIOTECNICA INSTRUMENTS S.p.A., Italy PT. RAJA ERBA INDOCHEM<br />
AKL<br />
20101113334 01/12/2011 TOSOH AIA-Pack Calibrator Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20207113332 01/12/2011<br />
AKL<br />
20306113331 01/12/2011<br />
FRA Fructosamine COBAS<br />
INTERGRA cobas csystems<br />
CA 15-3 II CalSets Elecsys and<br />
cobas e analyzer<br />
AKL<br />
11603113222 30/11/2011 ONE TOUCH UltraSoft Lancets<br />
AKL<br />
20101113059 28/11/2011<br />
AKL<br />
10203113009 22/11/2011<br />
AKL<br />
10203113012 22/11/2011<br />
AKL<br />
10203113010 22/11/2011<br />
AKL<br />
20102113124 29/11/2011<br />
AKL<br />
10203113011 22/11/2011<br />
VITROS Immunodiagnostic Products<br />
Prolactin Calibrators<br />
HESTION ERM-3100 Semi<br />
Automatic Microtome<br />
HESTION ERM-3100 Semi<br />
Automatic Microtome<br />
HESTION ERM-4000 Fully<br />
Motorized Rotary Microtome<br />
VITROS 4600 Chemistry System<br />
and Accessories<br />
HESTION CM-2850 Cryostat<br />
Micorotome<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
FACET TECHNOLOGIES,LLC,USA untuk<br />
LIFE SCAN INC., USA<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD P.R. China untuk<br />
DAINTREE SCIENTEFIC ,Australia<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD P.R. China untuk<br />
DAINTREE SCIENTEFIC ,Australia<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD P.R. China untuk<br />
DAINTREE SCIENTEFIC ,Australia<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
CHANGZHOU HAOSILIN MEDICAL<br />
INSTRUMENTS CO., LTD P.R. China untuk<br />
DAINTREE SCIENTEFIC ,Australia<br />
AKL<br />
20305113081 28/11/2011 LIAISON Anti-HBs DIASORIN S.p.A., Italy<br />
AKL<br />
20101113085 28/11/2011 ISE Solution 3 COBAS INTERGA<br />
AKL<br />
20101113083 28/11/2011 ISE Solution 1 COBAS INTERGA<br />
AKL<br />
20306113080 28/11/2011<br />
LIAISON Multi-Control Tumour<br />
Markers<br />
AKL<br />
20101113084 28/11/2011 ISE Solution 2 COBAS INTEGRA<br />
AKL<br />
10102113090 29/11/2011<br />
SOEVALL WX Ultra Centrifuge<br />
Series and Accessories<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
DIASORIN S.p.A., Italy<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
THERMO ELECTRON LED GmbH,Germany<br />
melalui THERMO FISHER SCIENTIFIC PTE.,<br />
LTD, Singapore a subsidiary<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SARANA MEDICA OPTINDO<br />
PT. SARANA MEDICA OPTINDO<br />
PT. SARANA MEDICA OPTINDO<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SARANA MEDICA OPTINDO<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
10101113311 01/12/2011<br />
VITROS Immunodiagnostic Products<br />
Testtoterone Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101113310 01/12/2011<br />
AKL<br />
20305113273 01/12/2011<br />
AKL<br />
20101113276 01/12/2011<br />
AKL<br />
20306113274 01/12/2011<br />
VITROS Immunodiagnostic Products<br />
Testtoterone Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
TOSOH AIA-PACK IgE Calibrator<br />
Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA- PACK<br />
Testosterone Calibrator Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK AFP Calibrator<br />
Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306113282 01/12/2011 TOSOH ST AIA - PACK CA19-9 TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113335 01/12/2011<br />
AKL<br />
20101113336 01/12/2011<br />
PDF Creator - PDF4Free v3.0<br />
TOSOH AIA-PACK PRL Calibrator<br />
Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK LH II<br />
Calibrator Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
http://www.pdf4free.com
AKL<br />
20101113337 01/12/2011<br />
TOSOH AIA-PACK E2 Calibrator<br />
Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306113293 01/12/2011 TOSOH ST AIA-PACK AFP TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101113418 02/12/2011<br />
TERTAMED Tes LH Kesuburan<br />
Midstream-Urine<br />
AKL<br />
20101113245 30/11/2011 ISE Calibrator Direct<br />
AKL<br />
20303113236 30/11/2011<br />
AKL<br />
10101113089 28/11/2011<br />
TERTAMED Tes Malaria Cassette-<br />
Darah<br />
VITROS Immunodiagnostic Products<br />
Prolactin Reagent Pack<br />
Guang ZHOU WONDFO BIOTECH CO.,<br />
LTD,P.R. China<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
Guang ZHOU WONDFO BIOTECH CO.,<br />
LTD,P.R. China<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. ROCHE INDONESIA<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10101113088 28/11/2011<br />
AKL<br />
10302113086 28/11/2011<br />
AKL<br />
20306113275 01/12/2011<br />
VITROS Immudiagnostic Products<br />
Progesterone Reagent Pavk<br />
RENOK Rehydator / Inuculator<br />
system and accessories<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
TOSOH AIA - PACK CEA Calibrator<br />
Set TOSOH CORPORAYION ,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101113058 28/11/2011<br />
VITROS Immudiagnostic Products<br />
Progesterone Calibrators<br />
AKL<br />
10101113057 28/11/2011 HGH Elecsys and Cobas e analyzers<br />
AKL<br />
20305113122 29/11/2011<br />
AKL<br />
20305113123 29/11/2011<br />
AKL<br />
20101113121 29/11/2011<br />
VITROS Immunodiagnostic Products<br />
Total Thyroid Control<br />
VITROS Immunodiagnostic Products<br />
Free Thyroid Control<br />
VITROS Immunodiagnostic product<br />
Cortisol Calibratos<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20207113216 30/11/2011 VIDAS D-Dimer Exclusion II BIOMERIEUX S.A France PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20102113350 01/12/2011<br />
AKL<br />
20102113351 01/12/2011<br />
AKL<br />
20101113238 30/11/2011 S2 Fluid Pack<br />
DIMENSION EXL With LM System<br />
and Accessories<br />
DIMENSION EXL 200 System and<br />
Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG, Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG, Germany<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10101113241 30/11/2011<br />
VITROS Immunodiagnostic Products<br />
Estradiol ( E2 ) Reagent Pack<br />
AKL<br />
20101113243 30/11/2011 ISE calibrator Indirect / Urine<br />
AKL<br />
20101113244 30/11/2011 ISE Reference Electrolyte<br />
AKL<br />
20301702292 18/07/2011<br />
AKL<br />
20103112970 21/11/2011<br />
AKL<br />
20103112969 21/11/2011<br />
AKL<br />
20103112971 21/11/2011<br />
AKL<br />
20103112972 21/11/2011<br />
AKL<br />
20103112973 21/11/2011<br />
AKL<br />
20103112968 21/11/2011<br />
BD PHOENIX 100 Instrument and<br />
accessories<br />
ABON MET One Step<br />
Methampetamine Test Strip ( urine )<br />
ABON MOP One Step Morphine<br />
Test Strip ( Urine )<br />
ABON COC One Step Cocaine test<br />
strip ( Urine )<br />
ABON AMP One Step<br />
Amphetamine Test strip ( urine )<br />
ABON THC One Step Marijuan Test<br />
Strip ( urine )<br />
ABON PCP One Step Phencyclidine<br />
Test device ( Urine )<br />
ORTHO-CLINICAL DIAGNOSTICS,INC., UK<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH,Germany<br />
melalui ROCHE DIAGNOSTICS INTL,LTD.,<br />
Switzerland<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
Singapore<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103112967 21/11/2011<br />
AKL<br />
20103112965 21/11/2011<br />
AKL<br />
20103112964 21/11/2011<br />
AKL<br />
20103112966 21/11/2011<br />
AKL<br />
20306113220 30/11/2011<br />
AKL<br />
20306113217 30/11/2011<br />
AKL<br />
20306113212 30/11/2011<br />
AKL<br />
20305113218 30/11/2011<br />
AKL<br />
20102113101 29/11/2011<br />
ABON PPX One Step Propoxyphene<br />
Test device( Urine )<br />
ABON MTD One Step Methadone<br />
Test Device ( Urine )<br />
ABON BZO One Step<br />
Benzodiazepines test device ( urine )<br />
ABON BAR One Step Barbiturate<br />
Test Device ( Urine )<br />
IMMULITE 2000 System OV OM-MA<br />
(CA125)<br />
IMMULITE 2000 System GIM GI-MA<br />
(CA 19-9)<br />
IMMULITE 2000 System fPSA Free<br />
PSA<br />
IMMULITE 2000 Thyroglobulin<br />
Sample Diluent<br />
AVL 9180 Electrolyte Analyzer and<br />
Accessories<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
ABON BIOPHARMA ( HANGZHOU ) CO.<br />
LTD., China untuk ALERE SANDIEGO INC.,<br />
USA melalui INVERNES MEDICAL<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20306113281 01/12/2011 TOSOH ST AIA-PACK CA 15-3 TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306113283 01/12/2011 TOSOH ST AIA-PACK CA 125 TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305113623 28/12/2011 QUANTA Lite ccp3.1 IgG/IgA ELISA INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20306113566 28/12/2011<br />
AKL<br />
20306113567 28/12/2011<br />
AKL<br />
20306113568 28/12/2011<br />
AKL<br />
20103113542 28/12/2011<br />
AKL<br />
20103113543 28/12/2011<br />
AKL<br />
20101113458 21/12/2011<br />
VYSIS CEP 12 Spectrumrange DNA<br />
Probe Kit with Control Slides (20<br />
Assays)<br />
VYSIS CEP X SpectrumOrange / Y<br />
SpectrumGreen DNA Probe Kit with<br />
Control Slides (20 Assays)<br />
VYSIS CEP 8 SpectrumOrange<br />
DNA Probe kit wit Control Slides (20<br />
Assays)<br />
ABON COC One Step Cocaine Test<br />
Device (Urine)<br />
ABON MQL One Step Methaqualone<br />
Test Device (Urine)<br />
RBC Folate Hemolyzing Reagent<br />
Elecsys and cobas e analyzer<br />
AKL<br />
10102113489 09/12/2011 RAJAWALI Chemistry Analyzer<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA untuk ALERE SAN DIEGO INC., USA<br />
melalui INVERNESS MEDICAL I<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA untuk ALERE SAN DIEGO INC., USA<br />
melalui INVERNESS MEDICAL I<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
AKL<br />
20305113624 28/12/2011 QUANTA Lite h-tTG IgA ELISA INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20209113638 28/12/2011 AFFIRMAGEN ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
AKL<br />
10204113581 28/12/2011<br />
AKL<br />
20303113582 28/12/2011<br />
AKL<br />
20303113583 28/12/2011<br />
COBAS AMPLICOR Wash Buffer<br />
(WB)<br />
COBAS AMPLICOR Conjugate<br />
Detection Reagent (CN4)<br />
COBAS AMPLICOR Detection<br />
Reagent Kit (DK)<br />
AKL<br />
20302702293 07/03/2011 BD Pheonix ID Broth<br />
AKL<br />
20302113576 07/03/2011 BD Tube Pheonix AST-Broth<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. RAJAWALI NUSINDO<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA
AKL<br />
20302113577 30/03/2011 BD Tube Pheonix NID<br />
AKL<br />
20302113578 30/03/2011 BD Tube Pheonix AST-S Broth<br />
AKL<br />
20104113242 30/11/2011<br />
AKL<br />
20306113461 21/12/2011<br />
AKL<br />
20306113462 21/12/2011<br />
VITROS Immunodiagnostic Products<br />
Signal Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
CA 19-9 Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
CA 19-9 Calibrator<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
20301113569 28/12/2011 ESBL Plus ESBL Confirmation Panel INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301113570 28/12/2011 MICROSTREP Plus Panel Type 1<br />
AKL<br />
10101113460 21/12/2011<br />
AKL<br />
20101113459 21/12/2011<br />
AKL<br />
20102113290 01/12/2011<br />
VITROS Immunodiagnostic Products<br />
FSH Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
FSH Calibrator<br />
REFLOTRON PLUS and<br />
Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10204113457 21/12/2011 ELITECH System Solution SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20305113079 28/11/2011 LIAISON Control Anti-HBc DIASORIN S.p.A., ITALY<br />
AKL<br />
21106113573 28/12/2011 VITROLIFE Hatching Micropipettes<br />
AKL<br />
21106113574 28/12/2011 VITROLIFE Holding Micropipettes<br />
VITROLIFE INC., USA melalui VITROLIFE<br />
SWEDEN AB., SWEDEN<br />
VITROLIFE INC., USA melalui VITROLIFE<br />
SWEDEN AB., SWEDEN<br />
AKL<br />
21106113575 28/12/2011 SWEMED Denutation Pipette VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106113572 28/12/2011 SWEMED Transfer Pipettes VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106113571 28/12/2011 VITROLIFE Injection Micropipettes<br />
VITROLIFE INC., USA melalui VITROLIFE<br />
SWEDEN AB., SWEDEN<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20101113463 21/12/2011<br />
AKL<br />
20101113464 21/12/2011<br />
AKL<br />
20306113342 01/12/2011<br />
AKL<br />
10101113291 01/12/2011<br />
VITROS Immunodiagnostic Products<br />
Troponin I ES Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
Troponin I ES Calibrator<br />
VITROS Immunodiagnostic Products<br />
PSA Reagent Pack<br />
Precocontrol Varia Elecsys and<br />
cobas e analyzers<br />
AKL<br />
10204113272 01/12/2011 REFLOTRON Clean + Check<br />
AKL<br />
20103113270 01/12/2011<br />
ARCHITECT Valproid Acid Reagent<br />
Kit<br />
AKL<br />
10101113286 01/12/2011 DIALINE BIONORM<br />
AKL<br />
10101113288 01/12/2011 DIALINE BIONORM L<br />
AKL<br />
20101113289 01/12/2011 DIALINE BIOCAL<br />
AKL<br />
20101113271 01/12/2011<br />
ARCHITECT Valproid Acid<br />
Calibrators<br />
AKL<br />
10101113287 01/12/2011 DIALINE BIOPATH<br />
AKL<br />
20303113279 01/12/2011<br />
AKL<br />
20303113278 01/12/2011<br />
BLUE CROSS BIO-MEDICAL One<br />
Step Anti-TP Test Strip<br />
BLUE CROSS BIO-MEDICAL One<br />
Step Anti-TP Test Cassette<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT LABORATORIES, USA<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
DENKA SEIKEN CO. LTD., JAPAN untuk<br />
ABBOTT LABORATORIES, USA<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. ABBOTT INDONESIA<br />
PT. DIATRON PROMEDIKA<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ABHIMATA MANUNGGAL<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305113277 01/12/2011<br />
BLUE CROSS BIO-MEDICAL One<br />
Step Anti-HCV Cassette<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20305113280 01/12/2011<br />
BLUE CROSS BIO-MEDICAL One<br />
Step Anti-HCV Test Strip<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
PT. ABHIMATA MANUNGGAL<br />
AKL<br />
10102113588 28/12/2011<br />
CLINITEK Advantus Analyzer and<br />
Accessories<br />
KIMBALL ELECTRONICS POLAND Sp.z.OO,<br />
POLAND untuk SIEMENS HEALTHCARE<br />
DIAGNOSTICS INC., USA melalui SI<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20209113663 28/12/2011 FAST CORMAY D-Dimer Control PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101113662 28/12/2011 FAST PRESTIGE 24i ACP PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101113661 28/12/2011 FAST CORMAY CK-MB calibrator PZ. CORMAY S.A., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10302113585 28/12/2011 MICROSCAN Rapid Yeast ID Panel<br />
AKL<br />
10302113586 28/12/2011<br />
AKL<br />
20303113664 28/12/2011<br />
MICROSCAN Rapid Aneroid ID<br />
Panel<br />
IMMULITE 2000 Systems CMM<br />
CMV IgG<br />
AKL<br />
10302113587 28/12/2011 MICROSCAN HNID Panel<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204113660 28/12/2011 TOSOH AIA-PACK Substrate Set II TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10302113590 28/12/2011 BBL Tripticase Soy Broth<br />
AKL<br />
10302113589 28/12/2011 BD BACTED Myco/F Lytic<br />
AKL<br />
20303113111 29/11/2011<br />
ABON Malaria Pan / p.f Rapid Test<br />
Device (Whole Blood)<br />
BECTON DICKINSON AND COMPANY USA,<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY USA,<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA untuk ALERE SAN DIEGO INC., USA<br />
melalui INVERNESS MEDICAL I<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20207113621 28/12/2011 DIAZYME SMART HbA1c Assay DIAZYME LABORATORIES, USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
10204113584 28/12/2011<br />
AKL<br />
20101113637 28/12/2011<br />
AKL<br />
20101113636 28/12/2011<br />
AKL<br />
10208113659 28/12/2011<br />
AKL<br />
10204113678 28/12/2011<br />
AKL<br />
20101113656 28/12/2011<br />
AKL<br />
20101113655 28/12/2011<br />
AKL<br />
20101113657 28/12/2011<br />
AKL<br />
20101113658 28/12/2011<br />
AKL<br />
20103113642 28/12/2011<br />
AKL<br />
20103113641 28/12/2011<br />
AKL<br />
20103113640 28/12/2011<br />
AKL<br />
20103113639 28/12/2011<br />
SDR II COBAS FP Sample Dilution<br />
Reagent II<br />
PTH (1-84) Elecsys and cobas e<br />
analyzers<br />
PTH (1-84) CalSet Elecsys and<br />
cobas e analyzers<br />
ROCHE DIAGNOSTICS GMBG, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBG, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBG, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
TOSOH AIA-PACK Diluent<br />
Concentrate TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK Wash<br />
Concentrate TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK T4 Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK TT3 Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK FT4 Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK TSH 3RD Gen<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
SYVA Emit II Plus Ecstasy Calibrator<br />
/ Control Level 2<br />
SYVA Emit II Plus Ecstasy Calibrator<br />
/ Control Level 3<br />
SYVA Emit II Plus Ecstasy Calibrator<br />
/ Control Level 4<br />
SYVA Emit Calibrator / Control Level<br />
4<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103113643 28/12/2011<br />
AKL<br />
10101113667 28/12/2011<br />
AKL<br />
10101113679 28/12/2011<br />
AKL<br />
20303113647 28/12/2011<br />
AKL<br />
20303113646 28/12/2011<br />
AKL<br />
20303113644 28/12/2011<br />
AKL<br />
20305113645 28/12/2011<br />
AKL<br />
20101113199 30/11/2011<br />
AKL<br />
10101113302 01/12/2011<br />
AKL<br />
10101113303 01/12/2011<br />
SYVA Emit II Plus Ecstasy Calibrator<br />
/ Control Level 1<br />
HBDH Roche / Hitachi a -<br />
Hydroxybutarate Dehydrogenase<br />
Optimized<br />
BeneCheck Plus Total Cholesterol<br />
Test Strip<br />
IMMULITE 2000 System RUM<br />
Rubella IgM<br />
IMMULITE 2000 System aHA Total<br />
Anti-HAV<br />
IMMULITE 2000 System HAM Anti-<br />
HAV IgM<br />
IMMULITE 2000 System HAM HBS<br />
HBsAg<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBG, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MITRAASA PRATAMA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
FREESTYLE Optium Blood Glucose<br />
Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
FREESTYLE Optium H Blood B<br />
Ketone Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
FREESTYLE Optium Blood B<br />
Ketone Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
AKL<br />
10101113213 30/11/2011<br />
ADVIA Centaur Systems Vitamins D<br />
QC<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20303113211 30/11/2011<br />
IMMULITE 2000 Systems CVG<br />
CMV IgG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101113219 30/11/2011<br />
ADVIA Chemistry CO2 Reagents<br />
Concentrate<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204113676 28/12/2011 FURUNO Washing Solution No. 9<br />
NIHON KEMIKOTO CO. LTD., JAPAN untuk<br />
FURUNO ELECTRIC CO. LTD., JAPAN<br />
melalui SYSMEX ASIA PASIFIC PTE<br />
AKL<br />
10101113627 28/12/2011 SEKISUI Apo-II Auto N "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101113626 28/12/2011 SEKISUI Apo C-III Auto N "DAIICHI" SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
10101705509 28/12/2011<br />
AKL<br />
20102113619 28/12/2011<br />
AKL<br />
10102113294 01/12/2011<br />
SEKISUI Apoprotein Control Serum<br />
"DAIICHI" High & Low<br />
DIESTRO 103AP Electrolyte<br />
Analyzer<br />
ACURA Manual XS 826<br />
Micropipettes<br />
SEKISUI MEDICAL CO. LTD., JAPAN<br />
JS MEDICINA ELECTRONICA SRL.,<br />
ARGENTINA<br />
SOCOREX ISBA S.A., SWITZERLAND<br />
PT. SYSMEX INDONESIA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. CITRAMED INDONESIA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
AKL<br />
20102113621 28/12/2011<br />
AKL<br />
20305113686 28/12/2011 DIASOURCE HBsAg ELISA<br />
DIAZYME SMART Point of Care<br />
System 700/340 and Accessories DIAZYME LABORATORIES, USA PT. MEGA UTAMA MEDICA<br />
DIASOURCE IMMUNOASSAYS S.S.,<br />
BELGIUM<br />
AKL<br />
10101113580 28/12/2011 SEKISUI LPL ELISA DAIICHI SEKISUI MEDICAL CO. LTD., JAPAN<br />
AKL<br />
20101113060 28/11/2011<br />
AKL<br />
20304113349 01/12/2011<br />
AKL<br />
20205113512 12/12/2011<br />
AKL<br />
20101805144 10/07/2012<br />
AKL<br />
20101805143 13/07/2012<br />
AKL<br />
20207210895 21/03/2012<br />
AKL<br />
20209805126 02/10/2012<br />
PDF Creator - PDF4Free v3.0<br />
ONE TOUCH Verio Blood Glucose<br />
Monitoring System<br />
THERMOBRITE Slide<br />
Denaturation/Hybrization System 240<br />
VAC and Accessories<br />
WP-360 Automated Hematology<br />
Analyzer<br />
PLEXUS MANUFACTURING SDN. BHD.<br />
MALAYSIA untuk LIFESCAN EUROPE,<br />
SWITZERLAND<br />
IRIS SAMPLE PROCESSING, USA melalui<br />
ABBOTT LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
PT. ABHIMATA MANUNGGAL<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
SD CHECK Gold Blood Glucose<br />
Test System SD BIOSENSOR INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
SD CHECK Gold Blood Glucose<br />
Monitoring System SD BIOSENSOR INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
ALERE INRatio 2 PT/INR Test<br />
Strips ALERE SAN DIEGO INC., USA PT. MEDQUEST JAYA GLOBAL<br />
BD VACUTAINER K2 EDTA (K2E)<br />
Plus Blood Collection Tube<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE.LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. ANUGRAH ARGON MEDICA
AKL<br />
20101805092 15/08/2012 ELITECH Amylase SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101805090 15/08/2012 ELITECH Calcium Arzeno SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101805089 15/08/2012 ELITECH Chloride SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101805088 15/08/2012 ELITECH Glucose HK SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101805093 15/08/2012 ELITech CK-MB Control SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801370 09/02/2012<br />
AKL<br />
20305804975 15/08/2012<br />
AKL<br />
20305804976 15/08/2012<br />
AKL<br />
20305804973 15/08/2012<br />
ABBOTT Clinical Chemistry<br />
Creatinine ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
IMMULITE / IMMULITE 1000<br />
Systems IL-2R<br />
IMMULITE 2000 Systems Allergen<br />
Specific IgE Universal Kit<br />
IMMULITE / IMMULITE 1000<br />
Systems Anti-TG Ab (Anti<br />
Thyroglobulin Ab)<br />
AKL<br />
20101804977 15/08/2012 IMMULITE2000ACTH<br />
AKL<br />
20101804978 15/08/2012<br />
AKL<br />
20305804954 15/08/2012<br />
AKL<br />
10102805017 01/11/2012<br />
AKL<br />
10102805016 01/11/2012<br />
AKL<br />
10102805013 01/11/2012<br />
IMMULITE 2000 Systems CAL<br />
Calcitonin<br />
COBAS AMPLICOR Internal Control<br />
Detection Kit (IC DK)<br />
BD-FALCON Round-Bottom Tube,<br />
Polystrene<br />
BD-FALCON Round-Bottom Tube,<br />
Polypropylene<br />
BD-FALCON Blue Max Jr. 15ml<br />
Polystrene Conical Tube<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
10302805001 15/08/2012 BD BBL Endo Agar BECTON DICKINSONAND COMPANY, USA PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
10102805010 01/11/2012<br />
BD-FALCON 5 ml Polystyrene<br />
Round-Botton Tube<br />
AKL<br />
10302805000 21/03/2012 BD BBL V-C-N Inhibator<br />
AKL<br />
10302804999 21/03/2012 BD BBL Iso Vitalex Enrichment<br />
AKL<br />
10302804997 21/03/2012 BD BBL Salmonella shigella Agar<br />
AKL<br />
10302804996 21/03/2012 BD BBL Mannitol Salt Agar<br />
AKL<br />
10302804998 15/08/2012 BD BACTO Tryptic Soy Broth<br />
AKL<br />
10302804994 20/03/2012 BD BBL Brain Heart Infusion<br />
AKL<br />
10302804995 20/03/2012 BD BBL MacConkey ll Agar<br />
AKL<br />
10302804993 21/03/2012 BD BBL MacConkey Agar<br />
AKL<br />
10302804992 21/03/2012<br />
AKL<br />
20101804988 13/07/2012<br />
PDF Creator - PDF4Free v3.0<br />
BD BBL Cary and Blair Transport<br />
Medium<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
EASYLYTE Calcium Analyzer and<br />
Accessories MEDICA CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
http://www.pdf4free.com
AKL<br />
20101804989 13/07/2012<br />
AKL<br />
20101804991 13/07/2012<br />
AKL<br />
10102804957 22/05/2012 ZENIK-120 Urine Analyzer<br />
AKL<br />
20101804990 13/07/2012<br />
AKL<br />
10303804951 15/08/2012<br />
AKL<br />
20303804953 15/08/2012<br />
AKL<br />
20303804955 15/08/2012<br />
AKL<br />
20305804969 30/05/2012<br />
AKL<br />
20305804968 25/05/2012<br />
AKL<br />
20101804781 23/11/2012<br />
EASYLYTE Plus Na/K/CI Analyzer<br />
and Accessories MEDICA CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
EASY Blood Gas Analizer and<br />
Accessories MEDICA CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
RAYTO LIFE AND ANALYTICAL SCIENCE<br />
CO. LTD., CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
EASYSTAT Analyzer and<br />
accessories MEDICA CORPORATION, USA PT. ALAM PUTRA SAKTI<br />
COBAS AMPLICOR Chlamydia<br />
Trachomatis Detection Kit (CT DK)<br />
AMPLICOR CT/NG Amplification Kit<br />
(CT/NG AMP)<br />
COBAS AMPLICOR Neisseria<br />
Gonorhoeae Detection Kit (NG DK)<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
for ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
ABBOTT AXSYM AUSAB Reagent<br />
pack hepatitis B Surface Antigen ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM HCV version 3,0<br />
Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
MAJOR ll Blood Glucose Monitoring<br />
System MAJOR BIOSYSTEM CORP., TAIWAN PT. WACANA INDO MITRA<br />
AKL<br />
20101804782 23/11/2012 MAJOR ll Blood Glucose test Strips MAJOR BIOSYSTEM CORP., TAIWAN PT. WACANA INDO MITRA<br />
AKL<br />
20101804836 15/08/2012 ADVIA Chemistry ALP (DEA)<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804829 13/07/2012 ADVIA Chemistry IgG2 2 Reagent<br />
AKL<br />
20305804832 15/08/2012<br />
ADVIA Chemistry Transferrin<br />
Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804834 15/08/2012 ADVIA Chemistry Creatinine<br />
AKL<br />
20101804833 10/07/2012<br />
ADVIA Chemistry Microalbumin<br />
calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804828 15/08/2012<br />
ADVIA Chemistry Direct Bilirubin 2<br />
(DBIL-2)<br />
AKL<br />
20101804830 15/08/2012 ADVIA Chemistry ALP (AMP)<br />
AKL<br />
20101804831 15/08/2012 ADVIA Chemistry Amylase Reagent<br />
AKL<br />
10101804835 15/08/2012 ADVIA Chemistry APO B Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804827 15/08/2012 DIAGON Bilirubin Auto Direct (DCA) DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101804823 15/08/2012 DIAGON Calcium (AS) DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20205804788 23/10/2012 HELENA ADP Reagent HELENA LABORATORIES, USA<br />
AKL<br />
20205804785 23/10/2012 HELENA Collagen reagent HELENA LABORATORIES, USA<br />
AKL<br />
20205804786 23/10/2012 HELENA Epinephrine Reagent HELENA LABORATORIES, USA<br />
AKL<br />
20205804783 31/10/2012 HELENA Scale Set Solution HELENA LABORATORIES INC., USA<br />
AKL<br />
20208804746 13/07/2012 TECOTE Control N<br />
AKL<br />
20207804747 15/08/2012 TEClot PT-B Kit<br />
AKL<br />
20207804744 13/07/2012<br />
DIMEX Jr D-Dimer Analyzer and<br />
Accessories<br />
AKL<br />
20207804743 15/08/2012 TECO Dimex D-Dimer Kit-100<br />
PDF Creator - PDF4Free v3.0<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION & TRADING GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION & TRADING GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION & TRADING GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION & TRADING GmbH.,<br />
GERMANY<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
http://www.pdf4free.com<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA
AKL<br />
20207804739 15/08/2012 TECO D-Dimer Aglutination Kit<br />
AKL<br />
20207804740 13/07/2012 TEClot FIB Kit 10<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GmbH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION & TRADING GmbH.,<br />
GERMANY<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20205804749 13/07/2012<br />
TECO COATRON M1 Coagulation<br />
Analyzer and Accessories<br />
AKL<br />
10208804751 02/08/2012 TECO IBS Buffer<br />
TECO MEDICAL INSTRUMENT PRODUCTS<br />
& TRADING GMBH., GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION & TRADING GmbH.,<br />
GERMANY<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20305804737 25/05/2012 ABBOTT AXSYM Hbe 2.0 Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305804734 30/05/2012<br />
AKL<br />
20305804732 30/05/2012<br />
AKL<br />
20305804733 30/05/2012<br />
AKL<br />
20305804728 25/05/2012<br />
AKL<br />
20305804729 30/05/2012<br />
AKL<br />
20303804736 30/04/2012<br />
AKL<br />
20305804727 30/04/2012<br />
AKL<br />
20303804726 30/04/2012<br />
AKL<br />
20303804735 30/04/2012<br />
ABBOTT AXSYM AUSAB Controls<br />
Hepatitis B Surface Antigen ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System HBsAg<br />
Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM HBsAg (V2)<br />
Reagent Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM HCV version 3.0<br />
Reagent Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM AUSAB Standard<br />
Calibrators Hepatitis B Surface<br />
Antigen ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Toxo IgM Reagent<br />
Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM HBsAg<br />
Confirmatory Reagent Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Toxo IgM Index<br />
Calibrator ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Toxo IgG Reagent<br />
Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20303804725 30/04/2012 ABBOTT AXSYM Toxo IgM Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305804690 15/08/2012<br />
AKL<br />
20305804698 15/08/2012<br />
IMMULITE 2000 Systems High<br />
Sensitivy CRP<br />
IMMULITE / IMMULITE 1000<br />
Systems IL-1 Beta I1B<br />
10102212228 13/07/2012 ADVIA LabCell Automation Solution<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ATS AUTOMATION TOOLING SYSTEMS,<br />
INC., CANADA untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS INC., USA<br />
melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804654 11/07/2012 ADVIA Chemistry IgM 2<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG, GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804650 15/08/2012 ADVIA Chemistry Calcium<br />
AKL<br />
20101804660 10/07/2012<br />
ADVIA Chemistry urea Nitrogen<br />
Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804658 15/08/2012 ADVIA Chemistry Albumin<br />
AKL<br />
10101804657 10/07/2012<br />
ADVIA Chemistry Uric Acid<br />
Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS INC., USA melalui SIEMENS<br />
AG, GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101804659 22/05/2012<br />
AKL<br />
10101804655 15/08/2012<br />
ADVIA CENTAUR TSTO Ready<br />
Pack<br />
ADVIA Chemistry Inorganic<br />
Phosporus Reagents<br />
AKL<br />
10101804652 10/07/2012 ADVIA Chemistry Iron 2 Reagents<br />
AKL<br />
10101804648 10/07/2012<br />
ADVIA Chemistry Magnesium<br />
Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101804649 15/08/2012<br />
PDF Creator - PDF4Free v3.0<br />
ADVIA Chemistry ALT (GPT)<br />
Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA
AKL<br />
20207213665 30/10/2012<br />
IMMULITE/ IMMULITE 1000 System<br />
EPN EPO<br />
AKL<br />
20208213076 24/08/2012 HEMOLYZER-3-Control (L, N, H)<br />
AKL<br />
20305804699 15/08/2012<br />
AKL<br />
20305804694 15/08/2012<br />
AKL<br />
20305804689 15/08/2012<br />
AKL<br />
20305804685 15/08/2012<br />
AKL<br />
20305804683 15/08/2012<br />
AKL<br />
20305804682 15/08/2012<br />
AKL<br />
20305804679 15/08/2012<br />
AKL<br />
20305804678 15/08/2012<br />
AKL<br />
20101804681 15/08/2012<br />
AKL<br />
10101804692 15/08/2012<br />
AKL<br />
10101804693 15/08/2012<br />
AKL<br />
10101804695 15/08/2012<br />
AKL<br />
10101804687 15/08/2012<br />
AKL<br />
10101804680 15/08/2012<br />
IMMULITE / IMMULITE 1000<br />
Systems II IL-6<br />
IMMULITE / IMMULITE 1000<br />
Systems TG Thyroglobulin<br />
IMMULITE 2000 Systems Ferritin<br />
FER<br />
IMMULITE 2000 Systems TG<br />
Thyroglobulin<br />
IMMULITE / IMMULITE 1000<br />
Systems IL-8<br />
IMMULITE 2000 Systems Total IgE<br />
TIE<br />
IMMULITE / IMMULITE 1000<br />
Systems MYO Myoglobin<br />
IMMULITE / IMMULITE 1000<br />
Systems RMY Myoglobin Turbo<br />
IMMULITE / IMMULITE 1000<br />
System RCK CK-MB Turbo<br />
IMMULITE / IMMULITE 1000<br />
Systems DPD Pyrilinks - D<br />
IMMULITE / IMMULITE 1000<br />
Systems LH<br />
IMMULITE / IMMULITE 1000<br />
Systems GH Growth Hormone (hGH)<br />
IMMULITE 2000 Systems DPD<br />
Pyrilinks-D<br />
IMMULITE / IMMULITE 1000<br />
Systems IGI IGF - 1<br />
AKL<br />
10101804688 15/08/2012 IMMULITE 2000 Systems FSH<br />
AKL<br />
10101804686 15/08/2012 IMMULITE 2000 Systems LH<br />
AKL<br />
10101804677 15/08/2012<br />
AKL<br />
10101804676 15/08/2012<br />
IMMULITE / IMMULITE 1000<br />
Systems SHBG<br />
IMMULITE 2000 System E2<br />
Estradiol<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. DJEMBATAN DUA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804644 13/07/2012 Cystatin C Tina-quant Roche/Hitachi ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20305804645 13/07/2012<br />
AKL<br />
20303804646 26/07/2012<br />
AKL<br />
20101804643 26/07/2012<br />
Anti-CCP Elecsys and Cobas e<br />
analyzers<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
B.R.A.H.M.S PCT Elecsys and<br />
Cobase analyzer ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
CYSC Tina-quant [a] Cystatin C<br />
Cobas Integra Cobas C Systems ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10204804665 15/08/2012 DIAGON Diacatch-Sys-Diluent DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20305804558 13/07/2012 Ferritin Tina-quant[a] Roche/Hitachi ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20207213418 04/10/2012 VARIANT II Turbo HbA1c Kit - 2.0<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE., LTD., SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20305804555 13/07/2012 C4 Tina-quant Roche/Hitachi ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20208212311 13/07/2012 Precipath HBA1C Roche Systems<br />
AKL<br />
20101804562 13/07/2012<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
CO2-L Roche/Hitachi (Bicarbonate<br />
Liquid) ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10101804561 13/07/2012 ACCU-CHECK Advantage ll Control<br />
AKL<br />
10204804565 10/07/2012<br />
ISE Cleaning Solution/SysClean<br />
Roche/Hitachi Elecsys<br />
AKL<br />
20101804559 13/07/2012 TSH Elecsys and Cobas e Analyzer<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305804546 07/06/2012 ADVIA CENTAUR aHBs QC<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804548 07/06/2012<br />
ADVIA CENTAUR Anti HBc Total<br />
(HBcT) Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
20305804545 07/06/2012 ADVIA CENTAUR HCV Ready Pack INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804543 12/03/2012<br />
ADVIA Centaur HBc IgM Quality<br />
Control<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804540 07/06/2012<br />
ADVIA CENTAUR HBc IgM Ready<br />
Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10102806788 28/06/2012<br />
KUBOTA Compact Table Top<br />
Refrigerator Centrifuge 2810 and<br />
Accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
PT. DEMKA SAKTI<br />
AKL<br />
20101804538 15/08/2012<br />
DCA System<br />
Microalbumin/Creatinine<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20102212297 13/07/2012<br />
AKL<br />
10101804541 13/07/2012<br />
ADVIA 1800 Chemistry System and<br />
Accessories<br />
IMMULITE/IMMULITE 1000<br />
Systems E2 Estradiol<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804530 13/07/2012 CRPLX Tina-quanta Roche/Hitachi ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101804531 13/07/2012 Combur 10 Test M ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
10302804528 10/07/2012 OXOID TINSDALE Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302804527 10/07/2012<br />
OXOID Toxin Detection VET-RPLA<br />
Toxin Detection Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302804526 10/07/2012 OXOID TINSDALE Agar Base OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302211985 26/06/2012<br />
AKL<br />
20305804445 11/07/2012<br />
TECAN HYDROFLEX Platform and<br />
Accessories<br />
Tg Confirmatory Test Elecsys and<br />
Cobas e Analyzers<br />
TECAN AUSTRIA GMBH., AUSTRIA melalui<br />
TECAN SALES INTERNATIONAL GMBH.,<br />
AUSTRIA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305804447 20/03/2012 SD BIOLINE HBsAg Fast STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101804448 20/03/2012 SD BIOLINE hCG Fast STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10102804451 26/06/2012<br />
AKL<br />
20306804696 15/08/2012<br />
FREEDOM EVO Clinical and<br />
Accessories TECAN SCHWEIZ AG., SWITZERLAND PT. MEDQUEST JAYA GLOBAL<br />
IMMULITE / IMMULITE 1000<br />
System TNA TNF-alpha<br />
AKL<br />
20208804401 30/04/2012 EQAS Hemaglobin Program<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101804399 30/04/2012<br />
AKL<br />
10101804398 30/04/2012<br />
AKL<br />
20101804423 25/05/2012<br />
AKL<br />
20303804410 13/07/2012<br />
AKL<br />
20101804426 25/05/2012<br />
EQAS Immunoassay (Monthly)<br />
Program<br />
EQAS Clinical Chemistry (Monthly)<br />
Program<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
ABBOTT Clinical Chemistry CO2<br />
Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System CMV IgM<br />
Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry HDL<br />
Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20205804415 25/05/2012<br />
AKL<br />
20101804407 25/05/2012<br />
AKL<br />
20101804419 25/05/2012<br />
AKL<br />
20101804416 25/05/2012<br />
AKL<br />
20305804409 22/05/2012<br />
AKL<br />
20305804408 04/06/2012<br />
AKL<br />
20303804310 06/07/2012<br />
AKL<br />
20303804311 06/07/2012<br />
AKL<br />
20303804308 06/07/2012<br />
AKL<br />
20303804309 06/07/2012<br />
AKL<br />
20303804307 06/07/2012<br />
ABBOTT CELL DYN 1800 System<br />
and Accessories + Spareparts ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Total T3 Reagent<br />
Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Bilirubin<br />
Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Lipase<br />
Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Anti Hbe 2.0<br />
Reagent Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Anti-Hbe<br />
2.0 Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
a - SHIELD Salmonella H Antigen,<br />
Antigen C<br />
a - SHIELD Salmonella H Antigen,<br />
Antigen D (Typhi H)<br />
a - SHIELD Salmonella H Antigen,<br />
Antigen A<br />
a - -SHIELD Salmonella H Antigen,<br />
Antigen B<br />
a-SHIELD Salmonella O, Antigen D<br />
(Typhi O)<br />
AKL<br />
20303804306 06/07/2012 a-SHIELD Salmonella O, Antigen C<br />
AKL<br />
20303804305 06/07/2012 a-SHIELD Salmonella O, Antigen B<br />
AKL<br />
20303804304 06/07/2012 a-SHIELD Salmonella O, Antigen A<br />
AKL<br />
20209804313 22/06/2012 a - SHIELD Anti A Monoclonal<br />
AKL<br />
20209804315 22/06/2012 a - SHIELD Anti AB Monoclonal<br />
AKL<br />
20209804314 22/06/2012 a - SHIELD Anti B Monoclonal<br />
AKL<br />
20209804312 22/06/2012 a - SHIELD Anti D Monoclonal<br />
AKL<br />
20306804281 04/07/2012<br />
AKL<br />
20306804215 13/07/2012<br />
AKL<br />
20103804211 04/07/2012<br />
AKL<br />
20306804212 04/07/2012<br />
PDF Creator - PDF4Free v3.0<br />
IMMULITE /IMMULITE 1000 System<br />
BRM BR-MA (CA 15-3)<br />
IMMULITE / IMMULITE 1000<br />
System AF AFP<br />
IMMULITE /IMMULITE 1000 System<br />
CMP Carbamazepine<br />
IMMULITE /IMMULITE 1000<br />
Systems CEA<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
http://www.pdf4free.com<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
20101804209 13/07/2012 IMMULITE 2000 System T3 Total T3 PRODUCTS LTD., UK<br />
AKL<br />
10101804214 10/07/2012<br />
AKL<br />
10101804210 10/07/2012<br />
IMMULITE/IMMULITE 1000 System<br />
INS Insulin<br />
IMMULITE/ IMMULITE 1000 System<br />
FSH<br />
AKL<br />
20303804206 06/07/2012 a-SHIELD RPR Test Kit Special<br />
AKL<br />
20303804205 06/07/2012 a-SHIELD RPR test Kit<br />
AKL<br />
20303804204 06/07/2012 a-SHIELD Microsyph TP 200<br />
AKL<br />
20303804203 06/07/2012<br />
a - SHIELD Syphscreen - RPR<br />
Antigen Suspension<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK a division of LAB 21 HEALTHCARE<br />
LIMITED., UK<br />
AKL<br />
10102804136 25/07/2012 ADALITIS ECLECTICA Analyzer Adaltis Italia S.p.A, Italy<br />
AKL<br />
20306804160 13/07/2012<br />
AKL<br />
20207804156 13/07/2012<br />
IMMULITE / IMMULITE 1000<br />
System GIM GI-MA (CA 19-9)<br />
IMMULITE / IMMULITE 1000<br />
System EPO<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. WHIRA PITOE USAHA<br />
BERSAMA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804161 10/07/2012<br />
IMMULITE / IMMULITE 1000<br />
sYSTEM HSc High Sensitivity CRP<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101804162 13/07/2012<br />
IMMULITE / IMMULITE 1000<br />
System T4 Total T4<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204804159 13/07/2012<br />
AKL<br />
10101804157 10/07/2012<br />
IMMULI/IMMULITE 1000<br />
Chemiluminescent Substrare Module<br />
IMMULITE/IMMULITE 1000 Sytem<br />
DHS DHEA-SO4<br />
AKL<br />
20305804144 10/07/2012 iMMULITE 1000 System FER Feritin<br />
AKL<br />
20305804142 04/07/2012<br />
AKL<br />
20101804143 04/07/2012<br />
AKL<br />
20101804141 10/07/2012<br />
IMMULITE /IMMULITE 1000 System<br />
TIE Total IgE<br />
IMMULIE /IMMULITE 1000 Systems<br />
T3 Total T3<br />
IMMULITE 2000 System iPT Intact<br />
PTH<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10303804148 20/03/2012 SD Bioline TB Ag MPT64 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10303804146 20/03/2012 SD Bioline TB IgG/IgM STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10303804147 22/03/2012 SD BIOLINE Chlamydia STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20103804154 13/07/2012<br />
AKL<br />
20208804043 25/05/2012<br />
SPEEDYTEST-DOA 3 Drugs Panel<br />
Test (MOP/THC/AMP) IND DIAGNOSTIC INC., USA PT. LANIROS DIAN PHARMA<br />
SYSMEX Hematology Calibrator<br />
SCS-1000<br />
AKL<br />
10208804048 22/05/2012 STROMATOLYSER - FB<br />
AKL<br />
10208804046 13/06/2012 SULFOLYSER<br />
AKL<br />
20208804037 30/04/2012 EIGHTCHECK 3WP<br />
AKL<br />
10208804049 22/05/2012 Stromatolyser -3 WP<br />
AKL<br />
10208804045 22/05/2012 STROMATOLYSER - 4 DS<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE untuk SYSMEX<br />
CORPORATION., JAPAN<br />
SYSMEX ASIA PACIFIC PTE., LTD.,<br />
SINGAPORE untuk SYSMEX<br />
CORPORATION, JAPAN<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE untuk SYSMEX<br />
CORPORATION, JAPAN<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE untuk SYSMEX<br />
CORPORATION., JAPAN<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208804044 07/05/2012<br />
AKL<br />
10208804039 22/05/2012 CELLPACK<br />
SYSMEX QUICKYSER - II QLS-<br />
200A<br />
AKL<br />
10208804040 22/05/2012 STROMATOLYSER - WH<br />
AKL<br />
10208804041 07/05/2012<br />
SYSMEX RET-SEARCH (II) RED-<br />
700A<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE untuk SYSMEX<br />
CORPORATION., JAPAN<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE untuk SYSMEX<br />
CORPORATION., JAPAN<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20305804126 12/03/2012 ADVIA Centaur HBsAg Confirmatory<br />
AKL<br />
20305804130 12/03/2012 ADVIA Centaur HBsAg Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804129 12/03/2012 ADVIA Centaur aHBs Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305804125 12/03/2012 ADVIA Centaur HBsAg QC<br />
AKL<br />
20205212287 13/07/2012<br />
AKL<br />
10101804131 10/07/2012<br />
ADVIA 120 ADVIA 2120i Sheath /<br />
Rinse<br />
IMMULITE/IMMULITE 1000 System<br />
CPE C-Peptide<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTH DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG,<br />
GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20205212299 13/07/2012 ADVIA 120 ADVIA 2120i autoRETIC<br />
AKL<br />
10101804127 13/07/2012<br />
IMMULITE 2000 SYSTEMS PRL<br />
Prolactin Sample Diluent<br />
AKL<br />
20101804035 11/07/2012 BIL-T Roche/Hitachi Bilirubin DPD<br />
AKL<br />
20101804036 07/05/2012 P-AMYL Roche/Hitachi<br />
AKL<br />
20305804031 11/07/2012<br />
Anti-HBc IgM Elecsys and Cobas e<br />
Analyzers<br />
AKL<br />
10101804034 12/06/2012 ACCUTREND Control TG1<br />
AKL<br />
10101804032 12/06/2012 ACCUTREND Control CH1<br />
AKL<br />
20101804029 21/03/2012<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG, GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS LTD., UK<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
RAJAWALI Urea (Liquid Enzymatic<br />
UV-Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20207804021 21/03/2012 RAJAWALI Hemoglobin HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101804026 21/03/2012<br />
RAJAWALI Urea (Enzymatic<br />
Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101804025 21/03/2012 RAJAWALI Total Protein HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
10101804028 22/03/2012<br />
AKL<br />
10101804022 22/03/2012<br />
AKL<br />
10101804020 22/03/2012<br />
AKL<br />
10101804027 22/03/2012<br />
RAJAWALI Uric Acid (Enzymatic<br />
Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
RAJAWALI LDL Cholesterol (Direct<br />
Enzymatic Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
RAJAWALI Iron (Photometric<br />
Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
RAJAWALI Triglycerides (Enzymatic<br />
Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
10101804024 22/03/2012 RAJAWALI TIBC HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
10101804017 22/03/2012<br />
AKL<br />
10101804018 22/03/2012<br />
PDF Creator - PDF4Free v3.0<br />
RAJAWALI GPT (ALAT) (Liquid UV<br />
Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
RAJAWALI HDL Cholesterol (Direct<br />
Enzymatic Colometric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
http://www.pdf4free.com
AKL<br />
10101804019 22/03/2012<br />
RAJAWALI HDL Cholesterol<br />
(Precipitant and Standard) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20303804112 22/03/2012 SD BIOLINE Leptospira IgG/IgM STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20206804113 20/03/2012 SD BIOLINE FOB (Multi) STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10101804060 13/07/2012 ABX Pentra LDL Direct CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101804064 05/06/2012 ABX PENTRA P Control HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101804051 13/07/2012 ABX Pentra Cholesterol CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101804055 13/07/2012 ABX Pentra Phosphorus CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101804054 13/07/2012 ABX Pentra Iron CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101804058 13/07/2012 ABX Pentra HDL Direct CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101804088 30/04/2012<br />
AKL<br />
10101804087 30/04/2012<br />
AKL<br />
20208804079 22/03/2012<br />
AKL<br />
20208804080 22/03/2012<br />
AKL<br />
10101804082 22/03/2012<br />
AKL<br />
10101804081 22/03/2012<br />
LYPHOCHECK Urine Metal Control<br />
Level 2<br />
LYPHOCHECK Urine Metal Control<br />
Level 1<br />
LIQUICHEK Hematology Control (A)<br />
High<br />
LIQUICHECK Hematology Control<br />
(A) Normal<br />
LIQUICHECK Blood Gas Plus E<br />
Control Level 1<br />
LIQUICHECK Blood Gas Plus E<br />
Control Level 2<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20103805176 08/08/2012<br />
AKL<br />
20305804077 06/07/2012 a-SHIELD CRP latex test Kit<br />
AKL<br />
20303804076 06/07/2012 a-SHIELD RA Latex Test Kit<br />
AKL<br />
10303804078 12/06/2012<br />
INTEC ADVANCED QUALITY One<br />
Step THC / Cannabinoid Test card INTEC PRODUCTS INC., CHINA PT. MITRASAMAYA SEJATI<br />
a - Shield Diagnostics ASO Latex<br />
Test Kit<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A DIVISION OF LAB 21<br />
HEALTHCARE LIMITED, UK LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK A division of LAB 21 HEALTHCARE<br />
LIMITED, UK<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
PT. ALAM PUTRA SAKTI<br />
AKL<br />
20207803999 13/07/2012 Antithrombin lll ROCHE/HITACHI ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20305803931 19/06/2012<br />
AKL<br />
20305803929 19/06/2012<br />
AKL<br />
20305803930 21/06/2012<br />
Anti-HBc Elecsys and Cobas e<br />
Analyzers<br />
Anti-HBs Elecsys and Cobas e<br />
Analyzers<br />
HBsAg Confirmatory Test Elecsys<br />
and Cobas e Analyzers<br />
AKL<br />
20305803928 19/06/2012 ROCHE Cardiac M Myoglobin<br />
AKL<br />
20305803882 13/07/2012<br />
AKL<br />
20305803883 28/06/2012<br />
AKL<br />
20305803877 12/07/2012<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
APOBT Tina-Quant [a] Apoliprotein<br />
B ver.2 Cobas Integra Cobasc<br />
System ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AAT2 Tina-Quant [a] a 1-Antitrypsin<br />
ver.2 COBAS INTEGRA cobas c<br />
systems<br />
Ferritin Elecsys and Cobas e<br />
Analyzer<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305803881 13/07/2012<br />
AKL<br />
20305803874 28/06/2012<br />
AKL<br />
20101803932 28/06/2012<br />
APOAT Tina-Quant [a] ApoliproteinA-<br />
1ver.2Cobas Integra Cobasc System ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
FERR2 Ferritin Gen.2 COBAS<br />
INTEGRA<br />
COMBUR TEST (pH, Protein,<br />
Glucose)<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20102803937 28/06/2012 Roche/Hitachi 902 and Accessories<br />
AKL<br />
20101803926 07/05/2012 CREA Roche/Hitachi<br />
AKL<br />
20101803879 28/06/2012<br />
GLUC2 Glucose HK COBAS<br />
INTEGRA Cobas c Systems<br />
AKL<br />
20101803875 21/06/2012 T4 Elecsys and Cobas e Analyzers<br />
AKL<br />
20101803871 04/07/2012<br />
AKL<br />
10303803878 08/06/2012<br />
AKL<br />
10303211728 04/06/2012<br />
AKL<br />
20305211931 19/06/2012<br />
HITACHI HIGH TECHNOLOGIES CORP.,<br />
JAPAN untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOST<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
LACT2 Lactate Gen.2 Cobas Integra<br />
Cobas c Systems ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
ASO Antistreptolysin O COBAS<br />
INTEGRA<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
HUMAN EBV (Epstein-Barr-Virus)<br />
IgM ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PreciControl Anti-HBs Elecsys and<br />
cobas e analyzer<br />
AKL<br />
10101803927 07/05/2012 GGT Roche/Hitachi<br />
AKL<br />
10101803880 08/06/2012<br />
TRIGL Triglycerides COBAS<br />
INTEGRA cobas e Systems<br />
AKL<br />
10101803873 08/06/2012 Precinorm PUC Roche Systems<br />
AKL<br />
10101803872 08/06/2012<br />
AKL<br />
10101803869 31/05/2012<br />
AKL<br />
10101803870 31/05/2012<br />
UA2 Uric Acid ver.2 COBAS<br />
INTEGRA Cobasc Systems<br />
LIPC Lipase Colorimetric COBAS<br />
INTEGRA Cobas c System<br />
PHOS2 Phosphate (Inorganic) Ver.2<br />
COBAS INTEGRA Cobas c Systems<br />
AKL<br />
10101803868 08/06/2012 Precipath PUC Roche Systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101803923 30/05/2012 DIALAB Cystatin C Calibrator series DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20208803842 13/07/2012 ADVIA 120/ADVIA 2120i OPTIpoint<br />
AKL<br />
20205803850 13/07/2012<br />
ADVIA 120 ADVIA 2120i Diff<br />
Timepac<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC,<br />
USA melalui SIEMNES AG, GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20205803846 13/07/2012<br />
ADVIA 120 ADVIA 2120i CBC<br />
TIMEPAC<br />
AKL<br />
10204803844 10/07/2012 ADVIA 120 ADVIA 2120/ Defoamer<br />
AKL<br />
10204803843 10/07/2012 ADVIA120 ADVIA 2120i EzKleen<br />
AKL<br />
20101803820 22/05/2012 LH Elecsys and cobas e analyzer<br />
AKL<br />
20101803819 07/05/2012 T3 Elecsys and Cobas e Analyzers<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101803818 19/06/2012<br />
AKL<br />
20101803817 19/06/2012<br />
AKL<br />
20101803816 19/06/2012<br />
AKL<br />
20101803813 19/06/2012<br />
HCG Stat CalSet Elecsys and Cobas<br />
e Analyzers<br />
T-Uptake Calset Elecsys and Cobas<br />
e Analyzers<br />
T-Upteka Elecsys and Cobas e<br />
Analyzers<br />
FT3 CalSet Elecsys and Cobas e<br />
Analyzers<br />
AKL<br />
10101803809 07/05/2012 Fe Roche/Hitachi<br />
AKL<br />
20101803811 19/06/2012<br />
AKL<br />
20101803812 07/05/2012<br />
AKL<br />
20101803814 19/06/2012<br />
AKL<br />
20101803810 07/05/2012<br />
CK-MB Calset Elecsys and Cobas e<br />
Analyzers<br />
LH Calset ll Elecsys and Cobas e<br />
Analyzers<br />
CK-MB Elecsys and Cobas e<br />
Analyzers<br />
Troponin T CalSet Elecsys and<br />
Cobas eAnalyzers<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101803807 13/07/2012 DIAGON Urea(UV) DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803806 13/07/2012<br />
AKL<br />
10101803808 13/07/2012<br />
DIAGON ALKALINE<br />
PHOSPHATASE DIAGON KFT., HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
DIAGON-<br />
GLUTAMILTRANSERASE(-GT) DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803805 13/07/2012 DIAGON ALBUMIN DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20209803854 15/08/2012<br />
J.MITRA anti-AB Monoclonal<br />
AntiBodies J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20306803664 04/06/2012 AFP Elecsys and Cobas e Analyzers<br />
AKL<br />
20305803732 08/06/2012 REFLOTRON Hemoglobin<br />
AKL<br />
20207803671 12/07/2012 FRUC Fructosamine Roche/Hitachi<br />
AKL<br />
20207803678 04/07/2012<br />
AKL<br />
20208803648 21/06/2012<br />
AKL<br />
20103803652 08/06/2012<br />
AT (Antithrombin) COBAS<br />
INTEGRA Cobas c Systems<br />
Precimat Chromogen Roche<br />
Systems<br />
Opiates II ONLINE DAT<br />
Roche/Hitachi<br />
AKL<br />
20101803736 08/06/2012 REFLOTRON Alkal. Phosphatase<br />
AKL<br />
20101803735 08/06/2012 REFLOTRON CK<br />
AKL<br />
20101803730 06/07/2012 REFLOTRON Bilirubin<br />
AKL<br />
20101803679 08/06/2012<br />
CO2-L (Bicarbonate Liquid) COBAS<br />
INTEGRA Cobas c Systems<br />
AKL<br />
20101803676 08/06/2012 TP (Total Protein) Roche/Hitachi<br />
AKL<br />
20101803729 08/06/2012 REFLOTRON GOT (AST)<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101803674 08/06/2012<br />
P-AMYL (Pancreatic a -Amylase<br />
Liquid) Roche/Hitachi<br />
AKL<br />
20101803669 08/06/2012 U/CSF Protein Roche/Hitachi<br />
AKL<br />
20101803663 12/07/2012 ISE Compensator Roche/Hitachi<br />
AKL<br />
20101803665 04/06/2012<br />
Troponin T Elecsys and Cobas e<br />
Analyzers<br />
AKL<br />
20101803657 19/06/2012 BIL-T Roche/Hitachi<br />
AKL<br />
20101803654 04/07/2012<br />
ALB Plus Roche/Hitachi Albumin<br />
BCG Method<br />
AKL<br />
20101803653 08/06/2012 c.f.a.s CK-MB Roche Systems<br />
AKL<br />
20101803647 04/07/2012<br />
AKL<br />
20101803646 13/07/2012<br />
AKL<br />
20101803644 13/07/2012<br />
AKL<br />
20101803645 13/07/2012<br />
ACP Acid Phosphatase<br />
Roche/Hitachi<br />
AKL<br />
10101803733 04/06/2012 REFLOTRON Triglycerides<br />
AKL<br />
20101803643 19/06/2012<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
Free ² hcG Calset Elecsys and<br />
Cobase Analyzer ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
Free ² hcG Elecsys and Cobase<br />
Analyzer ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
PAPP-A Calset Elecsys and Cobase<br />
Analyzers ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
PAPP-A Elecsys and Cobas e<br />
Analyzers<br />
AKL<br />
10101803734 12/06/2012 REFLOTRON Cholesterol<br />
AKL<br />
10101803731 19/06/2012 REFLOTRON Precinorm U<br />
AKL<br />
10101803680 07/05/2012<br />
NH3L Ammonia COBAS INTEGRA<br />
Cobas c systems<br />
AKL<br />
10101803728 12/06/2012 REFLOTRON HDL Cholesterol<br />
AKL<br />
10101803675 04/06/2012<br />
AKL<br />
10101803677 04/06/2012<br />
AKL<br />
10101803672 01/05/2012<br />
AKL<br />
10101803673 22/05/2012<br />
AKL<br />
10101803667 05/06/2012<br />
AKL<br />
10101803666 14/06/2012<br />
CHOL2 Cholesterol Gen.2 COBAS<br />
INTEGRA Cobas c Systems<br />
GGT-2 y-Glutamyl-Transferase ver.2<br />
COBAS INTEGRA cobas c systems<br />
PRECICONTROL Anti-HCV Elecsys<br />
and Cobas e analyzers<br />
CHOL Cholesterol CHOD-PAP<br />
Roche/Hitachi<br />
Precipath U Roche<br />
SystemsROCHEPrecipathURocheSy<br />
stem<br />
Precinorm Fructosamine Roche<br />
Systems<br />
AKL<br />
10101803662 05/06/2012 Precipath Protein Roche Systems<br />
AKL<br />
10101803659 05/06/2012 Precinorm U Roche Systems<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
10101803642 22/05/2012<br />
AKL<br />
10101803658 13/07/2012<br />
PreciControl Maternal Care Elecsys<br />
and cobas e analizers<br />
AKL<br />
10101803656 19/06/2012 ACCUTREND Triglycerides<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
Lipoprotein(a) Tina-Quant[a]<br />
Roche/Hitachi ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20208803564 13/07/2012<br />
ADVIA 120 TESTPOINT<br />
Hematology Control Normal<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG, GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20208803563 02/08/2012<br />
ADVIA 120 TESTPOINT<br />
Hematology Control High<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20208803562 13/07/2012<br />
AKL<br />
20205803566 26/07/2012<br />
AKL<br />
20101803573 26/07/2012<br />
AKL<br />
20101803568 26/07/2012<br />
ADVIA 120 SETpoint Hematology<br />
Calibrator<br />
RAPIDPOINT 400 Critical Care<br />
Analyzer and Accessories<br />
RAPIDPOINT 400 Measurement<br />
Cartridge<br />
RAPIDPOINT 405 Measurement<br />
Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD.,UK melalui<br />
SIEMENS AG, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD.,UK melalui<br />
SIEMENS AG, GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305212269 13/07/2012 SEBIA Minicap Immunotyping Kit SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10101803567 19/06/2012<br />
AKL<br />
20306803620 13/07/2012<br />
RAPIDSYSTEMS Automatic QC<br />
Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
S100 Calset Elecsys and cobas e<br />
analyzers ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20306803623 13/07/2012 S100 Elecsys and Cobas e Analyzer ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20306803594 04/06/2012<br />
AKL<br />
20306803619 13/07/2012<br />
AKL<br />
20306803593 04/06/2012<br />
AKL<br />
20305803628 06/07/2012<br />
AKL<br />
20101803617 04/06/2012<br />
Total PSA Calset ll Elecsys and<br />
Cobas e Analyzers<br />
PreciControl S100 Elecsys and<br />
cobas e analyzers<br />
Total PSA Calset ll Elecsys and<br />
Cobas e Analyzers<br />
Roche CARDIAC Control Myoglobin<br />
Cardiac Reader<br />
Total P1NP Calset Elecsys and<br />
Cobas e Analyzers<br />
AKL<br />
20101803627 08/06/2012 C.f.a.s PUC Roche Systems<br />
AKL<br />
20101803592 28/06/2012<br />
COMBUR TEST (Specific Gravity,<br />
pH, Leukocytes, Nitrite, Protein,<br />
Glucose, Ketones, Urobilinogen,<br />
Bilirubin, Blood)<br />
AKL<br />
20101803609 19/06/2012 ROCHE Control-Test M<br />
AKL<br />
10208803616 22/05/2012<br />
AKL<br />
10204803612 05/06/2012<br />
AKL<br />
10101803629 22/05/2012<br />
AKL<br />
10101803615 22/05/2012<br />
Diluent MultiAssay Elecsys and<br />
Cobas e analyzers<br />
ProbeWash M Modular Analytics<br />
E170 and Cobas e 601 analyzer<br />
C-Peptide Elecsys and Cobas e<br />
analyzers<br />
PRECICONTROL MultiAnalyte<br />
Elecsys and cobas e analyzer<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101803621 04/06/2012 Precinorm U Plus Roche Systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10101803626 11/07/2012 Precipath U Plus Roche Systems ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101803605 21/03/2012<br />
AKL<br />
20101803606 20/03/2012<br />
AKL<br />
20101803604 21/03/2012<br />
GIESSE DIAGNOSTICS Calcium<br />
OCPC GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Direct<br />
Bilirubin GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS CK-NAC<br />
SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
20101803602 20/03/2012 GIESSE DIAGNOSTICS Albumin GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
20101803601 21/03/2012<br />
AKL<br />
20101803600 21/03/2012<br />
AKL<br />
20101803599 21/03/2012<br />
AKL<br />
20101803598 20/03/2012<br />
AKL<br />
10101803603 20/03/2012<br />
AKL<br />
10101803607 20/03/2012<br />
AKL<br />
20101803579 25/05/2012<br />
AKL<br />
10101803576 04/06/2012<br />
GIESSE DIAGNOSTICS Total<br />
Bilirubin (B) GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS LDH-P<br />
(SL) GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Total<br />
Proteins GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Alkaline<br />
Phosphatase SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Gamma<br />
GT - SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Precise<br />
Norm ControlPRECISENORM GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
ABBOTT Clinica lChemistry<br />
Urine/CSF Protein Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System<br />
Testosterone Reagent pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20101803583 22/05/2012<br />
AKL<br />
20101803586 10/07/2012<br />
AKL<br />
20303803581 22/05/2012<br />
ABBOTT AXSYM Testosterone<br />
Standard Calibrators<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry LDH<br />
Lactate Dehydrogenase ABOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Toxo IgG<br />
Calibrators ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20303803582 22/05/2012 ABBOTT AXSYM Toxo IgG Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
10303803480 16/04/2012<br />
AKL<br />
20305803474 30/05/2012<br />
AKL<br />
10302803477 07/05/2012<br />
AKL<br />
20101803470 22/03/2012<br />
AMPLICOR Chlamydia Trachomatis<br />
Detection Kit (CT MWP DK)<br />
AMPLICOR HCV Amplification Kit<br />
ver.2 (HCV AMP)<br />
AMPLICOR STD Swab Specimen<br />
Collection and Transport Kit<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL GMBH.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL GMBH.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
RAJAWALI Alpha Amylase<br />
(Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803471 21/03/2012 RAJAWALI GOT (ASAT) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803467 22/03/2012<br />
AKL<br />
20101803469 22/03/2012<br />
RAJAWALI Bilirubin D + T<br />
(Photometric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
RAJAWALI Albumin (Colorimetric<br />
Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803463 21/03/2012 RAJAWALI CK-MB (NAC-act) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803464 21/03/2012 RAJAWALI CK (NAC-act) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803465 22/03/2012<br />
RAJAWALI Calcium (Photometric<br />
Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803468 22/03/2012<br />
RAJAWALI Alkaline Phosphatase<br />
(AP) (Colorimetric est) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803461 21/03/2012 RAJAWALI Creatinine HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
10101803466 22/03/2012<br />
AKL<br />
20101803459 22/03/2012<br />
RAJAWALI Cholesterol (Enzymatic<br />
Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
RAJAWALI Acid Phosphatase (AcP)<br />
(Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20101803460 20/03/2012 RAJAWALI Glucose HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101803462 22/03/2012<br />
RAJAWALI y-GT (GGT)<br />
(Colorimetric Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
AKL<br />
20306803554 22/05/2012 ABBOTT CA 125 Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20306803550 22/05/2012 ABBOTT CA 125 Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101803549 04/06/2012 ABBOTT FSH Control ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101803521 07/05/2012<br />
AKL<br />
10208211249 30/04/2012<br />
AKL<br />
20101803523 07/05/2012<br />
ABBOTT AXYSYM B12 Standard<br />
Calibrators<br />
ABBOTT AXSYM B12 Specimen<br />
Diluent<br />
ABBOTT AXSYM Folate Reagent<br />
Pack<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
AKL<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
20101803519 07/05/2012 ABBOTT AXSYM B12 Reagent pack IRELAND<br />
AKL<br />
10208211251 30/04/2012<br />
ABBOTT AXSYM Folate Specimen<br />
Diluent<br />
AKL<br />
20101803516 07/05/2012 ABBOTT Folate Lysis Reagent<br />
AKL<br />
20101803514 07/05/2012<br />
AKL<br />
10208211248 30/04/2012<br />
AKL<br />
10101803518 30/04/2012<br />
ABBOTT AXSYM Folate Standard<br />
Calibrators<br />
ABBOTT AXSYM Folate RBC<br />
Protein Diluent<br />
ABBOTT AXSYM Folate Medium<br />
Control<br />
AKL<br />
10101803520 30/04/2012 ABBOTT AXSYM B12 Controls<br />
AKL<br />
10101803524 30/04/2012<br />
AKL<br />
10102213079 24/08/2012<br />
ABBOTT AXSYM Folate Low and<br />
High Controls<br />
EPPENDORF Research Plus (Fixed<br />
Volume) Pipette<br />
AKL<br />
10102803400 19/06/2012 COMBI Scan 100<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION,<br />
IRELAND<br />
EPPENDORF AG., GERMANY melalui<br />
EPEENDORF ASIA PACIFIC SDN., BHD.,<br />
MALAYSIA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
20102212620 15/08/2012<br />
PALIO 100 Clinical Chemistry<br />
Analyzer and Accessories<br />
AKL<br />
20101803412 07/05/2012 ARCHITECT Tacrolimus Calibrators<br />
AKL<br />
20101803411 07/05/2012<br />
AKL<br />
20101803410 01/05/2012<br />
ARCHITECT Tacrolimus Reagent<br />
Kit<br />
ARCHITECT Tacrolimus Whole<br />
Blood Precipitation Reagent<br />
AKL<br />
20101803407 07/05/2012 ARCHITECT Sirolimus Calibrators<br />
AKL<br />
20101803408 07/05/2012 ARCHITECH Sirolimus Reagent Kit<br />
AKL<br />
20101803365 18/12/2012 BLUE CROSS HCG Strip<br />
AKL<br />
20101803364 18/12/2012 BLUE CROSS HCG Card<br />
AKL<br />
20305803354 30/04/2012<br />
AKL<br />
20305803353 30/05/2012<br />
AKL<br />
20101803356 07/05/2012<br />
Transferin ver.2 Tina-Quant<br />
Roche/Hitachi<br />
COBAS Amplicor HCV Detection Kit,<br />
version 2.0 (HCV DK)<br />
ROCHE CARDIAC READER and<br />
accessories<br />
ISE S.r.l., ITALY untuk SCLAVO<br />
DIAGNOSTICS INTERNASIONAL S.r.l., ITALY PT. BUANA MITRA SUKSES<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION., USA<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION., USA<br />
FUJIREBIO DIAGNOSTICS, USA for<br />
ABBOTT LABORATORIES., USA<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION., USA<br />
FUJIREBIO DIAGNOSTICS INC., USA untuk<br />
ABBOTT LABORATORIES DIAGNOSTICS<br />
DIVISION., USA<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101803350 22/05/2012 ADVIA CENTAUR Calibrators D<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20207803336 15/08/2012 HUMAN Glycohemoglobin HbA1 HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303803333 08/06/2012 HUMAN Humatex Toxo HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101803340 08/06/2012 HUMAN Albumin Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101803339 15/08/2012 HUMAN LDH SCE Mod. LiquiUV HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101803338 15/08/2012 HUMAN Chloride Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101803341 13/06/2012 HUMAN Iron Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101803337 15/08/2012 HUMAN Calcium Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305803343 18/12/2012 BLUE CROSS HbsAg Test Strip<br />
AKL<br />
20305803277 13/07/2012<br />
AKL<br />
20303803275 06/07/2012<br />
AKL<br />
20305803268 06/07/2012<br />
IMMULITE System TIE Total IgE<br />
Control<br />
IMMULITE /IMMULITE 1000 System<br />
RUM Rubella IgM<br />
IMMULITE /IMMULITE 1000<br />
Systems HBS HBsAg<br />
AKL<br />
20101803269 19/06/2012 IMMULITE 2000 Systems HCG<br />
AKL<br />
20101803267 19/06/2012<br />
AKL<br />
20101803262 12/07/2012<br />
AKL<br />
20101803264 10/07/2012<br />
AKL<br />
20101803260 10/07/2012<br />
AKL<br />
10101803261 13/07/2012<br />
SIEMENS 2000 Systems TSH Third<br />
Generation TSH<br />
IMMULITE/IMMULITE 1000 System<br />
HCG<br />
IMMULITE 2000 Systems COR<br />
Cortisol<br />
IMMULITE 200 Systems FOL Folic<br />
Acid<br />
IMMULITE 2000 System TES Total<br />
Testosteron<br />
AKL<br />
20208803315 30/04/2012 D-Dimer Calibrator Roche Systems<br />
AKL<br />
20208803316 30/04/2012 D-Dimer Control l/ll Roche Systems<br />
AKL<br />
20208803314 30/04/2012 PRECICHROM I/II Roche/Hitachi<br />
AKL<br />
20101803325 03/05/2012 LDH (LDH Optimized) Roche/Hitachi<br />
AKL<br />
20101803324 07/05/2012 CA Roche/Hitachi<br />
AKL<br />
20101803323 03/05/2012<br />
AKL<br />
20101803313 03/05/2012<br />
AKL<br />
20101803312 03/05/2012<br />
CK-MB (Creatine Kinase-MB Liquid)<br />
Roche/Hitachi<br />
ROCHE Cardiac T Quantitative<br />
Troponin T<br />
NSE Calset Elecsys and Cobas e<br />
Analyzers<br />
AKL<br />
20101803322 07/05/2012 AST (ASAT/GOT) Roche/Hitachi<br />
AKL<br />
20101803320 16/04/2012<br />
AKL<br />
20101803310 16/04/2012<br />
ALP Alkaline Phosphatase Optimized<br />
Roche/Hitachi<br />
SHBG CalSet Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20101803309 30/04/2012 CK Roche/Hitachi<br />
PDF Creator - PDF4Free v3.0<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LIMITED, UK melalui SIEMENS<br />
AG, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ABHIMATA MANUNGGAL<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
10101803329 30/04/2012 Mg Magnesium Roche/Hitachi<br />
AKL<br />
10101803321 30/04/2012<br />
AKL<br />
10101803319 01/05/2012<br />
AKL<br />
10101803308 01/05/2012<br />
AKL<br />
10101803311 30/04/2012<br />
AKL<br />
20101803305 13/07/2012<br />
AKL<br />
20101803301 13/07/2012<br />
AKL<br />
20101803300 13/07/2012<br />
ALT (ALAT/GPT) Alanine<br />
Aminotransferase acc to IFCC<br />
Roche/Hitachi<br />
LDL-C Plus (LDL-Cholesterol 2nd<br />
Generation) Roche/Hitachi<br />
ROCHE Cardiac Control Troponin T<br />
Cardiac Reader<br />
SHBG Elecsys and Cobas e<br />
Analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
ELITECH Triglycerides standard 200<br />
mg/dl SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
ELITECH Uric Acid Standard 6<br />
mg/dl SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
ELITECH Creatinine standard 2<br />
mg/dl SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101803298 13/07/2012 ELITECH Urea Standard 50 mg/dl SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101803295 10/07/2012 ELITECH Iron Chromazural SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101803296 10/06/2012 ELITECH Magnesium Calmagite SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101803292 06/07/2012 ELITECH HDL Cholesterol SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101803291 23/05/2012 AVICO Strip UjiKehamilan Pribadi<br />
AKL<br />
20305803342 18/12/2012 BLUE CROSS Anti Hbs Strip<br />
AKL<br />
20303803283 05/06/2012<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., P.R. CHINA<br />
BLUE CROSS BIO-MEDICAL (BEIJING) CO.<br />
LTD., P.R. CHINA<br />
PT. AVICO INTERNATIONAL<br />
INDONESIA<br />
PT. ABHIMATA MANUNGGAL<br />
ABBOTT AXSYM CMV IgG Reagent<br />
Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101803290 01/05/2012 ABBOTT FSH Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20305803281 01/05/2012<br />
ABBOTT AXSYM Hbe 2.0 Reagent<br />
Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20101803961 13/07/2012 DIAGON Bilirubin Direct DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803964 13/07/2012 DIAGON Glucose PAP DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803962 13/07/2012 DIAGON Bilirubin Total DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803960 13/07/2012 DIAGON Creatinine DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803959 13/07/2012 DIAGON Total Protein DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803954 13/07/2012 DIAGON AST/GOT DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101803965 13/07/2012 DIAGON Cholesterol HDL DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101803956 10/07/2012 DIAGONAL ALT/ GPT DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101803963 10/07/2012 DIAGON Triglycerides DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101803955 10/07/2012 DIAGON Cholesterol PAP DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
10101803957 10/07/2012 DIAGON Uric Acid DIAGON KFT, HUNGGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20101803229 07/05/2012<br />
AKL<br />
20101803227 07/05/2012<br />
AKL<br />
20101803226 07/05/2012<br />
AKL<br />
20101011225 07/05/2012<br />
AKL<br />
10101803234 30/04/2012<br />
ABBOTT Clinical Chemistry CO2C<br />
(Carbon Dioxide) Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Bilit<br />
(Total Bilirubin) Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Bil D<br />
(Direct Bilirubin) Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry TP<br />
(Total Protein)Reagent ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry GGT<br />
(Gamma_glutamyl Transferase)<br />
Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101803220 07/05/2012 ABX PENTRA Calcium CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101803218 07/05/2012 ABX PENTRA Albumin CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20306803191 04/07/2012 IMMULITE 2000 Systems PSA<br />
AKL<br />
20303803200 28/06/2012<br />
AKL<br />
20101803199 04/07/2012<br />
AKL<br />
20101803194 04/07/2012<br />
IMMULITE /IMMULITE 1000<br />
Systems CMV IgG<br />
IMMULITE 2000 System TU Thyroid<br />
Uptake<br />
IMMULITE 2000 Vitamin VB Vitamni<br />
B12<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS, USA<br />
AKL<br />
SIEMENS MEDICAL SOLUTIONS<br />
20101803187 04/07/2012 IMMULITE 2000 System F$ Free T4 DIAGNOSTICS, USA<br />
AKL<br />
10208803198 19/06/2012 IMMULITE 2000 System MD1<br />
AKL<br />
10208803197 19/06/2012 IMMULITE 2000 Systems MD2<br />
AKL<br />
10204212298 13/07/2012<br />
AKL<br />
10101803186 19/06/2012<br />
AKL<br />
20306803138 30/04/2012<br />
AKL<br />
20306803137 21/03/2012<br />
AKL<br />
20306803133 09/03/2012<br />
AKL<br />
20306803136 21/03/2012<br />
ADVIA CENTAUR Acid/Base<br />
Reagent<br />
IMMULITE 2000 System PRG<br />
Progesterone<br />
CEA CalSet Elecsys and cobas e<br />
analyzers<br />
Free PSA CalSet Elecsys and cobas<br />
e analyzers<br />
CYFRA 21-1 Elecsys and cobas e<br />
analyzers<br />
Free PSA Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20306803129 30/04/2012 CEA Elecsys and cobas e analyzers<br />
AKL<br />
20306803131 22/03/2012<br />
AKL<br />
20101803173 09/03/2012<br />
AKL<br />
20101803174 09/03/2012<br />
CYFRA 21-1 CalSet Elecsys and<br />
Cobas e analyzers<br />
C.f.a.s Lipids Calibrator for<br />
Automated Systems<br />
Preciset Serum Proteins Roche<br />
Systems<br />
AKL<br />
20101803175 09/03/2012 PRECIBIL lRoche Systems<br />
AKL<br />
20305803165 09/03/2012 RF II Tina-quant [a] Roche/Hitachi<br />
AKL<br />
20101803163 09/03/2012<br />
ALP Roche/Hitachi Alkaline<br />
Phosphatase Liquid acc. to IFCC<br />
AKL<br />
20101803167 09/03/2012 GLU Gluco-quant Roche / Hitachi<br />
AKL<br />
20101803166 09/03/2012 TP Total Protein Roche / Hitachi<br />
AKL<br />
20101803156 21/03/2012 REFLOTRON Urea<br />
AKL<br />
20101803158 20/03/2012 REFLOTRON Creatinine<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101803152 20/04/2012 REFLOTRON K<br />
AKL<br />
20101803154 21/03/2012 REFLOTRON Pancreatic Amylase<br />
AKL<br />
20101803150 20/03/2012 REFLOTRON Glucose<br />
AKL<br />
20101803139 21/03/2012<br />
FSH CalSet ll Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20101803135 22/03/2012 FT4 Elecsys and cobas e analyzers<br />
AKL<br />
10101803171 22/03/2012<br />
AKL<br />
20101803132 03/05/2012<br />
Tg Roche/Hitachi Triglycerides GPO-<br />
PAP<br />
T4 Calset Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20101803127 21/03/2012 FT3 Elecsys and cobas e analyzers<br />
AKL<br />
20101803130 22/03/2012<br />
AKL<br />
20101803134 22/03/2012<br />
AKL<br />
10101803170 22/03/2012<br />
FT4 CalSet Elecsys and cobas e<br />
analyzers<br />
T3 CalSet Elecsys and cobas e<br />
analyzer<br />
UA Pus Roche/Hitachi Uric Acid<br />
Plus<br />
AKL<br />
10101803168 22/03/2012 Mg Magnesium Roche/Hitachi<br />
AKL<br />
10101803169 22/03/2012 Lp (a) Control Set Roche Systems<br />
AKL<br />
10101803153 09/03/2012 REFLOTRON GPT (ALT)<br />
AKL<br />
10101803140 21/03/2012 FSH Elecsys cobas e analyzers<br />
AKL<br />
10101803151 09/03/2012 REFLOTRON GGT<br />
AKL<br />
10101803155 09/03/2012<br />
AKL<br />
10101210806 14/03/2012<br />
AKL<br />
10101210807 14/03/2012<br />
AKL<br />
20207803185 09/03/2012<br />
AKL<br />
10101803182 12/03/2012<br />
AKL<br />
10101803183 14/03/2012<br />
AKL<br />
10101803180 12/03/2012<br />
AKL<br />
10101803181 12/03/2012<br />
PDF Creator - PDF4Free v3.0<br />
REFLOTRON Uric Acid<br />
ReflotronUricAcid<br />
LIQUICHEK Cardiac Markers Plus<br />
Control LT Level 2<br />
LIQUICHEK Cardiac Markers Plus<br />
Control LT Level 1<br />
VARIANT ll Hemoglobin A1c<br />
Program Reorder Pack<br />
LIQUICHEK Ethanol/Ammonia<br />
Control Level 1<br />
LIQUICHEK Pediatric Control Level<br />
2<br />
LIQUICHEK Ethanol/Ammonia<br />
Control Level 3<br />
LIQUICHEK Ethanol/Ammonia<br />
Control Leve l2<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO
AKL<br />
10101803148 14/03/2012<br />
AKL<br />
20101803146 20/03/2012<br />
LIQUICHEK Pediatric Control Level<br />
1<br />
BD Vacutainer Serum Plus Blood<br />
Collection Tubes<br />
AKL<br />
10302803148 20/02/2012 BD BACTEC Plus Anaerobic/F<br />
AKL<br />
10302803149 20/02/2012<br />
BD BACTEC Plus Aerobic /F Culture<br />
Vials<br />
AKL<br />
10302803145 20/03/2012 BD BBL CultureSwab Plus<br />
AKL<br />
10302803143 20/02/2012<br />
BD BACTEC PEDS PLUS /F<br />
Culture Vials<br />
AKL<br />
10302803142 21/03/2012 BD BBL Columbia Agar base<br />
AKL<br />
10302803144 20/03/2012 BD BBL Blood Agar Base<br />
AKL<br />
10302803141 21/03/2012 BD BBL Crystal ANR<br />
AKL<br />
20301803125 09/03/2012<br />
AKL<br />
20301803126 20/02/2012<br />
AKL<br />
20301803124 20/02/2012<br />
AKL<br />
20301803122 09/03/2012<br />
AKL<br />
20301803123 20/02/2012<br />
AKL<br />
20301803121 09/03/2012<br />
AKL<br />
20301803120 20/02/2012<br />
AKL<br />
20301803119 20/02/2012<br />
AKL<br />
20301803118 20/02/2012<br />
AKL<br />
20101803117 20/02/2012<br />
AKL<br />
20301803115 09/03/2012<br />
AKL<br />
20301803114 09/03/2012<br />
BD BBL SENSI-DISC Meropenem<br />
10 ug<br />
BD BBL SENSI-DISC Penicilin 10<br />
IU/IE/UI<br />
BD BBL SENSI-DISC Ciprofloxacin<br />
5 ug<br />
BD BBL SENSI-DISC Nalidixid 30<br />
ug<br />
BD BBL SENSI-DISC Cefotaxime 30<br />
ug<br />
BD BBL SENSI-DISC Ampicillin /<br />
Sulbactam 20 ug<br />
BD BBL SENSI-DISC Cefoperazone<br />
75 ug<br />
BD BBL SENSI-DISC Cefepime 30<br />
ug<br />
BD BBL SENSI-DISC Norfloxacin 10<br />
ug<br />
BD MICROTAINER Tubes with K2E<br />
(K2EDTA)<br />
BD BBL SENSI-DISC Gentamicin 10<br />
ug<br />
BD BBL SENSI-DISC Amoxicillin /<br />
Clavulanic Acid 30 ug<br />
AKL<br />
10303803116 20/02/2012 BD BACTEC MycoPrep Kit<br />
AKL<br />
20306803108 30/04/2012<br />
AKL<br />
20101803111 09/03/2012 BM - Lactate<br />
PDF Creator - PDF4Free v3.0<br />
PRECICONTROL Tumor Marker<br />
Elecsys and cobas e analyzers<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
COPAS FOR BECTON DICKINSON AND<br />
COMPANY, USA melalui BECTON<br />
DICKINSON HOLDINGS PTE. LTD.,<br />
SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20101803109 09/03/2012 ACCUTREND Glucose<br />
AKL<br />
10101803110 09/03/2012 ACCUTREND Cholesterol<br />
AKL<br />
10101803105 09/03/2012<br />
PRECIPATH HDL / LDL-C Roche<br />
Systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20306803066 20/03/2012 ADVIA Centaur CA 125 ll Calibrator<br />
AKL<br />
20306803062 20/03/2012<br />
ADVIA Centaur CEA<br />
(Carcinoembryonic Antigen)Ready<br />
Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103803064 22/03/2012 ADVIA Centaur PHNB ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103803067 21/06/2012<br />
ADVIA Centaur TOBR (Tobramycin)<br />
Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103803074 20/03/2012 ADVIA Centaur CARB ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103803061 22/03/2012 ADVIA Centaur DGTN ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101803063 20/03/2012 ADVIA Centaur CKMB ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101803069 20/03/2012 SIEMENS CAL Calibrator Q<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101803071 20/03/2012 SIEMENS CAL Calibrator E<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
20306210873 20/03/2012 ADVIA Centaur CA 19-9 Ready Pack INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101803073 20/03/2012 SIEMENS QC Ligand Plus 1, 2, 3<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204803075 20/03/2012 ADVIA Centaur Wash 1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101803068 20/03/2012 ADVIA Centaur PRL ReadyPack<br />
AKL<br />
20303803078 13/07/2012<br />
AKL<br />
20303803079 13/07/2012<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
HUMATEX Febrile Antigens<br />
Salmonella Paratyphi CO HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMATEX Febrile Antigens<br />
Salmonella Paratyphi BO HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20207803082 13/07/2012 HUMAN Hemoglobin Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303803077 13/07/2012<br />
HUMAN Humatex Febrile Antigens<br />
Salmonella Paratyphi BH HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303803076 13/07/2012<br />
HUMAN Humatex Febrile Antigens<br />
Salmonella Paratyphi CH HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20206803083 13/07/2012 HUMAN Hexagon OBScreen HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101803081 13/06/2012 HUMAN FSH ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101803080 13/07/2012 HUMAN Combina Glucose HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101803085 15/08/2012 HUMAN Humalyte Reagent Pack HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205802571 12/03/2012<br />
SYSMEX Automated Hematology<br />
Analyzer XT-1800i<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20205802573 12/03/2012<br />
SYSMEX Automated Hematology<br />
Analyzer KX-21<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20205802572 12/03/2012<br />
SYSMEX Automated Hematology<br />
Analyzer XT-2000i<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20306802794 22/03/2012<br />
ADVIA Centaur PSA Prostate<br />
Specific Antigen ReadyPack<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20306802800 22/03/2012 ADVIA Centaur aPSA ReadyPack<br />
AKL<br />
20306802566 31/05/2012<br />
IMMULITE 1000 Systems fPS Free<br />
PSA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305802717 22/03/2012 ADVIA Centaur aTPO Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305802795 22/03/2012 ADVIACCentaur FER Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103802790 22/03/2012<br />
ADVIA Centaur GENT Reagent<br />
ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305802569 31/05/2012<br />
IMMULITE 1000 Systems aHB Anti<br />
HBs<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103802789 22/03/2012 ADVIA Centaur DIG ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802801 22/03/2012 ADVIA Centaur T3 ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802953 22/03/2012 ADVIA Centaur T4 ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802799 22/03/2012 ADVIA Centaur ThCG ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802796 22/03/2012 ADVIA Centaur TSH-3 ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802791 22/03/2012<br />
ADVIA Centaur TUp Reagent<br />
ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802716 22/03/2012 ADVIA Centaur FT3<br />
AKL<br />
20101802565 28/06/2012<br />
IMMULITE /IMMULITE 1000<br />
Systems iPT Intact PTH<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101802793 09/04/2012 ADVIA Centaur PRGE Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101802798 20/03/2012 ADVIA CENTAUR FSH Ready pack<br />
AKL<br />
10101802568 19/06/2012<br />
IMMULITE / IMMULITE 1000<br />
System PGN Progesterone<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101802788 20/03/2012 ADVIA Centaur E2 - 6 Ready Stock<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101802787 09/04/2012 ADVIA Centaur LH Ready Pack<br />
AKL<br />
20207802700 22/05/2012 TECO Factor XI Deficient Plasma<br />
AKL<br />
20207802701 22/05/2012 TECO Factor XII Deficient Plasma<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20207802697 16/04/2012 TECO Factor VIII Deficient Plasma<br />
AKL<br />
20207802699 22/05/2012 TECO Factor X Deficient Plasma<br />
AKL<br />
20207802698 22/05/2012 TECO Factor IX Deficient Plasma<br />
AKL<br />
20207802694 16/04/2012 TECO Factor II Deficient Plasma<br />
AKL<br />
20207802696 16/04/2012 TECO Factor Vll Deficient Plasma<br />
AKL<br />
20207802695 16/04/2012 TECO Factor V Deficient Plasma<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
TECO MEDICAL INSTRUMENTS<br />
PRODUCTION + TRADING GMBH.,<br />
GERMANY<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103802751 21/03/2012 QUICKSCREEN THC Dipstick Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103802750 21/03/2012 QUICKSCREEN Opiate Dipstick kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103802748 20/03/2012<br />
AKL<br />
20103802745 20/03/2012<br />
QUICKSCREEN Metamphetamine<br />
Cassette Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Benzodiazepine<br />
Cassette kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103802746 21/03/2012 QUICKSCREEN Opiate Cassette kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103802747 20/03/2012<br />
AKL<br />
20103802744 20/03/2012<br />
AKL<br />
20103802743 21/03/2012<br />
AKL<br />
20103802742 21/03/2012<br />
AKL<br />
20103802741 21/03/2012<br />
AKL<br />
20103802740 21/03/2012<br />
AKL<br />
20303802730 04/07/2012<br />
QUICKSCREEN Metamphetamine<br />
Dipstcik Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Benzodiazepine<br />
Dipstick kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Barbiturate Dipstick<br />
Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Barbiturate<br />
Cassette kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Amphetamine<br />
Dipstick kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
QUICKSCREEN Amphetamine<br />
Cassette kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
HUMAN Humatex Febrile Antigens<br />
Salmonella Typhi H HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306802737 13/07/2012 HUMAN CEA ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20103802734 05/06/2012 HUMAN Humadrug Opiates (OPI) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303802732 05/06/2012<br />
HUMAN HSV (Herpes Simplex<br />
Virus) IgM ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303802643 02/05/2012 HUMAN Rubella IgM ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802739 13/07/2012 HUMAN Cortisol ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802736 05/06/2012 HUMAN TSH ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802735 05/06/2012 HUMAN Creatinine Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303211752 05/06/2012<br />
HUMAN Humatex Febrile Antigens /<br />
Salmonella Typhi O HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802647 04/07/2012 HUMAN Bilirubin D+T Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802645 04/07/2012<br />
AKL<br />
20101802648 04/07/2012<br />
AKL<br />
20101802642 02/05/2012<br />
HUMAN Combina 10 M (Specific<br />
Gravity, Leucocytes, Nitrite, pH,<br />
Protein, Glucose, Ketones,<br />
Urobilinogen, Bilirubin Blood) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN a-Amylase Liquicolor<br />
Humazym Test HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN Combina 3 (pH, Protein,<br />
Glucose) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802644 04/07/2012 HUMAN Urea LiquiUV HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802639 02/05/2012 HUMAN T3 ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101802640 02/05/2012 HUMAN T4 ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101211287 02/05/2012 HUMAN Estradiol ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101802646 20/03/2012 HUMAN LH ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802718 13/07/2012 ELITECH Creatinine PAP SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101802726 06/07/2012 ELITECH Triglycerides SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101802661 02/02/2012<br />
ONE TOUCH SURESTEP Test<br />
Strip<br />
LIFESCAN INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101802660 02/02/2012<br />
ONE TOUCH SURESTEP Complate<br />
Blood Glucose Monitoring System<br />
and Accessories<br />
AKL<br />
20101802658 09/02/2012 ONE TOUCH HORIZON Test Strip<br />
LIFESCAN INC., division of JOHNSON &<br />
JOHNSON INTERNATIONAL., USA<br />
LIFESCAN SCOTLAND LTD., UK untuk<br />
LIFESCAN INC., USA<br />
AKL<br />
20101802656 09/02/2012 ONE TOUCH ULTRA Test Strip LIFESCAN, UK untuk LIFESCAN INC., USA<br />
AKL<br />
20101802657 20/03/2012<br />
AKL<br />
10101802653 20/03/2012<br />
AKL<br />
10101802649 20/03/2012<br />
AKL<br />
10101802652 20/03/2012<br />
ONE TOUCH HORIZON Blood<br />
Glucose Monitoring System and<br />
Accesories<br />
SURESTEP Hospital Glucose<br />
Control Solution High<br />
SURESTEP Hospital Glucose<br />
Control Solution Normal<br />
SURESTEP Hospital Glucose<br />
Control Solution Low<br />
AKL<br />
20101802715 28/02/2012 DSI Calcium CPS FS<br />
AKL<br />
20101802714 28/02/2012 DSI Glucose Hexokinase FS<br />
AKL<br />
20208802562 22/03/2012<br />
DIAGON D-CHECK 18 Hematologi<br />
Control<br />
LIFESCAN INC., USA<br />
LIFESCAN INC., USA<br />
LIFESCAN INC., USA<br />
LIFESCAN INC., USA<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
GERMANY<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
DIAGON LTD., HUNGARY<br />
AKL<br />
20208802561 22/03/2012 DIAGON D-CHECK SYS DIAGON LTD., HUNGGARY<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DEC HEALTHCARE<br />
INDONESIA<br />
PT. DEC HEALTHCARE<br />
INDONESIA<br />
AKL<br />
10303802003 06/07/2012 OXOID Staphylase test OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302802000 06/07/2012 OXOID Gelatin Bacteriological OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302212113 06/07/2012 OXOID Agar Technical (Agar No. 3) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801999 22/06/2012 OXOID Half Fraser Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801998 22/06/2012<br />
OXOID Campylobacter Selective<br />
Supplement (Skirrow) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801996 22/06/2012 OXOID Horse Serum OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801992 22/06/2012<br />
AKL<br />
10303801960 06/07/2012<br />
AKL<br />
10302801961 10/07/2012<br />
AKL<br />
10302801962 10/07/2012<br />
AKL<br />
10302801959 06/07/2012<br />
AKL<br />
20303801813 06/01/2012<br />
AKL<br />
20303801814 06/01/2012<br />
AKL<br />
20303801812 06/01/2012<br />
AKL<br />
20303801811 06/01/2012<br />
OXOID Pseudomonas CN<br />
Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID TST-RLPA Toxin Detection<br />
Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Yersenia Selective<br />
Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Streptoccus Selective<br />
Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID SET-RPLA Toxin Detection<br />
Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
ARCHITECT CMV IgG Avidity<br />
Reagent Kit<br />
ARCHITECT CMV IgG Avidity<br />
Calibrator and Controls<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
ARCHITECT Toxo IgG Avidity<br />
Calibrator and Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ARCHITECT Toxo IgG Avidity<br />
Reagent Kit ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20306801653 01/11/2012 IDL TPS Elisa IDS Biotech AB., SWEDEN<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
AKL<br />
10101210986 27/03/2012 HUMAN Serodos HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101801413 28/02/2012<br />
AKL<br />
20303801396 22/03/2012<br />
HUMAN Combina 9 SG (Specific<br />
Gravity, Nitrite, Ph Protein, Glucose,<br />
Ketones, Urobilinogen, Bilirubin HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN VZV (Varicella-Zoster Virus)<br />
IgG HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303801397 20/03/2012 HUMAN Measles IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303801403 20/03/2012 HUMAN Mumps Virus IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102801395 20/03/2012 HUMAPETTE Fixed HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801334 07/05/2012 ELITech Creatinine Jaffe SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801333 07/05/2012 ELITech Urea UV SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801332 07/05/2012 ELITech Glucose PAP SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801329 21/06/2012 ELITech AST/GOT 4+1 SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801327 21/06/2012 ELITech ALP (DEA) SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101801340 30/04/2012 ELITech Uric Acid Mono SL SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10103801337 30/04/2012 ELITech Cholinesterase SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801325 21/06/2012 ELITech Albumin (ALB) SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101801335 30/04/2012 ELITech Triglycerides Mono SL New SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101801338 30/04/2012 ELITech Cholesterol SL SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101801331 14/06/2012 ELITech ALT/GPT 4+1 SL SEPPIMS.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101801346 06/01/2012 ARCHITECT Estradiol Calibrators<br />
AKL<br />
10101801347 11/01/2012 ARCHITECT Estradiol Controls<br />
AKL<br />
20101801344 06/01/2012 ARCHITECT Total T4 Reagent Kit<br />
AKL<br />
10101801354 06/01/2012 ARCHITECT Total T4 Controls<br />
AKL<br />
20101801352 06/01/2012<br />
AKL<br />
10101801353 06/01/2012<br />
ARCHITECT Progesteron<br />
Calibrators<br />
ARCHITECT Progesterone Reagent<br />
Kit<br />
AKL<br />
20101801355 06/01/2012 ARCHITECT Total T4 Calibrators<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101801351 06/01/2012<br />
ABBOTTARCHITECTTMProgestero<br />
neControl<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101801348 06/01/2012<br />
ABBOTTARCHITECTTMEstradiolRe<br />
agentkit<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20305801127 02/02/2012 IMTEC-Cardiolipin-Antibodies IgG HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801126 02/02/2012 IMTEC-Cardiolipin-Antibodies IgM HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801051 02/02/2012 HUMAN Dengue IgM HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801052 22/03/2012 HUMAN H.Pylori IgA ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801053 22/03/2012 HUMAN H. Pylori IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801045 02/02/2012 HUMAN Anti HCV HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801043 03/02/2012<br />
AKL<br />
20305801022 02/02/2012<br />
HUMAN Apolipoprotein A1/B<br />
Standard HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
IMTEC-Phosphatidylserine-<br />
Antibodies Combi HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801016 02/02/2012 IMTEC-RF IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20208801050 13/07/2012 HUMAN HC-Control HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801015 02/02/2012 IMTEC-Cardiolipin-Antibodies Combi HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801048 02/02/2012 HUMAN hCG HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801046 02/02/2012<br />
AKL<br />
10101801014 17/02/2012<br />
AKL<br />
20301801063 11/07/2012<br />
AKL<br />
20301801062 11/07/2012<br />
AKL<br />
20301801064 11/07/2012<br />
AKL<br />
20301801061 10/07/2012<br />
AKL<br />
20301801060 11/07/2012<br />
AKL<br />
20301801059 11/07/2012<br />
AKL<br />
20301801058 11/07/2012<br />
HUMAN Humazym M-Test Acid<br />
Phosphatase HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PRECIPATH CK-MB Roche<br />
Systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
OXOID Cefriaxone 30 mcg<br />
Antimicrobial Susceptibility discs OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Chloramphenicol 30 mcg<br />
Antimicrobial Susceptibility Discs OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Cefotaxime 30 mcg<br />
Antimicrobial Susceptibility Discs OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Erythromycin Antimicrobial<br />
Susceptibility DiscS OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Gentamycin 10 MCG<br />
Antimicrobial Susceptibility DiscS OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Kanamycin Antimicrobial<br />
Susceptibility DiscS OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Tetracycline 30 mcg<br />
Antimicrobial Susceptibility Discs OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801065 06/07/2012 OXOID Hemoglobin Powder Soluble OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801057 06/07/2012<br />
OXOID Listeria Enrichment Broth<br />
Base OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801055 22/06/2012 OXOID Egg Yolk Emulsion OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801054 06/07/2012 OXOID MRS Agar OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101800899 28/02/2012 DIALAB LDL Cholesterol Calibrator DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800897 30/05/2012<br />
DIALAB Diacal Auto (Assayed<br />
Universal Calibration Serum) DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800896 09/02/2012 DIALAB Lipoprotein (a) DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800854 12/07/2012<br />
AKL<br />
20101800853 12/07/2012<br />
AKL<br />
10102800850 06/02/2012<br />
PTS Panels Chol+HDL+Glu Panel<br />
Test Strip<br />
PTS Panels Metabolic Chemistry<br />
Panel Test Strip<br />
SFRI BSA 3000 Semi Biochemistry<br />
Analyzer<br />
POLYMER TECHNOLOGY SYSTEM INC.,<br />
USA<br />
POLYMER TECHNOLOGY SYSTEM INC.,<br />
USA<br />
SFRI Sarl., FRANCE<br />
PT. MITRAASA PRATAMA<br />
PT. MITRAASA PRATAMA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20101502898 31/05/2012 DIALAB Glucose Hexokinase DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305800834 03/02/2012<br />
IMTEC Phosphatidylserine<br />
Antibodies IgA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305800830 03/02/2012 IMTEC - RF IgA ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305800825 03/02/2012 IMTEC Cardiolipin Antibodies IgA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305800828 03/02/2012 IMTEC ANA Screen HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305800829 03/02/2012 IMTEC - RF IgM ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10305800832 02/02/2012<br />
AKL<br />
10305800833 02/02/2012<br />
AKL<br />
10305800826 02/02/2012<br />
IMTEC-B2-Glycoprotein I-Antibodies<br />
IgG HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
IMTEC-B2-Glycoprotein I-Antibodies<br />
IgM HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
IMTEC-B2-Glycoprotein I-Antibodies<br />
IgA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305800798 03/02/2012 IMTEC- dsDNA - Antibodies HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10305800789 02/02/2012 IMTEC-B- Lactoglobulin Antibody HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
30305800746 07/05/2012<br />
SIGNAL HIV Flow Through HIV 1+2<br />
Spot / Immunodot Test KitTestKit SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101800549 03/10/2012 Albumin Tina-quant Roche/Hitachi<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA
AKL<br />
10303800511 22/03/2012<br />
AKL<br />
10101800513 22/03/2012<br />
ONCOPROBE H. Pylori Rapid Test<br />
Card<br />
ONCOPROBE hCG (Pragnancy<br />
test)<br />
CAL-TECH DIAGNOSTICS INC., USA a<br />
subsidiary of CDI/BIOCHEM CORP., USA<br />
CAL-TECH DIAGNOSTICS INC / BIOCHEM<br />
CORP., USA<br />
AKL<br />
10208800476 14/02/2012 SFRI Diluterge H18 SFRI Sarl., FRANCE<br />
AKL<br />
10208800475 14/02/2012 SFRI Diluton H18 SFRI Sarl., FRANCE<br />
AKL<br />
10208800474 14/02/2012 SFRI Diluclair H18 SFRI Sarl., FRANCE<br />
AKL<br />
10208800477 14/02/2012 SFRI Lysoglobine H18 SFRI Sarl., FRANCE<br />
PT. ONCOPROBE UTAMA<br />
PT. ONCOPROBE UTAMA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
10102800407 22/05/2012 MICROLAB 300 LX VITAL SCIENTIFIC B.V., NETHERLAND PT. MRK DIAGNOSTICS<br />
AKL<br />
10302800404 08/02/2012<br />
BD Bactec 9120 Instrument and<br />
Accessories<br />
AKL<br />
10302800402 08/02/2012 BD Difco CHROMagar Orientation<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20205800306 18/01/2012 ABX Pentra 80 HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208800308 18/01/2012 ABX Minilyse HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101800305 27/01/2012 ABX Pentra CK-MB RTU HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10204800310 18/01/2012 ABX Minoclair HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208800311 18/01/2012 ABX Minilyse LMG HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10204800313 18/01/2012 ABX Miniclean HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10204800314 18/01/2012 ABX Cleaner HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208800315 18/01/2012 ABX Basolyse ll HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20208800307 18/01/2012 ABX Minocal HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208800309 18/01/2012 ABX Minidil LMG HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101503017 02/10/2012 DIALABLipaseColorimetric DIALAB GESM.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101604061 21/03/2012 DIALAB Albumin Standard DIALAB GMBH., AUTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101604060 02/08/2012 DIALAB Triglycerides Standard DIALAB GESM.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800259 30/04/2012 DIALAB Calcium CPG DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101502894 30/05/2012 DIALAB Bilirubin Auto Total DCA DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101502893 21/03/2012 DIALAB Bilirubin Direct DIALAB GMBH., AUTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800263 27/02/2012 DIALAB GOT (AST), Mod. IFCC DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800262 15/08/2012 DIALAB Urea, Colorimetric DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800266 15/08/2012 DIALAB Uric Acid DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800267 02/08/2012 DIALAB Glucose GOD - PAP DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800268 02/08/2012<br />
DIALAB Cholesterol (Total), CHOD-<br />
PAP DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800261 15/08/2012 DIALAB Protein Total DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101603968 21/03/2012 DIALAB Chloride Standard DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800260 27/02/2012 DIALAB GPT (ALT), Mod. IFCC DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800271 15/08/2012 DIALAB Creatinine Mod. Jaffe DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800277 15/08/2012 DIALAB Gamma GT DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101800282 13/07/2012 DIALAB Albumin BCG DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101800274 28/02/2012 DIALAB Cholesterol HDL Direct DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101800280 21/03/2012 DIALAB Chloride DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20103800296 16/01/2012 SD BIOLINE COC STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
30305800285 09/02/2012 SD HIV 1/2 ELISA 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20103800292 16/01/2012 SD BIOLINE MET STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20306800284 24/01/2012 SD BIOLINE PSA STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20306800291 24/01/2012 SD BIOLINE AFP STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20306800290 24/01/2012 SD BIOLINE CEA STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10101800298 24/01/2012 SD BIOLINE LH STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20103800297 16/01/2012 SD BIOLINE AMP STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20103800286 16/01/2012 SD BIOLINE MOP STANDARD DIAGNOSTIC INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305800294 30/01/2012 SD HCV ELISA 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305800295 30/01/2012 SD HBsAg ELISA 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303800300 30/01/2012 SD Syphilis Elisa 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305800299 30/01/2012 SD Anti HBs Elisa 3.0 STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20103800293 16/01/2012 SD BIOLINE THC STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303800287 24/01/2012<br />
SD BIOLINE Malaria P.F /<br />
P.VMALARIA P.f / P.v STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20305800242 22/03/2012 HEPACARD J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20305800243 22/03/2012 HCV TRI-DOT 4th Generation J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20209800240 22/03/2012 J. MITRA Bovine Albumin J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20209800241 22/03/2012<br />
AKL<br />
20209800244 22/03/2012<br />
AKL<br />
20209800245 22/03/2012<br />
AKL<br />
20305800120 05/06/2012<br />
AKL<br />
20305800117 05/06/2012<br />
J. MITRA Anti-A Monoclonal<br />
Antibodies J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
J. MITRA Anti-B Monoclonal<br />
Antibodies J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
J.MITRA Anti-D Rh1 IgM Monoclonal<br />
Antibodies J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
SEBIA Antisera K & L Standard<br />
Mask SEBIA, FRANCE PT. RIOCA MEDICA<br />
SEBIA Antisera and Fixative<br />
(GAM,K,L) Standard Mask SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10101211117 16/04/2012 SEBIA Minicap Protein (E) 6 maxi kit SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10101211115 16/04/2012 SEBIA Minicap Protein (E) 6 SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10304800116 22/05/2012 SEBIA Fluidil SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20305211116 16/04/2012 SEBIA Minicap and Accessories SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20101800061 05/06/2012 BIONIME Blood Glucose Test Strip BIONIME CORPORATION, TAIWAN PT. SEKAR GUNA MEDIKA<br />
AKL<br />
20208800073 16/01/2012 DIALINE BIOCAL lHbA1c<br />
AKL<br />
20207210077 16/01/2012 DIALINE HbA1c<br />
AKL<br />
20208800071 16/01/2012 DIALINE BIOPATH HbA1c<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE., LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE., LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE., LTD., SINGAPORE<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208800072 16/01/2012<br />
DIALINE HbA1c Hemolyzing<br />
Solution<br />
AKL<br />
20208800069 16/01/2012 DIALINE BIONORM HbA1c<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE., LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTICS<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
AKL<br />
10101800067 21/03/2012<br />
DIALINE HDL Cholesterol<br />
Precipitant<br />
AKL<br />
10101800068 21/03/2012 DIALINE Magnesium<br />
AKL<br />
20205705659 14/02/2012<br />
AKL<br />
20205705658 14/02/2012<br />
DIAGON D-Cell 60 Hematology<br />
Analyzer<br />
DIAGON D-Cell 5D Haematology<br />
Analyzer<br />
DYASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY for DIALINE DIAGNOSTICS<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
DIAGON LTD., HUNGARY<br />
DIAGON LTD., HUNGARY<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DEC HEALTHCARE<br />
INDONESIA<br />
PT. DEC HEALTHCARE<br />
INDONESIA<br />
AKL<br />
10302705492 22/06/2012 OXOID Yeast Autolysate Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302705491 22/06/2012<br />
AKL<br />
20101210445 20/02/2012<br />
AKL<br />
20101705355 20/02/2012<br />
OXOID CLED Medium (with<br />
Andrade's Indicator) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
DIMENSION EXL Integrated<br />
Chemistry System LOCI Module Flex<br />
Reagent Cartridge TNI<br />
VACUETTE Safety Blood Collection<br />
Set + Luer Adapter<br />
AKL<br />
20101705356 20/02/2012 VACUETTE Blood Conection Set<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG<br />
HEALTHCARE SECTOR, GERMANY<br />
NIPRO (THAILAND) CORPORATION<br />
LIMITED, THAILAND untuk GREINER BIO-<br />
ONE GMBH., AUSTRIA<br />
NIPRO (THAILAND) CORPORATION<br />
LIMITED, THAILAND untuk GREINER BIO-<br />
ONE GMBH., AUSTRIA<br />
PT. SIEMENS INDONESIA<br />
PT. WIDYA MITRA PERSADA<br />
PT. WIDYA MITRA PERSADA<br />
AKL<br />
10208705350 04/06/2012 MEDONIC CA 620/530 Diluent BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20208705351 07/05/2012 BOULE Cal BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
10208705349 04/06/2012 MEDONIC CA 620/930 Lyse BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
20208705122 07/05/2012<br />
BOULE Con Hematology Control<br />
Boule Con Hematology Control BOULE MEDICAL AB., SWEDEN PT. MRK DIAGNOSTICS<br />
AKL<br />
21106704864 21/03/2012 VITROLIFE G MOPS PLus VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106704862 21/03/2012 VITROLIFE G-MOPS VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106704860 21/03/2012 VITROLIFE G-IVF VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
21106704861 21/03/2012 VITROLIFE G-IVF Plus VITROLIFE SWEDEN AB., SWEDEN<br />
AKL<br />
10101214193 28/12/2012 SYSMEX UA Reagent L<br />
AKL<br />
20103704651 11/01/2012<br />
AKL<br />
20103704648 11/01/2012<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. SYSMEX INDONESIA<br />
Speedytest One Step Style PCP<br />
(Phencyclidine) Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
Speedytest One Step Cassette Style<br />
BAR (Barbiturate) Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103704645 11/01/2012<br />
AKL<br />
20103704644 11/01/2012<br />
Speedytest One Step Cassette Style<br />
PCP (Phencyclidine) Testest IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
Speedytest One Step Strip Style<br />
Cocaine (COC) Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
10302704280 22/06/2012 OXOID Extraction Enzyme OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101213739 01/11/2012 SCLAVO Gamma - GT<br />
AKL<br />
20101213655 30/10/2012<br />
AKL<br />
20305213656 30/10/2012<br />
AKL<br />
10302702978 22/06/2012<br />
BECKMAN COULTER CK-MB<br />
Calibrator<br />
BECKMAN COULTER ITA Control<br />
Serum Level 1<br />
SCLAVO DIAGNOSTICS INTERNATIONAL<br />
S.r.l., ITALY<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
PT. BUANA MITRA SUKSES<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
OXOID Compylobacter Selective<br />
Supplement (Blaster-Wang) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103704640 11/01/2012<br />
AKL<br />
20103704638 11/01/2012<br />
AKL<br />
20103704634 11/01/2012<br />
AKL<br />
20103704630 11/01/2012<br />
AKL<br />
20103704628 11/01/2012<br />
AKL<br />
20305210319 09/02/2012<br />
AKL<br />
20303210328 09/02/2012<br />
Speedytest One Step Style Ectasy<br />
(MDMA) Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
Speedytest One Step trip Style MTD<br />
(Methadone) Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
Speedytest One step Cassette Style<br />
Cocaine (COC) Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
Speedytest One Step Strip Style<br />
BAR (Barbiturate) Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
Speedytest One Step Cassette Style<br />
MTD (Methadone) Test IND DIAGNOSTIC INC, Canada PT. LANIROS DIAN PHARMA<br />
Tg CalSet Elecsys and cobas e<br />
analyzers<br />
COBAS 4800 CT / NG Amplification<br />
/ Detection Kit<br />
AKL<br />
20101704624 01/02/2012 MULTICARE Glucose Test Strip<br />
AKL<br />
10101704622 09/02/2012 MULTICARE Cholesterol Strips<br />
AKL<br />
20101704621 01/02/2012<br />
MULTICARE Meter (Glucose,<br />
Cholesterol, Triglycerides)<br />
AKL<br />
10101704623 09/02/2012 MULTICARE Triglycerides Strip<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
BIOCHEMICAL SYSTEM INTERNATIONAL<br />
S.r.l., ITALY<br />
BIOCHEMICAL SYSTEM INTERNATIONAL<br />
S.r.l., ITALY<br />
BIOCHEMICAL SYSTEMS INTERNATIONAL<br />
S.r.l., ITALY<br />
BIOCHEMICAL SYSTEM INTERNATIONAL<br />
S.r.l., ITALY<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
PT. ISOTEKINDO INTERTAMA<br />
AKL<br />
20305702503 17/02/2012 SEBIA Hydragel 4 Bence Jones SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20305702498 17/02/2012 SEBIA Hydragel 1 Bence Jones SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20305702496 30/05/2012 SEBIA Hydragel Bence Jones K20 SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20101214116 12/12/2012 BECKMAN COULTER AST<br />
AKL<br />
20303214132 26/12/2012 SD Bioline Dengue NS1 Ag<br />
AKL<br />
20305214129 26/12/2012 SD Bioline HCV<br />
AKL<br />
20303214128 26/12/2012 SD Bioline Dengue Duo<br />
AKL<br />
20305214131 26/12/2012 SD Bioline Anti - HBs<br />
AKL<br />
20305214130 26/12/2012 SD Bioline HBsAg<br />
AKL<br />
10102214092 30/11/2012<br />
AKL<br />
20101801368 09/02/2012<br />
AKL<br />
20101801367 31/01/2012<br />
AKL<br />
20207701228 20/03/2012<br />
AKL<br />
20207701230 20/03/2012<br />
AKL<br />
20207701231 20/03/2012<br />
BIO-RAD PR40 - Microplate<br />
Washerand Accessories<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
BIO-RAD LABORATORIES, FRANCE melalui<br />
BIO-RAD LABORATORIES PTE,. LTD,.<br />
SINGAPORE<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
ABBOTT Clinical Chemistry Albumin<br />
BCP ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Albumin<br />
BCG Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
SEBIA Hydrogel Acid (E)<br />
Hemoglobin (E) K-20 BEBIA, France PT. RIOCA MEDICA<br />
SEBIA Hydrogel 15 Acid (E)<br />
Hemoglobin (E) BEBIA, France PT. RIOCA MEDICA<br />
SEBIA Hydrogel 7 Acid (E)<br />
Hemoglobin (E) SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20101801369 09/02/2012 ABBOTT Clinical Chemistry Amylase ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101801371 09/02/2012 ABBOTT Clinical Chemistry Calcium ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10102701071 11/07/2012<br />
AKL<br />
20303214187 28/12/2012<br />
BIOHIT Proline Mechanical Single-<br />
Channel Variable Pipettes BIOHITOVJ, Finland PT. MULTI SARANA MEDIKA<br />
SD BIOLINE Malaria Ag p.f/Pan<br />
POCT<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
PT. ALERE HEALTH<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303214184 28/12/2012 SD BIOLINE Influenza Antigen<br />
AKL<br />
20305214185 28/12/2012 SD BIOLINE HBeAg<br />
AKL<br />
10101808088 19/12/2012 SYSMEX T-Bil Reagent A<br />
AKL<br />
20101213804 08/11/2012 BECKMAN COULTER CK-MB<br />
AKL<br />
20101213805 08/11/2012<br />
BECKMAN COULTER Calcium<br />
oCPC<br />
AKL<br />
20305212242 13/07/2012 DIASOURCE Anti - HCV Elisa V 4.0<br />
AKL<br />
20303214186 28/12/2012 SD BIOLINE Malaria P.f/P.v<br />
AKL<br />
20305214083 30/11/2012 MONALISA Anti - HBc Plus<br />
AKL<br />
20305214085 30/11/2012 MONALISA HCV Ag-Ab Ultra<br />
AKL<br />
20305214082 30/11/2012<br />
AKL<br />
30305214232 28/12/2012<br />
AKL<br />
20303214084 30/11/2012<br />
MONALISA Anti - HCV Plus Version<br />
2<br />
COBAS HIV Combi PT Elecsys and<br />
Cobas e Analyzers<br />
BIO-RAD Syphilis EIA II total<br />
Antibody<br />
AKL<br />
20101213621 29/10/2012 CAL ToxAmmonia Calibrator<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
SYSMEX INTERNATIONAL REAGENT CO.<br />
LTD., JAPAN melalui SYSMEX ASIA PASIFIC<br />
PTE. LTD., SINGAPORE<br />
BECKMAN COULTER INC., USA melalui<br />
BECKMAN COULTER SINGAPORE PTE.<br />
LTD., SINGAPORE<br />
BECKMAN COULTER INC., USA melalui<br />
BECKMAN COULTER SINGAPORE PTE.<br />
LTD., SINGAPORE<br />
DIASOURCE IMMUNO ASSAYS S.A.,<br />
BELGIUM<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE., LTD.,<br />
SINGAPORE<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE., LTD.,<br />
SINGAPORE<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE., LTD.,<br />
SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
LAB21 HEALTHCARE LTD., UK untuk BIO-<br />
RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
MICROGENICS CORP, USA untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS INC., USA<br />
melalui SIEMENS AG., GERMANY<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. SYSMEX INDONESIA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ALERE HEALTH<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101213628 29/10/2012 ABX Pentra Bilirubin Total CP HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20205213618 29/10/2012 ADVIA 120/2120i CSF Reagent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101213620 29/10/2012<br />
ADVIA Chemistry AMM Ammonia<br />
Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101213615 29/10/2012 FAST Liquick Cor - Calcium PZ. CORMAY SA., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101213614 29/10/2012 FAST Liquick Cor - ALP PZ. CORMAY SA., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
20101213657 30/10/2012 FAST Liquick Cor - Amylase PZ. CORMAY SA., POLAND PT. SAPTA LARONA MUDA<br />
AKL<br />
10101213668 30/10/2012 SEIMITSU Lipid Control<br />
AKL<br />
10102213609 24/10/2012<br />
AKL<br />
10101213611 24/10/2012<br />
AKL<br />
10102213610 24/10/2012<br />
AKL<br />
20101213608 24/10/2012<br />
AKL<br />
20101213607 24/10/2012<br />
AKL<br />
20102213606 24/10/2012<br />
AKL<br />
20101213605 24/10/2012<br />
FUREIYA CO. LTD., JAPAN melalui YUSHI-<br />
SEIHIN CO. LTD., JAPAN<br />
PT. SEIMITSU DIAGNOSTICS<br />
DIRUI-800 Automated Urine<br />
Analyzer and accessories DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
DIRUI A10 Reagent Strips for<br />
Urinalysis<br />
DIRUI INDUSTRIAL CO., LTD., P.R. CHINA<br />
PT. ENSEVAL MEDIKA PRIMA,<br />
JAKARTA<br />
DIRUI-100 Urine Analyzer and<br />
Accessories DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
DIRUI H11 Reagent Strips for<br />
Urinalysis DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
DIRUI H14-Ca Reagent Strips for<br />
Urinalysis DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
DIRUI FUS-100 Urine Sediment<br />
Analyzer and Accessories<br />
AKL<br />
20101214188 28/12/2012 SD BIOLINE hCG<br />
PDF Creator - PDF4Free v3.0<br />
DIRUI INDUSTRIAL CO., LTD., P.R. CHINA<br />
PT. ENSEVAL MEDIKA PRIMA,<br />
JAKARTA<br />
DIRUI H13-Cr Reagent Strips for<br />
Urinalysis DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
PT. ALERE HEALTH<br />
http://www.pdf4free.com
AKL<br />
20207210896 21/03/2012<br />
AKL<br />
20305214081 30/11/2012 MONALISA Anti - HBsAg Ultra<br />
AKL<br />
10302604116 22/03/2012<br />
ALERE INRatio 2 PT/INR<br />
Profesional Testing Kit ALERE SAN DIEGO INC., USA PT. MEDQUEST JAYA GLOBAL<br />
BD BBL SENSI-DISC Designer<br />
Dispenser<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE., LTD.,<br />
SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101603969 02/08/2012 DIALAB Protein Total Standard DIALAB GESM.B.H., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101011577 11/07/2012<br />
BD Vacutainer plus sodium heparin (<br />
NH ) 95 USP unit plus blood<br />
collection tubes<br />
AKL<br />
20101807626 29/11/2012 ARCHITECT FSH Calibrators<br />
BECTON DICKINSON AND COMPANY , USA<br />
melalui BECTON DICKINSON AND<br />
COMPANY ,Singapore<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, CO., IRELAND<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101801970 22/03/2012 ADVIA Centaur VB - 12<br />
AKL<br />
10302504850 28/06/2012 BD Universal Viral Transport System<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON CO.,<br />
SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20205213619 29/10/2012<br />
AKL<br />
20101010249 29/11/2012<br />
AKL<br />
10101807839 29/11/2012<br />
AKL<br />
10101807812 31/10/2012<br />
AKL<br />
10101807799 29/11/2012<br />
AKL<br />
20103213648 30/10/2012<br />
ADVIA 120/2120/2120i TESTpoint<br />
CSF Controls<br />
BD VACUTAINER Lithium Heparin<br />
(LH) Plus Blood Collection Tubes<br />
ABBOTT ARCHITECT FSH<br />
Reagent Kit<br />
ABBOTT AXSYM System Total B-<br />
hCG Controls<br />
ABBOTT ARCHITECT FSH<br />
Controls<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
BECTON DICKINSON AND COMPANY (BD).,<br />
USA melalui BECTON DICKINSON<br />
HOLDINGS PTE., LTD., SINGAPORE<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, CO., IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, CO., IRELAND<br />
PT. SIEMENS INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AIM Drugtest Uri Met-Amphetamine<br />
Strip VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20301807789 03/10/2012 OXOID Novobiocin (NV 30) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807790 03/10/2012 OXOID Ticarcillin - TIC75 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807593 29/11/2012 OXOID Clarithromycin - CLR 15 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10204905043 22/05/2012 SYSMEX CA Clean<br />
AKL<br />
20209213651 30/10/2012<br />
Anti-A/ Anti-B/ Anti-A,B/ Anti-D/<br />
Control ORTHO BioVue System<br />
Cassette<br />
AKL<br />
20101213649 30/10/2012 SENTINEL Clin Chem Cal<br />
SYSMEX INTERNATIONAL REAGENTS CO.,<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA<br />
ORTHO-CLINICAL DIAGNOSTICS Inc., USA<br />
untuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
SENTINEL CH, SpA., ITALY distribusi oleh<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SYSMEX INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103213647 30/10/2012 AIM Drugtest Uri Cocaine Strip VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20101010625 11/07/2012<br />
BD Vacutainer PST Gel and Lithium<br />
Heparin (LH) 83 Units Plus Blood<br />
Collection Tubes<br />
BECTON DICKINSON & COMPANY, USA<br />
melalui BECTON DICKINSON & COMPANY,<br />
SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101213629 29/10/2012 GlucoDr Blood Glucose Test Meter ALMEDICUS CO. LTD., KOREA PT. MEDISINDO BAHANA<br />
AKL<br />
20101213650 30/10/2012 SENTINEL Pancreatic Amylase<br />
SENTINEL CH, SpA., ITALY distribusi oleh<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10102500596 28/12/2012 SEBIA Hydragel Double IF K20 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500599 28/12/2012 SEBIA Hydragel IF K20 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20301807794 03/10/2012 OXOID Cefoperazone OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807791 03/10/2012 OXOID Cloxacillin (OB5) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807464 03/10/2012 OXOID Sulphonamide (S3-300) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305214087 30/11/2012<br />
GLOBAL'S Rapid Test for Detection<br />
of Total Antibodies to HCV in Serum /<br />
Plasma / Whole Blood<br />
QUALPRO DIAGNOSTICS., INDIA untuk<br />
GLOBAL INVITRO LLP., UK<br />
PT. BESTARI SUKSES MAKMUR<br />
AKL<br />
20305214086 30/11/2012<br />
AKL<br />
20303214088 30/11/2012<br />
AKL<br />
10102214079 30/11/2012<br />
GLOBAL'S Rapid Test for Detection<br />
of IgM Antibodies to S.Typhi in Serum ZEPHYR BIOMEDICALS., INDIA untuk<br />
/ Plasma / Whole Blood<br />
GLOBAL INVITRO LLP., UK<br />
GLOBAL'S Rapid Test for Malaria<br />
Pan/Pf<br />
BIO-RAD PR 4100 Absorbance<br />
Microplate Reader and Accessories<br />
ZEPHYR BIOMEDICALS., INDIA untuk<br />
GLOBAL INVITRO LLP., UK<br />
TECAN AUSTRIA GMBH., AUSTRIA untuk<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
10302214036 29/11/2012<br />
MICROSCAN HNID Indole Reagent<br />
H - IND<br />
AKL<br />
20102214078 30/11/2012 EVOLIS System and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
STRATEE BIOMEDICAL SYSTEM AG.,<br />
GERMANY untuk BIO-RAD, FRANCE melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20205214074 30/11/2012 AMP Accos 380 AMEDA LABORATORIES GMBH., AUSTRIA PT. SETIA GUNA MEDIKA<br />
AKL<br />
20103805177 15/08/2012 INTEC One Step Opiate Test Card INTEC PRODUCTS INC., CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
10302801184 10/07/2012<br />
AKL<br />
10302801191 13/07/2012<br />
AKL<br />
20101213626 29/10/2012<br />
AKL<br />
20301801179 11/07/2012<br />
OXOID Listeria Primary Selective<br />
Enrichment Supplement (UVMI) OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
OXOID VCNT Selective Supplement<br />
SR 91 E OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
LDH Roche/Hitachi Lactate<br />
Dehydrogenase Liquid acc. to IFCC<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
OXOID Penicillin G Acid<br />
Antimicrobial Susceptibility Discs (P<br />
10) OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
21106213631 29/10/2012 COOK MEDICAL Holding Pipette<br />
AKL<br />
21106213633 29/10/2012<br />
AKL<br />
21106213632 29/10/2012<br />
AKL<br />
20101907138 28/06/2012<br />
AKL<br />
20101213144 28/08/2012<br />
AKL<br />
21106213634 29/10/2012<br />
COOK MEDICAL Micro-Injection<br />
Pipette<br />
COOK MEDICAL Flexipet Denuding<br />
Pipette<br />
COOK VASCULAR INCORPORATED (CVI),<br />
USA<br />
COOK VASCULAR INCORPORATED (CVI),<br />
USA<br />
COOK VASCULAR INCORPORATED (CVI),<br />
USA<br />
BECTON DICKINSON & COMPANY, USA<br />
BD Vacutainer Buffered Sodium melalui BECTON DICKINSON CON & CO.,<br />
Citrate: (9NC) Blood Collection Tubes SINGAPORE<br />
NEOBASE Non-derivatized MSMS<br />
Kit<br />
COOK MEDICAL Flexipet<br />
Manipulation Pipette<br />
AKL<br />
20103213625 20/10/2012 Preciset TDM I Roche Systems<br />
AKL<br />
10201214091 30/11/2012 BD PREPSTAIN Cytology Stain Kit<br />
AKL<br />
10302214080 30/11/2012 BIO-RAD IPS Microplate Incubator<br />
WALLAC OY., FINLAND untuk PERKIN<br />
ELMER INC., USA melalui PERKIN ELMER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
COOK VASCULAR INCORPORATED (CVI),<br />
USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
TRIPATH IMAGING., USA untuk BECTON<br />
DICKINSON & COMPANY (BD)., USA melalui<br />
BECTON DICKINSON HOLDING PTE. LTD.,<br />
SINGAPORE<br />
STRATEE BIOMEDICAL SYSTEM AG.,<br />
GERMANY untuk BIO-RAD, FRANCE melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
PT. PRIMA CENTRA<br />
MEDIKACITRA<br />
PT. PRIMA CENTRA<br />
MEDIKACITRA<br />
PT. PRIMA CENTRA<br />
MEDIKACITRA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. PRIMA CENTRA<br />
MEDIKACITRA<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
10302214041 29/11/2012<br />
MICROSCAN 10% Ferric Chloride<br />
TDA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302214042 29/11/2012<br />
MICROSCAN 0.8% sULFANILIC<br />
aCID nit 1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302214043 29/11/2012 MICROSCAN Kovac's Reagent<br />
AKL<br />
20208213671 30/10/2012 SEIMITSU HbA1c Calibrator<br />
AKL<br />
20101213670 30/10/2012 SEIMITSU Lipid Calibrator<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
FUREIYA CO. LTD., JAPAN melalui YUSHI-<br />
SEIHIN CO. LTD., JAPAN<br />
FUREIYA CO. LTD., JAPAN melalui YUSHI-<br />
SEIHIN CO. LTD., JAPAN<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SEIMITSU DIAGNOSTICS<br />
PT. SEIMITSU DIAGNOSTICS
AKL<br />
20101213669 30/10/2012 SEIMITSU HbA1c Control<br />
AKL<br />
20303213623 29/10/2012 NOVALISA Bordetella Pertussis IgG<br />
AKL<br />
20303213622 29/10/2012<br />
AKL<br />
20303213624 29/10/2012<br />
NOVALISA Herpes Simplex Virus 2<br />
(HSV 2) IgM<br />
NOVALISA Herpes Simplex Virus 2<br />
(HSV 2) IgG<br />
FUREIYA CO. LTD., JAPAN melalui YUSHI-<br />
SEIHIN CO. LTD., JAPAN<br />
NOVATEC IMMUNDIAGNOSTICA GMBH.,<br />
GERMANY<br />
NOVATEC IMMUNDIAGNOSTICA GMBH.,<br />
GERMANY<br />
NOVATEC IMMUNDIAGNOSTICA GMBH.,<br />
GERMANY<br />
PT. SEIMITSU DIAGNOSTICS<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. BESTARI SUKSES MAKMUR<br />
AKL<br />
20101213630 29/10/2012 EZ-hCG One Step Pregnancy Test BIOMERICA INC., USA PT. MITRA ASA PRATAMA<br />
AKL<br />
20208213358 02/10/2012<br />
DIAZYME SMART HbA1c ssay<br />
Control Level 1.2 DIAZYME LABORATORIES, USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20206213667 30/10/2012 AIM D-DIMER-Q Rapid Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20101213663 30/10/2012<br />
AKL<br />
20205213784 01/11/2012<br />
BIL MICRO METER AUTO-3 and<br />
Accessories FUCHU GIKEN INC., JAPAN PT. SETIA ANUGERAH MEDIKA<br />
BD FACSVerse Flow Cytometer<br />
Universal Loader and Accessories<br />
AKL<br />
20303213612 24/10/2012 TYPHIDOT RAPID IgM<br />
AKL<br />
10101806195 29/11/2012 SYSMEX UA Reagent<br />
AKL<br />
20209213881 27/11/2012<br />
AKL<br />
20209213883 27/11/2012<br />
AKL<br />
20209213880 27/11/2012<br />
AKL<br />
20209213882 27/11/2012<br />
MARQUEST Quick A.B.G rterial<br />
Blood Sampler<br />
MARQUEST Gasylite A.B.G Arterial<br />
Blood Sampler<br />
MARQUEST Aspirator A.B.G Arterial<br />
Blood Sampler<br />
MARQUEST Micro A.B.G Arterial<br />
Blood Sampler<br />
BECTON DICKINSON & COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE., LTD., SINGAPORE<br />
RESZON DIAGNOSTICS INTERNATIONAL<br />
SDN. BHD., MALAYSIA<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
VITAL SIGNS COLORADO INC., USA<br />
VITAL SIGNS COLORADO INC., USA<br />
VITAL SIGNS COLORADO INC., USA<br />
VITAL SIGNS COLORADO INC., USA<br />
AKL<br />
10208213738 31/10/2012 DIAMED ID-Diluent 2 DIAMED GMBH., SWITZERLAND<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. NELTA MULTI GRACIA<br />
PT. SYSMEX INDONESIA<br />
PT. GE OPERATIONS<br />
INDONESIA<br />
PT. GE OPERATIONS<br />
INDONESIA<br />
PT. GE OPERATIONS<br />
INDONESIA<br />
PT. GE OPERATIONS<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20305213789 01/11/2012<br />
AKL<br />
20305213787 01/11/2012<br />
AKL<br />
20305213790 01/11/2012<br />
BESURE HBsAg One Step Hepatitis<br />
B Surface Antigen Rapid Test Device<br />
(Serum/Plasma)<br />
BESURE HCV Hepatitis C Virus<br />
Rapid Test Device (Serum/Plasma)<br />
BESURE HBsAg One Step Hepatitis<br />
B Surface Antigen Test Strip<br />
(Serum/Plasma)<br />
ABON BIOPHARM (HANG ZHOU) CO., LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
CONSULTING SERVICES CO. LTD., CHINA<br />
ABON BIOPHARM (HANG ZHOU) CO., LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
CONSULTING SERVICES CO. LTD., CHINA<br />
ABON BIOPHARM (HANG ZHOU) CO., LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
CONSULTING SERVICES CO. LTD., CHINA<br />
PT. GOLDEN MEDIKA MANDIRI<br />
PT. GOLDEN MEDIKA MANDIRI<br />
PT. GOLDEN MEDIKA MANDIRI<br />
AKL<br />
20305213788 01/11/2012<br />
AKL<br />
20303213786 01/11/2012<br />
AKL<br />
20303213791 01/11/2012<br />
AKL<br />
20301400631 22/03/2012<br />
AKL<br />
10302801194 13/07/2012<br />
BESURE HBsAg Hepatitis B<br />
Surface Antigen Rapid Test Device<br />
(Whole Blood/Serum/Plasma)<br />
BESURE Syphilis Ultra Rapid Test<br />
Device (Whole Blood/Serum/Plasma)<br />
BESURE Syphilis Ultra Rapid Test<br />
Strip (Whole Blood/Serum/Plasma)<br />
SD BBL SENSI-DISC Trimetthoprim<br />
5 ug<br />
ABON BIOPHARM (HANG ZHOU) CO., LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
CONSULTING SERVICES CO. LTD., CHINA<br />
ABON BIOPHARM (HANG ZHOU) CO., LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
CONSULTING SERVICES CO. LTD., CHINA<br />
ABON BIOPHARM (HANG ZHOU) CO., LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
CONSULTING SERVICES CO. LTD., CHINA<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
PT. GOLDEN MEDIKA MANDIRI<br />
PT. GOLDEN MEDIKA MANDIRI<br />
PT. GOLDEN MEDIKA MANDIRI<br />
PT. ANUGRAH ARGON MEDICA<br />
OXOID Modified Semi-Solid<br />
Rappaport Vassiliadis Selective<br />
Supplement SR 161 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801684 22/06/2012 OXOID S.I.M Medium OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301212257 13/07/2012 OXOID 0129 Discs 150 ug OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801181 06/07/2012<br />
OXOID Muller-Kauffmann-<br />
Trathionate Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301801186 13/07/2012 OXOID Sulphonamide Discs OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302214037 29/11/2012<br />
MICROSCAN Ehrlich's Reagent A-<br />
IND2<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302214035 29/11/2012<br />
MICROSCAN 0.05 N Sodium<br />
Hydoxide NaOH<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302214040 29/11/2012<br />
AKL<br />
20303214089 30/11/2012<br />
AKL<br />
20102214077 30/11/2012<br />
MICROSCAN 40% Potassium<br />
Hydroxide VP1<br />
GLOBAL'S Rapid Test for Malaria<br />
Pan/Pv/Pf<br />
EVOLIS Twin Plus System and<br />
Accessories<br />
AKL<br />
20102214075 30/11/2012 RESPONS 910 ProLine<br />
AKL<br />
20101801738 09/02/2012 GLU Roche / Hitachi<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ZEPHYR BIOMEDICALS., INDIA untuk<br />
GLOBAL INVITRO LLP., UK<br />
STRATEE BIOMEDICAL SYSTEM AG.,<br />
GERMANY untuk BIO-RAD, FRANCE melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTICS SYSTEMS GMBH.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. SETIA GUNA MEDIKA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305802053 21/03/2012<br />
AKL<br />
20207805142 15/08/2012<br />
AKL<br />
20103805182 15/08/2012<br />
AKL<br />
20103805180 15/08/2012<br />
AKL<br />
20303603423 15/08/2012<br />
AKL<br />
20305805174 15/08/2012<br />
AKL<br />
10101805198 28/09/2012<br />
AKL<br />
10101805193 28/09/2012<br />
AKL<br />
10103805194 28/09/2012<br />
AKL<br />
20101805200 04/10/2012<br />
STFR Tina-quant [a] Soluble<br />
Transferin Receptor COBAS<br />
INTEGRA cobas c systemsCOBAS<br />
INTEGRA STFR Tina<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
TECO Dimex Manual 2-Channel<br />
Instrument Mainly for D-Dimer and<br />
Accessories TECO GmbH., GERMANY PT. SETIA ANUGERAH MEDIKA<br />
INTEC One Step THC Amphetamine<br />
Test Card INTEC PRODUCTS INC., CHINA PT. MITRASAMAYA SEJATI<br />
INTEC One Step Barbiturate Test<br />
Strip<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
INTEC Advance Quality Syphilis<br />
Diagnostic (TRUST) INTEC PRODUCTS INC., CHINA PT. MITRASAMAYA SEJATI<br />
INTEC Advanced Quality Rapid Anti<br />
HBsAb Test Card INTEC PRODUCTS INC (XIAMEN)., CHINA PT. MITRASAMAYA SEJATI<br />
ADVIA Chemistry TRIG<br />
Triglycerides Reagents<br />
ADVIA Chemistry CHOL Cholesterol<br />
Reagents<br />
ADVIA Chemistry CHE<br />
Cholinesterase Reagents<br />
ADVIA Chemistry TP Total Proteinll<br />
Reagents<br />
AKL<br />
20101805199 04/10/2012 ADVIA Chemistry LDPL Reagents<br />
AKL<br />
10204807908 04/10/2012<br />
AKL<br />
10303805163 28/09/2012<br />
AKL<br />
20205805197 04/10/2012<br />
AKL<br />
20101805164 02/10/2012<br />
AKL<br />
20101805168 02/10/2012<br />
AKL<br />
20101805165 04/10/2012<br />
AKL<br />
20101805169 04/10/2012<br />
AKL<br />
20306805162 02/10/2012<br />
AKL<br />
20305805161 02/10/2012<br />
AKL<br />
20303805364 04/10/2012<br />
AKL<br />
20303805366 29/10/2012<br />
SCCS (Special cell Cleaning<br />
Solution) cobas c system<br />
IMMULITEH/IMMULITE 1000<br />
System HPG H.Pylori IgG<br />
ADVIA 120 Hematology System and<br />
Accessories<br />
IMMULITE/IMMULITE 1000 System<br />
Free Beta BCG Kit<br />
IMMULITE/IMMULITE 1000<br />
Systems OCN Osteocalcin Kit<br />
IMMULITE/IMMULITE 1000<br />
Systems PAP<br />
IMMULITE 2000 System PAA PAPP-<br />
A<br />
IMMULITE/IMMULITE 1000 System<br />
TPS<br />
IMMULITE/IMMULITE 1000 System<br />
aBC Anti-HBC Kit<br />
PLASMATEC Salmonella H<br />
paratyphi B<br />
PLASMATEC Salmonella O<br />
Paratyphi B<br />
AKL<br />
20303805367 04/10/2012 PLASMATEC Salmonella Typhi H<br />
AKL<br />
20303805365 04/10/2012<br />
AKL<br />
20303805368 04/10/2012<br />
PDF Creator - PDF4Free v3.0<br />
PLASMATEC Salmonella H<br />
Paratyphi C<br />
PLASMATEC Salmonella H<br />
Paratyphi A<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
ROCHE DIAGNOSTICS GmbH., GERMANY,<br />
melalui ROCHE DIAGNOSTIC, INTL, LTD,<br />
SWITZERLAND<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS., USA<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOTICS, UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI
AKL<br />
20303805375 29/10/2012<br />
PLASMATEC Salmonella O<br />
paratyphi C<br />
AKL<br />
20303805369 04/10/2012 PLASMATEC Salmonella Typhi O<br />
AKL<br />
20303805374 29/10/2012<br />
PLASMATEC Salmonella O<br />
paratyphi A<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10101805358 15/08/2012 ELITech Phosphorus SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101805360 15/08/2012 ELITech Iron Ferrozine SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10204212219 12/10/2012<br />
AKL<br />
20209805445 29/10/2012<br />
AKL<br />
20209805443 04/10/2012<br />
AKL<br />
20209805444 04/10/2012<br />
AKL<br />
20209805446 29/10/2012<br />
IMMULITE2000ChemiluminescentSu<br />
bstrateModule<br />
PLASMATEC Anti B Monoclonal<br />
Reagent<br />
PLASMATEC Anti D IgM Monoclonal<br />
Reagent<br />
PLASMATEC Anti A,B Monoclonal<br />
Reagent<br />
PLASMATEC Anti A Monoclonal<br />
Reagent<br />
SIEMENS MEDICAL SOLUTIONS<br />
DIAGNOSTICS PRODUCT LIMITED., UK<br />
melalui SIEMENS AG, GERMANY<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PT. SIEMENS INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303805447 20/03/2012 SD Bioline Rubella IgG/IgM STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10303805448 20/03/2012 SD Bioline Strep A STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10101805560 19/06/2012<br />
AKL<br />
20101805557 04/07/2012<br />
AKL<br />
20101805558 04/07/2012<br />
ABBOTT Clinical Chemistry Lactic<br />
Acid ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry ICT<br />
Urine Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry ICT<br />
Serum Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101805559 04/07/2012 ABBOTT Clinical Chemistry Lact Cal ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10204805573 28/09/2012 SIEMENS 348 Wash Analyzer<br />
AKL<br />
20101805571 31/10/2012<br />
SIEMENS Cal Gas Calibration<br />
Standard<br />
AKL<br />
10204805576 28/09/2012 RAPIDLAB 348 Bufferpack<br />
AKL<br />
20101805579 23/10/2012<br />
SIEMENS Slope Gas Calibration<br />
Standard<br />
AKL<br />
20101213595 23/10/2012 RAPIDLAB 348 Blood Gas ANalyzer<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS, USA<br />
AIR LIQUID HEALTHCARE AMERICA<br />
CORPORATION, USA untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS Inc., USA<br />
melalui SIEMENS AG., GERMANY<br />
SIEMENS MEDICAL SOLUTION<br />
DIAGNOSTICS, USA<br />
AIR LIQUID HEALTHCARE AMERICA<br />
CORPORATION, USA untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS Inc., USA<br />
melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING. LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305805649 20/03/2012 SD BIOLINE HBeAG STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
10208805635 15/08/2012 SYSMEX pocH-pack L (ll)<br />
AKL<br />
10208805636 15/08/2012 SYSMEX pocH-Pack D (ll)<br />
AKL<br />
20205805642 15/08/2012<br />
AKL<br />
20205805646 15/08/2012<br />
AKL<br />
20205805643 15/08/2012<br />
SYSMEX Automated Hematology<br />
Analyzer POCH-100i<br />
SYSMEX Automated Blood<br />
Coagulation Analyzer CA-50<br />
SYSMEX Automated Blood<br />
Coagulation Analyzer CA-1500<br />
SYSMEX ASIA PACIFIC PTE., LTD.,<br />
SINGAPORE<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20205805647 15/08/2012<br />
SYSMEX Automated Blood<br />
Coagulation Analyzer CA-500 Series<br />
SYSMEX CORPORATION, Japan melalui<br />
SYSMEX ASIA PACIFIC PTE.LTD., Singapore<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20205805648 15/08/2012<br />
SYSMEX Automated Hematology<br />
Analyzer XE-2100<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20305805761 22/10/2012<br />
IMMULITE Systems 3gAllergy-<br />
Specific lgE Negative Control<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305805762 05/10/2012<br />
AKL<br />
20305212196 11/07/2012<br />
SYSTEM 340 bDNA Analyzer and<br />
Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
ABBOTT Axsym System Hepatitis B<br />
Virus Core Antigen (Recombinant)<br />
CORE Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
20305805899 11/07/2012<br />
AKL<br />
20208805918 13/07/2012<br />
AKL<br />
20208805917 13/07/2012<br />
AKL<br />
20207805916 13/07/2012<br />
AKL<br />
20208805919 13/07/2012<br />
AKL<br />
20208805920 13/07/2012<br />
ABBOTT Axsym System Hepatitis B<br />
Virus Core Antigen (Recombinant)<br />
CORE Reagent Pack ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
LYPHOCHEK Hemoglobin A2<br />
Control Bilevel<br />
LIQUICHEK Hemoglobin Control (A)<br />
Level L<br />
VARIANT B Thallassemia Short<br />
Program Recorder Pack<br />
LYPHOCHEK Diabetes Control<br />
Bilevel<br />
LIQUICHEK Hematology Control (A)<br />
Trilevel<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20101211901 15/06/2012 AIM CK-MB 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20303211783 07/06/2012 ADVANTAGE Typhi IgM & IgG Card J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
10102806095 13/07/2012<br />
AKL<br />
20101806098 11/07/2012<br />
ARCHITECT c8000 System and<br />
Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Axsym System<br />
Homocystein Reagent Pack<br />
AKL<br />
10302806107 29/11/2012 BD BBL MGIT OADC<br />
AKL<br />
10302806102 18/12/2012 BBL TAXO<br />
AKL<br />
10302806110 29/11/2012<br />
BD BBL MGIT Mycobacterial Growth<br />
Indicator Tubes<br />
AKL<br />
10302806112 29/11/2012 BD BBL MGIT PANTA<br />
AKL<br />
20301806106 29/11/2012<br />
AKL<br />
20101806123 13/07/2012<br />
AKL<br />
20305806124 13/07/2012<br />
BD BBL Sensi-Disc Erythromycin E-<br />
15<br />
Folate lll Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20207806116 04/10/2012 IMMULITE 2000 Systems EPO<br />
AKL<br />
10101806114 03/10/2012<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK for<br />
ABBOTT GMBH & CO. KG., GERMANYES,<br />
USA<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GmbH, GERMANY<br />
CRPHS cobas c 111Cardiac C- malalui ROCHE DIAGNOSTICS, LTD,<br />
Reative Protein (Latex) High Sensitive SWIZERLAND<br />
IMMULITE 2000 Systems INS<br />
Insulin<br />
AKL<br />
20101806113 15/08/2012 IMMULITE 2000 Total T4<br />
AKL<br />
20102212638 15/08/2012<br />
ReLIA Immunoassay Diagnostic<br />
Instrument and Accessories<br />
AKL<br />
20305806119 15/08/2012 IMMULITE Anti TPO-Ab<br />
AKL<br />
20301806197 29/11/2012<br />
AKL<br />
20301806199 29/11/2012<br />
PDF Creator - PDF4Free v3.0<br />
BD BBL Sensi-Disc Cephalothin CF-<br />
30<br />
BD BBL Sensi-Disc Ampicillin AM-<br />
10<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
RELIA BIOTECHNOLOGIES (SHENZEN)<br />
INC., P.R. CHINA untuk RELIA DIAGNOSTIC<br />
SYSTEMS INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. ABBOTT INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. NELTA MULTI GRACIA<br />
PT. SIEMENS INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA
AKL<br />
10101806193 29/11/2012 SYSMEX ALT Reagent L<br />
AKL<br />
20101806190 29/11/2012 SYSMEX GLU Reagent L<br />
AKL<br />
10101806194 29/11/2012 SYSMEX T-CHO Reagent LA<br />
AKL<br />
20305806332 13/07/2012<br />
AKL<br />
20305806333 13/07/2012<br />
AKL<br />
20305806335 13/07/2012<br />
AEROSET/ARCHITECT Quantia<br />
Myoglobulin<br />
AEROSET/ARCHITECT Quantia<br />
IgE<br />
AEROSET/ARCHITECT Quantia<br />
Ferritin Standrad<br />
AKL<br />
10101806330 20/02/2012 ABBOTT Iron<br />
AKL<br />
10101806331 20/02/2012 ABBOTT Copper<br />
AKL<br />
20101806329 09/03/2012 ABBOTT Panreatic Amylase<br />
AKL<br />
20305806336 09/03/2012 ABBOTT Lambda Light Chains<br />
AKL<br />
10101806327 31/05/2012<br />
AKL<br />
10101806328 31/05/2012<br />
AKL<br />
10101806322 13/07/2012<br />
LYPHOCHEK Immunoassay Plus<br />
Control Trilevel<br />
LIQUICHEK Blood Gas Plus E<br />
Control Level 3<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
BIOKIT S. A., SPAIN for ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT S. A., SPAIN for ABBOTT<br />
LABORATORIES, USA<br />
BIOKIT S. A., SPAIN for ABBOTT<br />
LABORATORIES, USA<br />
SENTINEL CH S.p.A., ITALY for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
SENTINEL CH S.p.A., ITALY for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
SENTINEL CH., SpA., ITALY for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
SENTINEL CH., SpA., ITALY for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
ROCHE LACT2 cobas c 111 Lactate<br />
Gen.2 ROCHE DIAGNOSTICS GmbH, GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
10101806325 13/07/2012 ROCHE MG cobas c111 Magnesium ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20305806337 09/03/2012 ABBOOTT Kappa Light Chainds<br />
AKL<br />
20101806396 15/08/2012<br />
AKL<br />
20101806398 15/08/2012<br />
AKL<br />
20305806394 13/07/2012<br />
AKL<br />
20305806395 13/07/2012<br />
SENTINEL CH., SpA., ITALY for ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
ABBOTT Optium Point-Of-care<br />
Blood Glucose Test Strip ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
ABBOTT Optium Blood Glucose<br />
Electrodes ABBOTT DIABETES CARE LTD., UK PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System CORE-M<br />
Control<br />
ABBOTT AXSYM System CORE M-<br />
Reagent Pack<br />
AKL<br />
10201806371 29/11/2012 BD TB Quick Stain Kit<br />
AKL<br />
10201806370 29/11/2012 BD TB Stain Kit Zn<br />
AKL<br />
10201806372 29/11/2012<br />
BD Gram Stain Kit with Stabilized<br />
Iodine<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
ABBOTT IRELAND DIAGNOSTIC DIVISION.,<br />
IRELAND<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
AKL<br />
20205806472 15/08/2012 PARTEC CD4% Easy Count Kit PARTEC GMBH., GERMANY<br />
AKL<br />
20205806473 15/08/2012 PARTEC CD4 Easy Count Kit PARTEC GMBH., GERMANY<br />
AKL<br />
10101806503 03/10/2012<br />
IMMULITE System Intact PTH<br />
contro lModule<br />
AKL<br />
10101213469 05/10/2012 BECKMAN COULTER ALT<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
BECKMAN COULTER, inc, IRELAND melalui<br />
BECKMAN COULTER SINGAPORE, Pte,<br />
LTD, SINGAPORE<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. EUROP CONTINENTS<br />
INDONESIA<br />
PT. EUROP CONTINENTS<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
10101806511 05/10/2012<br />
ADVIA Chemistry LDL - Direct LDL<br />
Cholesterol Reagents<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10303806512 04/10/2012<br />
ADVIA Chemistry ASO_2-<br />
AntiStreptolysin-O_2 Reagents<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101806504 05/10/2012<br />
ADVIA Chemistry AST-AST (GOT)<br />
Reagent<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101806506 05/10/2012<br />
AKL<br />
20101806508 05/10/2012<br />
AKL<br />
20101806509 05/10/2012<br />
AKL<br />
20101806510 05/10/2012<br />
AKL<br />
20101806515 05/10/2012<br />
AKL<br />
20305806513 05/10/2012<br />
AKL<br />
20305806514 05/10/2012<br />
AKL<br />
10103213547 19/10/2012<br />
AKL<br />
20103213652 30/10/2012<br />
AKL<br />
20103806577 30/10/2012<br />
AKL<br />
20101806569 30/10/2012<br />
AKL<br />
20305806567 30/10/2012<br />
AKL<br />
20305806566 30/10/2012<br />
ADVIA Chemistry HDL/LDL<br />
Cholesterol Calibrator<br />
ADVIA Chemistry GLUCO - Glocose<br />
Oxidase PAP Reagents<br />
ADVIA Chemistry TBIL_2 Total<br />
Bilirubin 2 Reagents<br />
ADVIA Chemistry CKNAC-Creatine<br />
Kinase Reagents<br />
ADVIA Chemistry LDLPLD (L-<br />
P)Reagents<br />
ADVIA Chemistry IGA_2-IgA_2<br />
Reagents<br />
ADVIA Chemistry CRP_2-CRP_2<br />
Reagents<br />
RANDOX LABORATORIES LTD., UK untuk<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG, GERMANY<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
RANDOX LABORATORIES LTD., UK for<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
ABBOTT AXSYM System Digoxin III<br />
Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM SystemDigoxin III<br />
Calibrators ABBOTT MOLECULAR INC., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Digoxin lll<br />
Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry SPort<br />
Multi Call ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Complement C4 ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Complement C3 ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20209806585 04/10/2012 PLASMATEC Bovine Albumin 22%<br />
AKL<br />
20303806581 04/10/2012 PLASMATEC TPHA test kit<br />
AKL<br />
20303806580 29/10/2012 PLASMATEC RPR Test Kit<br />
AKL<br />
10303806584 04/10/2012 PLASMATEC ASO Latex Test Kit<br />
AKL<br />
20305806579 04/10/2012<br />
PLASMATEC Anti-Human Globulin<br />
Reagent<br />
AKL<br />
20305806582 29/10/2012 PLASMATEC CRP Latex test kit<br />
AKL<br />
20305806583 29/10/2012 PLASMATEC RA Latex test kit<br />
AKL<br />
20101806118 04/10/2012<br />
IMMULITE/IMMULITE 1000<br />
Systems TBG<br />
AKL<br />
21106806644 27/08/2012 TPC Injection Pipettes<br />
AKL<br />
21106806646 27/08/2012 TPC Holding Pipettes<br />
AKL<br />
21106806645 27/08/2012 TPC Injection Pipettes<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
PLASMATEC LABORATORY PRODUCTS<br />
LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
LTD., UK melalui SIEMENS HEALTHCARE<br />
DIAGNOSTIC LIMITED., HONGKONG<br />
THE PIPPETTE COMPANY PTY., LTD.,<br />
AUSTRALIA<br />
THE PIPPETTE COMPANY PTY., LTD.,<br />
AUSTRALIA<br />
THE PIPPETTE COMPANY PTY., LTD.,<br />
AUSTRALIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. SIEMENS INDONESIA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
AKL<br />
20209806661 30/10/2012 DIAGAST Anti-D (RH1) IgM DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209806664 23/10/2012 DIAGAST Anti-D (RH1) TOTEM DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209806662 30/10/2012 DIAGAST Anti-B (ABO2) DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209806665 30/10/2012 DIAGAST Anti-A (ABO1) DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209806663 30/10/2012 DIAGAST Anti-A,B (ABO3) DIAGAST, France PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20101806677 23/10/2012 ROCHE System Precimat Glycerol ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
AKL<br />
20101806678 28/09/2012<br />
AKL<br />
20101806679 28/09/2012<br />
PDF Creator - PDF4Free v3.0<br />
GLDH Glutamate dehydrogenase<br />
ROCHE/HITACHI ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
Total P1NP Elecsys and Cobas e<br />
analyzer ROCHE DIAGNOSTIC GmbH, Germany PT. ROCHE INDONESIA<br />
http://www.pdf4free.com
AKL<br />
10302806725 02/10/2012<br />
AKL<br />
10302806726 02/10/2012<br />
AKL<br />
10302806729 02/10/2012<br />
MERCK Anaerobic Agar acc.To<br />
Brewer MERCK KGaA., GERMANY PT. MERCK Tbk<br />
MERCK Candida Elective Agar<br />
acc.To Nickerson MERCK KGaA., GERMANY PT. MERCK Tbk<br />
MERCK BROLACIN-Agar (C.L.E.D.<br />
Agar) MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302806727 02/10/2012 MERCK Of Basal Medium MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20301806728 02/10/2012 MERCK Mueller - Hinton Broth MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20101806722 28/09/2012<br />
AKL<br />
10101806723 02/10/2012<br />
ALBU-XS Albumin Modular P<br />
Antigen Excess Reagent Tina-quant<br />
ROCHE/HITACHI ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
Cystatin C Contro lSet ROCHE<br />
Systems ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20101806724 28/09/2012 C.f.a.s Cystatin C ROCHE Systems ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20305806714 23/10/2012<br />
AKL<br />
10102806741 19/12/2012<br />
AKL<br />
10209806736 19/12/2012<br />
AKL<br />
10209806737 19/12/2012<br />
AKL<br />
10102806743 19/12/2012<br />
Versant 440 Molecular System &<br />
Spare Part<br />
EPPENDORF Reference Fixed and<br />
Accessories<br />
EPPENDORF Centrifuge and<br />
Accessories<br />
EPPENDORF Centrifuge MiniSpin<br />
Plus and Accessories<br />
EPPENDORF Reference Adjustable<br />
and Accessories<br />
AKL<br />
10209806734 19/12/2012 EPPENDORF Centrifuge 5804<br />
AKL<br />
10209806735 19/12/2012 EPPENDORF Centrifuge 5810<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
PT. SIEMENS INDONESIA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20205806748 04/10/2012 CoaguChek XS Plus + Accessories ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20101806750 28/06/2012 ZENIX Urinalysis Test Strip<br />
AKL<br />
10101803233 19/10/2012<br />
AKL<br />
10101803232 19/10/2012<br />
AKL<br />
20101803225 30/10/2012<br />
AKL<br />
10101803577 19/10/2012<br />
AKL<br />
20101803580 30/10/2012<br />
AKL<br />
20101804424 30/10/2012<br />
RAYTO LIFE AND ANALYTICAL SCIENCE<br />
CO. LTD., CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
ABBOTT Clinical Chemistry<br />
Phosphorus ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Magnesium (Mg) ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Creatine<br />
Kinase (CK) ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Iron<br />
Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry<br />
Urine/CSF Protein ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Iron/<br />
Magnesium Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101803584 19/10/2012 ABBOTT Clinical Chemistry Lipase ABOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101801373 30/10/2012<br />
ABBOTT Clinical Chemistry Urea<br />
Nitrogen ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20305807078 26/12/2012 AVITEX RF OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
AKL<br />
20305807077 26/12/2012 AVITEX CRP Latex Reagent OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
AKL<br />
20303807076 26/12/2012<br />
AKL<br />
20305807113 18/12/2012<br />
MICROPATH Salmonella O Antigen<br />
Group A OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
Signal HCV Flow Through Anti-HCV<br />
Spot Immunodot Test Kit SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20209807110 18/12/2012 SPANCLONE Anti A Monoclonal SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20208212312 13/07/2012<br />
ROCHE Cardiac Control D-Dimer<br />
Cardiac reader<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20208807260 13/07/2012 Sebia Hb A2 Normal Control SEBIA., FRANCE PT. RIOCA MEDICA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20207807262 13/07/2012<br />
Sebia Minicap Hemoglobin (E) Maxi<br />
Kit SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20207807261 13/07/2012 Sebia Minicap Hemoglobin (E) SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10303213429 04/10/2012<br />
COBAS Taqman CT Test V. 2.0<br />
(CTM CT v2.0)<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY, melalui ROCHE DIAGNOSTICS,<br />
INTL, LTD, SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305807841 13/07/2012 WANTAI HBsAg ELISA Kit<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO. LTD., P.R. CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
AKL<br />
20305807842 13/07/2012 WANTAI Anti-HCV ELISA Kit<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO. LTD., P.R. CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
AKL<br />
30305504659 31/07/2012 WANTAI Anti-HIV ELISA Kit<br />
AKL<br />
20303807893 29/11/2012<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO. LTD., P.R. CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
ARCHITECT HAVAb-IgM Reagent<br />
Kit ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20301807874 02/10/2012 OXOID Neomycin (N 30) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807639 03/10/2012 OXOID Bacitracin (B10) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303807608 21/03/2012 SD BIOLINE Rapid TB STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20301807590 03/10/2012 OXOID Pipercillin - PRL 100 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807589 03/10/2012 OXOID Tobramycin TOB 10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807588 29/11/2012 OXOID Spiramycin - SP 100 OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807586 03/10/2012 OXOID Fosfomycin (FOS50) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807585 03/10/2012 OXOID Pipemidic Acid - PIP 20 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
ROCHE DIAGNOSTICS OPERATIONS INC.,<br />
AKL<br />
20103807488 04/10/2012<br />
USA for ROCHE DIAGNOSTICS GmbH.,<br />
Total MPA Calibrator Roche Systems GERMANY<br />
AKL<br />
20103807487 04/10/2012<br />
MPA Mycophenolic Acid COBAS<br />
INTEGRA Cobas c System<br />
AKL<br />
10101808089 19/12/2012 SYSMEX D-Bil Reagent A<br />
AKL<br />
20101808090 19/12/2012 SYSMEX CK Reagent LB<br />
AKL<br />
20101808104 19/12/2012 SYSMEX ALB Reagent<br />
AKL<br />
20101808105 19/12/2012 SYSMEX Glucose-E Reagent<br />
AKL<br />
10101807477 29/11/2012 ARCHITECT Intact PTH Controls<br />
AKL<br />
20101807478 29/11/2012 ARCHITECT Intact PTH Reagent<br />
ROCHE DIAGNOSTICS OPERATIONS INC.,<br />
USA for ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
BIOKIT SA, SPAIN untuk ABBOTT GmbH &<br />
CO. KG., GERMANY<br />
BIOKIT SA, SPAIN untuk ABBOTT GmbH &<br />
CO. KG., GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20301807469 03/10/2012 OXOID Clindamycin (DA 2) OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302807473 29/11/2012<br />
OXOID Maximum Recovery Diluent<br />
(MRD) OXOID LTD., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807472 03/10/2012 OXOID Cephazolin (KZ 30) OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305807907 04/10/2012<br />
CRPL3 Tina-quant ROCHE/Hitachi<br />
(C-Reactive Protein Gen.3)<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20301807465 03/10/2012 OXOID Lincomycin (MY 2) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101808242 15/08/2012<br />
AKL<br />
20303801425 22/03/2012<br />
PDF Creator - PDF4Free v3.0<br />
IMMULITE / IMMULITE 1000<br />
Systems GAS Gastrin<br />
HUMATEX Febrile Antigen,<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
Salmonella Paratyphi AO HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
http://www.pdf4free.com
AKL<br />
10101801923 20/03/2012<br />
AKL<br />
20303801685 22/03/2012<br />
HUMAN Triglycerides Liquicolor<br />
mono HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN hUMATEX Febrile Antigen<br />
Salmonella Paratyphi AH HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101900288 19/12/2012 ONETOUCH SureStep Test Strip<br />
AKL<br />
20303805175 15/08/2012<br />
AKL<br />
20305805173 15/08/2012<br />
AKL<br />
20305805172 15/08/2012<br />
AKL<br />
30305801809 26/06/2012<br />
AKL<br />
20103801805 08/08/2012<br />
INTEC Advanced Quality One Step<br />
Syphilis (TP) Test Strip<br />
INTEC Advanced Quality Rapid<br />
HBsAG Test Card<br />
INTEC Advanced Quality Rapid<br />
HBsAg Test Strip<br />
INTEC Advanced Quality Rapid HIV<br />
Test Card<br />
LIFESCAN PRODUCTS LLC., PUERTO<br />
RICO, USA untuk LIFESCAN INC., USA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R.<br />
CHINA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
INTEC ADVANCED QUALITY One<br />
Step Cocaine Test Strip INTEC PRODUCTS INC (XIAMEN)., CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20103805179 02/10/2012 INTEC One Step Cocaine Test Card INTEC PRODUCTS INC (XIAMEN)., CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20101801918 20/03/2012 HUMAN Total Protein Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101802142 13/06/2012 HUMAN Uric Acid Liquicolor Plus HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20207801399 05/06/2012<br />
AKL<br />
20101802136 22/03/2012<br />
HUMAN Hemostat Thromboplastine -<br />
SI HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN GOT (ASAT) IFCC Mod.<br />
LiquiUV HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20103801806 08/08/2012<br />
INTEC ADVANCED QUALITY One<br />
Step Benzodiazepines Test Strip<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20103801803 15/08/2012 INTEC One Step Opiates Test Strip INTEC PRODUCTS INC, CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20103801802 08/08/2012<br />
INTEC ADVANCED QUALITY One<br />
Step THC / Cannabinoids Test Strip INTEC PRODUCTS INC, CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20103801807 08/08/2012<br />
AKL<br />
20103801804 08/08/2012<br />
AKL<br />
20103805181 15/08/2012<br />
AKL<br />
20103903911 15/08/2012<br />
INTEC ADVANCED QUALITY One<br />
Step Amphetamine Test Strip INTEC PRODUCTS INC, CHINA PT. MITRASAMAYA SEJATI<br />
INTEC ADVANCED QUALITY One<br />
Step Metamphetamine INTEC PRODUCTS INC, CHINA PT. MITRASAMAYA SEJATI<br />
INTEC One Step Benzodiazepines<br />
Test card<br />
INTEC ONE STEP<br />
Methamphetamine Test Card<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20205801687 08/06/2012 HUMACOUNT and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102801694 07/05/2012<br />
HUMAN Humapette Digital<br />
Adjustable HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10302214038 29/11/2012 MICROSCAN Peptidase PEP<br />
AKL<br />
20101801745 09/02/2012 ACCU-CHEK Go Test Strip<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10101214039 29/11/2012 MICROSCAN Reagent QC Kit<br />
AKL<br />
10101802194 19/06/2012<br />
AKL<br />
20101802199 31/05/2012<br />
AKL<br />
20101802196 31/05/2012<br />
AKL<br />
10301214064 29/11/2012<br />
IMMULITE / IMMULITE 1000<br />
System TES Total Testosterone<br />
IMMULITE 1000 System TSH Third<br />
Generation TSH<br />
IMMULITE 1000 Systems T3F Free<br />
T3<br />
MICROSCAN 0.5%<br />
N.N.Dimethylalphanaphthylamine<br />
NIT2<br />
AKL<br />
10302214062 29/11/2012 COMBIWASH and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
RAYTO LIFE and ANALYTICAL SCIENCES<br />
CO. LTD., P.R. CHINA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306801971 13/07/2012<br />
ADVIA Centaur CA 125 II Ready<br />
Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20306801975 13/07/2012<br />
ADVIA Centaur Systems CA 15-3<br />
Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10208604425 20/03/2012<br />
ADVIA CENTAUR Multi - Diluent 9<br />
Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10208604424 20/03/2012<br />
ADVIA Centaur Multi - Diluent 1<br />
Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10208604429 22/05/2012<br />
ADVIA CENTAUR Multi - Diluent 3<br />
Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10208604433 22/05/2012<br />
ADVIA CENTAUR Multi - Diluent 2<br />
Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20306801974 13/07/2012<br />
ADVIA Centaur CA 19-9 REady<br />
Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204214060 29/11/2012 MICROSCAN Mineral Oil<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302214065 29/11/2012<br />
MICROSCAN apid Indole Reagent-F<br />
R-IND<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302214063 29/11/2012 MICROSCAN Xylene A-IND1<br />
AKL<br />
10101214033 29/11/2012 DSI UIBC FS<br />
AKL<br />
10101214034 29/11/2012 DSI Iron FS Ferene<br />
AKL<br />
10101214032 29/11/2012 DSI Uric Acid FS Toos<br />
AKL<br />
20101803263 19/06/2012<br />
AKL<br />
10102806792 28/06/2012<br />
AKL<br />
20101213499 16/10/2012<br />
IMMULITE 2000 Systems FT3 Free<br />
T3<br />
KUBOTA Table Top Centrifuge 4000<br />
and Accessories<br />
ONE TOUCH VERIO Pro + Blood<br />
Glucose Monitoring System<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GMBH.,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GMBH.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK<br />
KUBOTA MANUFACTURING<br />
CORPORATION., JAPAN<br />
FLEXTRONIC IND (SHENZHEN) CO. LTD.,<br />
CHINA untuk LIFESCAN EUROPE,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. DEMKA SAKTI<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305801691 20/03/2012 HUMAN Humatex CRP HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205801636 08/06/2012 HUMACLOT DUO and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801914 16/04/2012 HUMAN Magnesium Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101802141 13/06/2012 HUMAN Uric Acid Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801915 22/03/2012 HUMAN Sodium Rapid HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20208802018 22/03/2012<br />
AKL<br />
10102801407 07/05/2012<br />
HUMAN Hemostat Control Plasma<br />
Normal HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMALYZER JUNIOR and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801920 20/03/2012 HUMAN CK NAC - Activated HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303801902 20/03/2012 HUMAN CMV IgM ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802137 22/03/2012 HUMAN Glucose Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801899 04/06/2012 HUMAN HDL Cholesterol Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101214076 30/11/2012 AMP Albumin BEG AMEDA LABORATORIES GMBH., AUSTRIA PT. SETIA GUNA MEDIKA<br />
AKL<br />
20101214117 12/12/2012<br />
BECKMAN COULTER Creatinine<br />
(Enzymatic)<br />
AKL<br />
20101214120 12/12/2012 BECKMAN COULTER ALP<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101214119 12/12/2012<br />
AKL<br />
20101214118 12/12/2012<br />
AKL<br />
20303802195 28/06/2012<br />
BECKMAN COULTER Direct<br />
Bilirubin<br />
BECKMAN COULTER Total<br />
Bilirubin<br />
IMMULITE /IMMULITE 1000<br />
Systems Toxoplasma Quantitative<br />
IgG<br />
AKL<br />
20101906652 11/07/2012 BD Vacutainer Plus Plastic Tubes<br />
AKL<br />
20101906655 04/07/2012<br />
BD Microtainer Z (No Additive<br />
Tubes)<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
BECTON DICKINSON CO, USA melalui<br />
BECTON DICKINSON CO, SINGAPORE<br />
BECTON DICKINSON CO, USA melalui<br />
BECTON DICKINSON CO, SINGAPORE<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. SIEMENS INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
10302807525 02/10/2012 URICULT ORION DIAGNOSTICA OY, FINLAND PT. PYRIDAM FARMA<br />
AKL<br />
20101802114 28/02/2012 DSI Bilirubin Jendrassik-Grof FS<br />
DIASYS DIAGNISTICS SYSTEM GMBH,<br />
GERMANY<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
AKL<br />
10302801174 06/07/2012 OXOID Peptone P OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801682 22/06/2012<br />
OXOID Rappaport - Vassiliadis (RV)<br />
Enrichment Broth OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301801188 13/07/2012 OXOID 0129 Discs 10 ug OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20101802032 28/02/2012 DSI Chloride FS<br />
AKL<br />
20101906428 15/08/2012<br />
AKL<br />
20305801810 02/08/2012<br />
IMMULITE/ IMMULITE 1000<br />
Systems RTI Troponin I Turbo<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. DIAGNOSTIKA SISTEM<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
INTEC Advanced Quality Rapid<br />
HBsAb Test Strip INTEC PRODUCTS INC (XIAMEN)., CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20101802792 22/03/2012 ADVIA Centaur FOL ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302802149 22/06/2012 OXOID Cooked Meat Medium OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302802150 13/07/2012<br />
AKL<br />
10302802148 22/06/2012<br />
OXOID ReinforceP Clostridial<br />
Medium (RCM) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Legionella MWY Selective<br />
Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303802147 13/07/2012 OXOID Streptococcal Grouping Kit OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
30305801808 24/07/2012 J. MITRA HIV TRI-DOT J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
10302802154 13/07/2012<br />
OXOID Lysine Decarboxylase Broth<br />
Tablets (Taylor Modification) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302802153 13/07/2012 OXOID Legionella CYE Agar Base OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801195 13/07/2012<br />
AKL<br />
10302801196 13/07/2012<br />
AKL<br />
10101801828 21/03/2012<br />
AKL<br />
20101801832 22/03/2012<br />
OXOID Clostridium Difficile Selective<br />
Supplement OXOID LTD, ENGLAND PT. DIPA PUSPA LABSAINS<br />
OXOID Chloramphenicol Selective<br />
Supplement OXOID LTD, ENGLAND PT. DIPA PUSPA LABSAINS<br />
GIESSE DIAGNOSTICS<br />
Triglycerides - SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIEESE DIAGNOSTICS AST/GOT-<br />
SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
20101801831 22/03/2012 GIEESE DIAGNOSTICS CK- MB GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
20101801830 22/03/2012 GIEESE DIAGNOSTICS Creatinine GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
10101801829 21/03/2012<br />
AKL<br />
10101801825 20/03/2012<br />
AKL<br />
10101801833 21/03/2012<br />
AKL<br />
20101801824 22/03/2012<br />
GIESSE DIAGNOSTICS HDL<br />
Cholesterol - SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS ALT / GPT<br />
- SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIESSE DIAGNOSTICS Cholesterol<br />
SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
GIEESE DIAGNOSTICS Glucose -<br />
SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
20101801827 22/03/2012 GIEESE DIAGNOSTICS Urea UV GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101801826 21/03/2012<br />
GIESSE DIAGNOSTICS Uric Acid<br />
SL GIESSE DIAGNOSTICSSNC, S.r.l ITALY PT. BETA PRIMA<br />
AKL<br />
20101802027 11/05/2012<br />
AKL<br />
10208802025 11/05/2012<br />
AKL<br />
10208802030 11/05/2012<br />
URITES Reagent Strip 13G (Nitrite,<br />
pH, Glucose, Protein, Occult Blood,<br />
Ketone, Urobilinogen, Specific<br />
Gravity, Leukocytes, Bilirubin,<br />
Microalbumin, Creatinine, calcium)<br />
URIT Lytic Hematology Analyzer<br />
Reagent<br />
URIT Diluent Hematology Analyzer<br />
Reagent<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
10102801696 30/04/2012 COMBILYZER VA and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802198 31/05/2012<br />
AKL<br />
20101802121 09/03/2012<br />
AKL<br />
20101802122 09/03/2012<br />
AKL<br />
10101802055 20/02/2012<br />
IMMULITE 1000 Systems F4 Free<br />
T4<br />
CA Calcium COBAS INTEGRA<br />
cobas c systems<br />
BIL-D Bilirubin Direct COBAS<br />
INTEGRA cobas c system<br />
MG Magnesium COBAS INTEGRA<br />
cobas c systems<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10302801681 31/07/2012 OXOID Anaerrobic Indicator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301801187 13/07/2012 OXOID Optochin Discs OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301801190 11/07/2012<br />
OXOID Amoxicillin 25 mcg<br />
Antimicrobial Susceptibility Discs OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801680 10/07/2012 OXOID ONPG Discs Selective OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10103214090 30/11/2012 ALCOSCAN AL 6000 SENTECH KOREA CORP., KOREA PT. SAMUDIA BAHTERA<br />
AKL<br />
10302801675 10/07/2012 OXOID Vitox Plus Diluen OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801192 13/07/2012<br />
AKL<br />
10302801185 06/07/2012<br />
OXOID Perferingens (TSC)<br />
Supplement OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
OXOID Agar Bacteriological (Agar<br />
No.1) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801172 06/07/2012 OXOID Urea Broth Base OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102801406 20/03/2012 HUMALYZER 2000 Clinical Analyzer HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102801638 07/05/2012<br />
AKL<br />
20101801916 22/03/2012<br />
HUMAN Automatic Washer ELISA<br />
Plate/Strip Washer and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN Alkaline Phosphatase<br />
Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801919 30/04/2012 HUMAN LDL Cholesterol Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802143 07/05/2012 HUMAN CK - NAC Liqui UV HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101211257 30/04/2012 HUMAN CK-MB NAC-Activated HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303802013 20/03/2012<br />
HUMAN Herpes-Simplex-Virus 2 IgG<br />
ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303801690 20/03/2012 HUMAN Syphilis RPR Test HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101802017 16/04/2012<br />
HUMAN COMBINA 11 S (Bilirubin,<br />
Urobilinogen, Ketones, Ascorbic Acid,<br />
Glucose, Protein, Blood, pH, Ni HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801922 30/04/2012 HUMAN Phosphorus Liquirapid HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303802012 13/06/2012 HUMAN HEXAGON TB HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303801900 04/06/2012 HUMAN Measles IgM ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303801904 20/03/2012 HUMAN Mumps Virus IgM ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801692 20/03/2012 HUMAN Fertitex Mono HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101210444 20/02/2012<br />
AKL<br />
20303210330 09/02/2012<br />
AKL<br />
10101802123 09/03/2012<br />
AKL<br />
20101802124 09/03/2012<br />
DIMENSION EXL Integrated<br />
Chemistry System LOCI Module<br />
LOCI TNI CAL<br />
COBAS 4800 System Control<br />
Diluent Kit<br />
LDL-C LDL-Cholesterol Plus 2<br />
generation COBAS INTEGRA cobas<br />
c system<br />
LDHL Lactate Dehydrogenase (P-L)<br />
COBAS INTEGRA cobas c systems<br />
AKL<br />
20303210329 09/02/2012 COBAS 4800 CT / NG Controls Kit<br />
AKL<br />
20305802051 09/03/2012<br />
C4-2 Tina-quant (a) Complement C4<br />
ver. 2 COBAS INTEGRA cobas c<br />
systems<br />
AKL<br />
20305801749 05/03/2012 RF Control Set Roche Systems<br />
AKL<br />
20305801743 05/03/2012 Preciset RF Roche / Hitachi<br />
AKL<br />
20101801739 09/02/2012 GLU Gluco-quant Roche / Hitachi<br />
AKL<br />
10101807919 26/12/2012 CELL-DYN 26 Plus Control<br />
AKL<br />
20205801360 14/03/2012<br />
AKL<br />
20205801361 14/03/2012<br />
AKL<br />
10302801177 10/07/2012<br />
AKL<br />
10302801176 10/07/2012<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG<br />
HEALTHCARE SECTOR, GERMANY<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
STRECK, USA untuk ABBOTT<br />
LABORATORIES., USA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
CELLDYN 3700CS Hematology<br />
Analyzer and Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
CELLDYN 3700SL Hematology<br />
Analyzer and Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
OXOID AMIES Agar Gel Single<br />
Applicator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
OXOID AMIES Agar Gel with<br />
Charcoal Single Aplicator OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801173 06/07/2012 OXOID Agar (Purified OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801199 22/06/2012 OXOID Palcam Agar Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801200 06/07/2012 OXOID Raka - Ray Agar Base OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801673 06/07/2012 OXOID Sodium Biselenite OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801674 06/07/2012 OXOID Soya Peptone Neutralized OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801677 10/07/2012<br />
OXOID Pseudomonas CFC<br />
Supplement OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302801683 22/06/2012 OXOID Sabouraud Maltose Agar OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10303802146 06/07/2012 OXOID Dryspot Staphytect Plus OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302802155 13/07/2012<br />
AKL<br />
20303802035 13/07/2012<br />
AKL<br />
20303802036 13/07/2012<br />
AKL<br />
20303802038 13/07/2012<br />
AKL<br />
20303802039 13/07/2012<br />
AKL<br />
20303802040 13/07/2012<br />
AKL<br />
20303802041 13/07/2012<br />
PDF Creator - PDF4Free v3.0<br />
OXOID Perfringens Agar Base (TSC<br />
& SFP) OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group B Monoclonal Antibody REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group C Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Polyvalent Agglutinating Serum<br />
(Group A-D) REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group W135 Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group X Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group Z Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
http://www.pdf4free.com
AKL<br />
20303802042 13/07/2012<br />
AKL<br />
20303802043 13/07/2012<br />
AKL<br />
20303802044 13/07/2012<br />
AKL<br />
20303802037 13/07/2012<br />
AKL<br />
10101803596 19/06/2012 ACCUTREND Control G<br />
AKL<br />
10101807917 26/12/2012 CELL-DYN 18 Plus Control<br />
AKL<br />
20101802200 28/06/2012<br />
REMEL Neisseria Meningitidis<br />
Group Y Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Polyvalent Group X, Y, Z, W135<br />
Agglutinating Serum REMEL EUROPE LTD., UK PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group A Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
REMEL Neisseria Meningitidis<br />
Group D Agglutinating Serum REMEL INC., USA PT. DIPA PUSPA LABSAINS<br />
IMMULITE /IMMULITE 1000 System<br />
VB Vitamin B12<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
STRECK., USA for ABBOTT<br />
LABORATORIES., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LIMITED, UK melalui SIEMENS<br />
AG., GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101802363 22/03/2012 HUMAN Potassium Liquirapid HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20103802749 21/03/2012 QUIKSCREEN THC Cassette Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20208801759 14/03/2012 MYT - 5D ORPHEE SA., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20208802073 28/03/2012 ABX Difftrol HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20208801758 09/03/2012 MYT - 3D ORPHEE SA., SWITZERLAND PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20101801689 20/03/2012 HUMAN fT 4 Elisa HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205213783 01/11/2012<br />
AKL<br />
20101802026 11/05/2012<br />
BD FACS Canto II Flow Cytometer<br />
and Accessories<br />
URITES Reagent Strip 10A<br />
(Leukocytes, Nitrite, Urobilinogen,<br />
Protein, pH Blood, Specific Gravity,<br />
Ke<br />
AKL<br />
20306804972 15/08/2012 IMMULITE 2000 Systems CEA<br />
BECTON DICKINSON & COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE., LTD., SINGAPORE<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101801973 22/03/2012 ADVIA Centaur Calibrator 28<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20306801972 22/03/2012 ADVIA Centaur BR ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305801969 22/03/2012<br />
ADVIA Centaur MYO - Myoglobin<br />
ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20207801964 26/03/2012 UNIPLASTIN System Pack TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20207801965 26/03/2012 LIQUICELLIN-E System Pack TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
101028014008 07/05/2012<br />
AKL<br />
20101802138 22/03/2012<br />
HUMAN Humareader Single Plus<br />
Microtiter Strip Reader and<br />
Accessories HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN Alkaline Phosphatase<br />
Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20207801398 22/03/2012 HUMAN Hemostat Thrombine Time HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101010358 29/11/2012<br />
BD MICROTAINER Tubes With FE<br />
(Sodium Fluoride/Disodium EDTA)<br />
Glycolitic Inhibator<br />
AKL<br />
20101010359 29/11/2012 BD MICROTAINER Tube Extender<br />
AKL<br />
20305213653 30/10/2012<br />
AKL<br />
BECKMAN COULTER ITA Control<br />
Serum Level 3<br />
BECTON DICKINSON CARIBE LTD.,<br />
PUERTO RICO untuk BECTON DICKINSON<br />
AND COMPANY (BD)., USA melalui BECTON<br />
DICKINSON HOLDING PTE. LTD.,<br />
SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. BEHRINDO NUSA PERKASA<br />
20303802145 07/05/2012 HUMAN Toxo IgG ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303211118 16/04/2012 HUMAN Dengue IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801905 16/04/2012 HUMAN Progeterone ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306801997 20/03/2012 HUMAN AFP ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303802014 22/03/2012 HUMAN HSV 1 IgG ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20209805170 03/10/2012<br />
AKL<br />
20101011146 29/11/2012<br />
AKL<br />
20101803625 13/07/2012<br />
J. MITRA Anti Human Serum<br />
(Coomb's Reagent) J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
BD Seditainer Blood Collection<br />
Tubes 1.8 ml<br />
AKL<br />
10101801906 09/03/2012 UIBC Roche/Hitachi<br />
AKL<br />
10101801742 17/02/2012 PRECINORM L Roche Systems<br />
AKL<br />
20101010822 29/11/2012<br />
BECTON DICKINSON & COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE., LTD., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
C - Peptide Calset Elecsys and<br />
Cobas e analyzer ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
BD VACUTAINER 1.8 ml Plus Blood<br />
Collection Tubes<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
10101801031 30/05/2012 DIALAB ASO Control DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101011430 29/11/2012<br />
AKL<br />
20101011431 29/11/2012<br />
BD Vacutainer CAT Plus Blood<br />
Collection Tubes<br />
BD VACUTAINER K3E 5.4mg Plus<br />
Blood Collection Tubes<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101802140 07/05/2012 HUMAN Bilirubin Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801416 20/03/2012 HUMAN fT3 HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801645 20/03/2012 HUMAN Estriol ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303801686 20/03/2012 HUMAN Syphilis TPHA Liquid HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102801697 30/04/2012 HUMALYZER 3000 and Accessories HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306801411 28/02/2012 HUMAN PSA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305213654 30/10/2012<br />
AKL<br />
20205012204 15/08/2012<br />
BECKMAN COULTER ITA Control<br />
Serum Level 2<br />
COATRON A4, 4-Channel Fully<br />
Automated Coagulation Analyzer and<br />
Accessories<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
LABITEC (LABOR BIOMEDICAL<br />
TECHNOLOGIES GMBH)., GERMANY<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20303802021 22/03/2012 HUMAN Rubella Virus IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801410 20/03/2012 HUMAN Testoterone ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801644 22/03/2012<br />
AKL<br />
20101802144 07/05/2012<br />
AKL<br />
20101801740 05/03/2012<br />
HUMAN COMBINA 2 (Glucose,<br />
Ketones) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN Humazym Acid<br />
Phosphatase HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
C.f.a.s Proteins Calibrators for<br />
Automated Systems<br />
AKL<br />
20305802046 09/03/2012 Tg Elecsys and Cobas e Analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL LTD.,<br />
SWITZERLAND<br />
AKL<br />
10208701871 31/10/2012 ID-Diluent 1 DIAMED GMBH., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20303802015 22/03/2012 HUMAN CMV IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10205801695 30/04/2012 HUMASED 20 and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101801741 01/02/2012 PRECIPATH Roche Systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
30305214126 18/12/2012 ARCHITECT HIV Ag/Ab Combo ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
AKL<br />
10102800405 22/05/2012 MICROLAB 300 VITAL SCIENTIFIC B.V., NETHERLAND PT. MRK DIAGNOSTICS<br />
AKL<br />
10103801907 09/02/2012 URISYS 2400 and Accessories<br />
AKL<br />
20305802076 09/02/2012<br />
Anti-Tg Elecsys and cobas e<br />
analyzer<br />
HITACHI HIGH-TECHNOLOGIES<br />
CORPORATION, JAPAN untuk ROCHE<br />
DIAGNOSTICS GMBH., GERMANY melalui<br />
ROCHE DI<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101802066 28/06/2012 ELITECH CK NAC SL SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101802067 28/06/2012 ELITECH Elical I2 SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20101802070 28/06/2012 ELITECH CK - MB SL SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101802069 28/06/2012 ELITECH Elitrol I SEPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
30305111261 14/02/2012<br />
INTEC One Step Anti-HIV (1 & 2) Tri<br />
Line Test Card<br />
INTEC PRODUCTS INC. (XIAMEN) P.R.,<br />
CHINA<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20303110674 04/06/2012 ADVANTAGE Dengue NS1 Ag Card J. MITRA & CO. PVT. LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20303110675 04/06/2012 DIAGNOS Dengue Card J. MITRA & CO. PVT. LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
AKL<br />
10204110227 22/03/2012 CONVERGYS Hypoclean CC<br />
AKL<br />
10206110228 22/03/2012 CONVERGYS Diff 5-P<br />
AKL<br />
10206110226 22/03/2012 CONVERGYS Lyse-5P<br />
AKL<br />
20101110705 01/02/2012 INTEC Simpdo Glucose Test Strip<br />
AKL<br />
20305110706 01/02/2012<br />
INTEC Advanced Quality HCV<br />
ELISA Test Kit<br />
CONVERGENT TECHNOLOGIES GMBH &<br />
CO. KG., GERMANY<br />
CONVERGENT TECHNOLOGIES GMBH &<br />
CO. KG., GERMANY<br />
CONVERGENT TECHNOLOGIES GMBH &<br />
CO. KG., GERMANY<br />
INTEC PRODUCTS INC. (XIAMEN) P.R.,<br />
CHINA<br />
INTEC PRODUCTS INC. (XIAMEN) P.R.,<br />
CHINA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. MITRASAMAYA SEJATI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
20303110695 13/07/2012<br />
WANTAI SYPHILIS TRUST ELISA<br />
KIT<br />
AKL<br />
10204110238 22/03/2012 CONVERGYS Cleaner<br />
AKL<br />
10208110240 22/03/2012 CONVERGYS Lyse Diff<br />
AKL<br />
10208110239 22/03/2012 CONVERGYS Dil Diff<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO, LTD, P.R. China<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
Co. KG, Germany<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
Co. KG, Germany<br />
CONVERGENT TECHNOLOGIES GmbH &<br />
Co. KG, Germany<br />
PT. SUMIFIN CITRA ABADI<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
AKL<br />
10101801896 04/06/2012 HUMAN Humatrol P HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306110790 13/07/2012<br />
AKL<br />
20306110789 13/07/2012<br />
INNOGENETICS INNO-LiPA HPV<br />
Genotyping Extra INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
INNOGENETICS INNO-LiPA HPV<br />
Genotyping Extra Amp. INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
20102111423 13/07/2012 Auto - LIPA 48 and Accessories INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
20304111421 13/07/2012<br />
AKL<br />
20305110791 13/07/2012<br />
INNOGENETICS INNO - LIA<br />
Syphilis Score INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
INNOGENETICS INNO-LIA HCV<br />
Score INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
10203111302 29/11/2012<br />
LEICA ST5020 Multistainer and<br />
Accessories<br />
LEICA BIOSYSTEMS (NUSSLOCH) GMBH.,<br />
GERMANY melalui LEICA MICROSYSTEM<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10203111301 29/11/2012<br />
LEICA CM1950 - Cryostat and<br />
Accessories<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MICROSYSTEM<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
30305111259 23/08/2012<br />
INTEC Advanced Quality HIV 1 & 2<br />
ELISA Test Kit (3rd Generation) INTEC PRODUCTS INC, P.R. CHINA PT. MITRASAMAYA SEJATI<br />
AKL<br />
20305110797 13/07/2012 WANTAI HBsAb ELISA Kit<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO. LTD., P.R. CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303111085 13/07/2012 WANTAI HAV-IgM ELISA Kit<br />
BEIJING WANTAI BIOLOGICAL PHARMACY<br />
ENTERPRISE CO. LTD., P.R. CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
AKL<br />
10203111388 29/11/2012<br />
AKL<br />
20205705087 21/02/2012<br />
LEICA DM 750 - Microscope and<br />
accessories<br />
SFRI H18 Light Hematology Blood<br />
Cell Counter<br />
AKL<br />
10203111787 05/10/2012 BD PrepStain Slide Processor<br />
AKL<br />
10203111786 05/10/2012<br />
AKL<br />
20305112126 13/07/2012<br />
BD PrepMate Automated and<br />
Accessories<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH,<br />
GERMANY melalui LEICA MICROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
SFRI Sarl., FRANCE<br />
TRIPATH IMAGING INC, USA a subsidiary of<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON<br />
TRIPATH IMAGING INC, USA a subsidiary of<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON<br />
PT. BIOGEN SCIENTIFIC<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
INNOGENETICS INNO-LiPA HBV<br />
DR v2 INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
10101802068 28/06/2012 ELITECH Elitrol II SAPPIM S.A.S., FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
10101606338 19/06/2012 ARCHITECT UIBC Liquid<br />
SENTINEL CH, SpA, ITALY untuk ABBOTT<br />
GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101800969 26/12/2012 MEDISAFE Blood Glucose Test Tip TERUMO CORPORATION, JAPAN PT. TERUMO INDONESIA<br />
AKL<br />
20101210094 17/01/2012<br />
AKL<br />
20101210092 17/01/2012<br />
INTHERMA Vacuum 3.8% Sodium<br />
Citrate<br />
INTHERMA Vacuum 3.2% Sodium<br />
Citrate<br />
AKL<br />
20101210093 17/01/2012 INTHERMA Vacuum Clot Activator<br />
AKL<br />
20101210091 17/01/2012 INTHERMA Vacuum Clot Activator<br />
AKL<br />
10204210023 06/01/2012<br />
ARCHITECT Progesterone Manual<br />
Diluent<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
WEIHAI HONGYU MEDICAL DEVICES CO.<br />
LTD., CHINA<br />
ABBOTT IRELAND DIAGNOSTICS DIVISION<br />
,Ireland<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101210074 16/01/2012 ABBOTT Uric Acid ABBOTT LABORATORIES,USA PT. ABBOTT INDONESIA<br />
AKL<br />
20209210075 16/01/2012<br />
AKL<br />
20305210076 16/01/2012<br />
AKL<br />
10204210022 06/01/2012<br />
AKL<br />
10204210025 11/01/2012<br />
AKL<br />
20103210034 11/01/2012<br />
AKL<br />
20103210035 11/01/2012<br />
Anti-A/ Anti-B/ Anti-D/ Control/<br />
Reverse Diluent Ortho BIOVE<br />
System<br />
Anti-IgG,-C3d; Polyspecific Ortho<br />
BIOVUE System<br />
ARCHITECT Probe Conditioning<br />
Solution<br />
ARCHITECT Estradiol Manual<br />
Diluent<br />
SYVA EMIT II Plus Phencyclidine<br />
Assay<br />
SYVA EMIT II Plus Ethyl Alcohol<br />
Assay<br />
AKL<br />
10103210033 11/01/2012 SYVA Oxidant Validity Test<br />
PT. JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA INDONESIA<br />
PT.JOHNSON & JOHNSON<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., USA INDONESIA<br />
ABBOTT IRELAND DIAGNOSTICS DIVISION<br />
,Ireland<br />
PT. ABBOTT INDONESIA<br />
ABBOTT IRELAND DIAGNOSTICS DIVISION<br />
,Ireland<br />
PT. ABBOTT INDONESIA<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany PT. SIEMENS INDONESIA<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany PT. SIEMENS INDONESIA<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany PT. SIEMENS INDONESIA<br />
AKL<br />
20103210036 11/01/2012 SYVA EMIT II Plus Ecstasy Assay<br />
AKL<br />
20103210037 11/01/2012<br />
AKL<br />
20306210032 11/01/2012<br />
AKL<br />
20306210030 11/01/2012<br />
AKL<br />
20306210031 11/01/2012<br />
AKL<br />
20306210029 11/01/2012<br />
SYVA EMIT II Plus Barbiturate<br />
Assay<br />
AKL<br />
10208210002 05/01/2012 CELLAPACK DCL<br />
AKL<br />
10208210001 05/01/2012 CELLPACK DST<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
TOSOH AIA-PACK CA15-3<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK CA 125<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK PSA II Calibrator<br />
Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK CA 19-9<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
SYSMEX INTERNATIONAL REAGENTS<br />
CO.,LTD., Japan melalui SYSMEX ASIA<br />
PACIFIC PTE., LTD., Singapore<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20306210027 11/01/2012<br />
VITROS Immunodiagnostic products<br />
Total B-hCG II ( B-hCG )<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20306210026 11/01/2012<br />
AKL<br />
20101210042 11/01/2012<br />
VITROS Immunodiagnostic products<br />
CA II ( CA 125 ) Calibrator<br />
SYVA Creatinine Validity Calibrator<br />
400<br />
AKL<br />
20101210041 11/01/2012 SYVA Creatinine Validity Test<br />
AKL<br />
20101210040 11/01/2012<br />
AKL<br />
20101210039 11/01/2012<br />
SYVA Creatinine Validity Calibrator<br />
100<br />
SYVA Creatinine Validity Calibrator<br />
20<br />
ORTHO-CLINICAL DIAGNOSTIC INC ., UK<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany<br />
SIEMENS HEALTCARE DIAGNOSTIC INC.,<br />
USA Melalui SIEMENS AG., Germany<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210038 11/01/2012 SYVA Creatinine Validity Calibrator 2<br />
AKL<br />
20303210242 06/02/2012<br />
AKL<br />
20101210196 02/02/2012<br />
AKL<br />
20101210197 02/02/2012<br />
OnSite Duo Dengue Ag+ IgG/IgM<br />
Duo Rapid Test<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG., GERMANY<br />
CTK BIOTECH INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
TOSOH AIA-PACK B12 Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK olate Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305210195 02/02/2012 TOSOH ST AIA-PACK HBsAG2 TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101210194 02/02/2012 TOSOH ST AIA-PACK Intact PTH TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305210192 02/02/2012 TOSOH ST AIA-PACK FER TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101210191 02/02/2012 TOSOH ST AIA-PACK ACTH TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101210193 02/02/2012 TOSOH AIA-PACK CORT TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20102210241 06/02/2012<br />
AKL<br />
20305210156 27/01/2012<br />
AKL<br />
20305210155 27/01/2012<br />
3M Integrated Cycler and<br />
Accessories<br />
KAPPA Tina-quant [a] Roche /<br />
Hitachi<br />
LAMBDA Tina-quant [a]<br />
Roche/Hitachi<br />
3M COMPANY, USA untuk FOCUS<br />
DIAGNOSTICS INC, USA<br />
ROCHE DIAGNOSTICS GMBH, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTSD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTSD.,<br />
SWITZERLAND<br />
PT. NELTA MULTI GRACIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20305210157 27/01/2012<br />
AKL<br />
10101210127 24/01/2012<br />
AKL<br />
10101210128 24/01/2012<br />
VITROS Immunodiagnostic Products<br />
HBsAg Confirmatory Kit<br />
VITROS Immunodiagnostic Products<br />
RE Controls<br />
VITROS Immunodiagnostic Products<br />
Testosterone Controls<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10302210121 24/01/2012 MERCKOPLATE Blood Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20306210028 11/01/2012<br />
AKL<br />
20305210126 24/01/2012<br />
AKL<br />
20101210129 24/01/2012<br />
AKL<br />
20306210130 24/01/2012<br />
AKL<br />
10204210134 24/01/2012<br />
VITROS Immunodiagnostics<br />
Products CA 15-3 Calibrators<br />
ORTHO HCV 3.0 ELISA Test<br />
System with Enhanced SAVe<br />
VITROS Immunodiagnostics<br />
Products Estradiol calibrator<br />
VITROS Immunodiagnostics<br />
Products CA 15-3 Reagent pack<br />
CLEAN Cleaner Cassette COBAS<br />
INTEGRA<br />
AKL<br />
10204210133 24/01/2012 NaCI Diluent 9% COBAS INTEGRA<br />
AKL<br />
20205210135 24/01/2012 Hemolyzer 5 and Accessories<br />
AKL<br />
20205210136 24/01/2012 Hemolyzer 3 and Accessories<br />
AKL<br />
20102210321 09/02/2012<br />
THERMO SCIENTIFIC INDIKO and<br />
Accessories<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ROCHE DIAGNOSTICS GMBH, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTSD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH, GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTSD.,<br />
SWITZERLAND<br />
DIATRON MI PLC., HUNGARY untuk<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
DIATRON MI PLC., HUNGARY untuk<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
THERMO FISHER SCIENTIFIC OY.,<br />
FINLAND<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. DJEMBATAN DUA<br />
PT. DJEMBATAN DUA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10204210175 31/01/2012<br />
VITROS Immunodiagnostics<br />
Products Universal Wash Reagent<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302210360 13/02/2012 KHB ST-36W Micro Plate Washer<br />
AKL<br />
10102210362 13/02/2012<br />
KHB L-3180 Semi Automated<br />
Clinical Chemistry Analyzer<br />
AKL<br />
10102210174 31/01/2012 HYDRO SPEED and Accessories<br />
AKL<br />
20101210284 09/02/2012<br />
AKL<br />
20101210283 09/02/2012<br />
AKL<br />
20101210285 09/02/2012<br />
AKL<br />
20102210207 02/02/2012<br />
AKL<br />
10305210212 02/02/2012<br />
AKL<br />
10305210211 02/02/2012<br />
AKL<br />
20101801381 09/02/2012<br />
AKL<br />
20102210371 14/02/2012<br />
AKL<br />
20102210809 14/03/2012<br />
AKL<br />
20101210446 20/02/2012<br />
AKL<br />
20101210448 20/02/2012<br />
AKL<br />
20101210447 20/02/2012<br />
DIMENSION EXL LOCI Module<br />
FT4L Flex Reagent Cartridge<br />
DIMENSION EXL LOCI Module FT3<br />
Flex Reagent Cartridge<br />
DIMENSION EXL LOCI Module Loci<br />
Thyr Cal<br />
VITROS 5600 Integrated System<br />
and Accessories<br />
SHANGHAI KEHUA LABORATORY SYSTEM<br />
CO. LTD., CHINA<br />
SHANGHAI KEHUA LABORATORY SYSTEM<br />
CO. LTD., CHINA<br />
TECAN AUSTRIA GMBH., AUSTRIA melalui<br />
TECAN SALES INTERNATIONAL GMBH.,<br />
AUSTRIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
IMTEC Phosphatidyserine<br />
Antibodies IgG HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
IMTEC - Phosphatidyserine<br />
Antibodies IgM HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
ABBOTT Clinical Chemistry<br />
Multiconstituent Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
DIAGON D-Chem 300 Auto<br />
Chemistry Analyzer<br />
DIAGON LTD., HUNGARY<br />
PT. DEC HEALTHCARE<br />
INDONESIA<br />
BIOSYSTEMS Random Access<br />
Analyzer A-15 BIOSYSTEMS S.A., SPAIN PT. ELGA TAMA<br />
DIMENSION EXL Integrated<br />
Chemistry System LOCI Module<br />
Reagent Cartridge TSHL<br />
DIMENSION EXL Integrated<br />
Chemistry System LOCI Module<br />
LOCI NTP CAL<br />
DIMENSION EXL Integrated<br />
Chemistry System LOCI Module Flex<br />
Reagent Cartridge NTP<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG<br />
HEALTHCARE SECTOR, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG<br />
HEALTHCARE SECTOR, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG<br />
HEALTHCARE SECTOR, GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10103210544 27/02/2012 DIALAB Cholinesterase test system DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10204210178 31/01/2012 AutoFISH Reagent A dan B ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10204210177 31/01/2012 AutoFISH Pretreatment Reagent ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10204210179 31/01/2012 AutoFISH Protease Buffer ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10101210187 01/02/2012<br />
PRECINORM PROTEIN Roche<br />
Systems<br />
AKL<br />
10303210186 01/02/2012 ASLO Tina-quant [a] Roche / Hitachi<br />
AKL<br />
10101210185 01/02/2012<br />
UIBC Unsaturated Iron Binding<br />
Capacity COBAS INTEGRA cobas c<br />
system<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10102210184 01/02/2012 URISYS 1100 and Accessories<br />
AKL<br />
10102210188 01/02/2012<br />
AKL<br />
20103704625 09/02/2012<br />
MICROSAN Turbidity Meter and<br />
Accessories<br />
77 ELEKTRONIKA MUSZERIPARI KFT.,<br />
HUNGARY untuk ROCHE DIAGNOSTICS<br />
GMBH., GERMANY melalui ROCHE DIAGNO<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG<br />
HEALTHCARE SECTOR, GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
SPEEDYTEST One Step Cassette<br />
Style TCA (Triclyclic Antidepresent)<br />
Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103704632 09/02/2012<br />
SPEEDYTEST One Step Strip Style<br />
TCA (Triclyclic Antidepresent) Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
20103704627 09/02/2012 SPEEDYTEST Alcohol Test IND DIAGNOSTIC INC., CANADA PT. LANIROS DIAN PHARMA<br />
AKL<br />
10101210324 09/02/2012 AIM HDL-Cholesterol 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101210322 09/02/2012 AIM Glucose-PAP 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
10101210323 09/02/2012 AIM Uric Acid (UA) 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101210325 09/02/2012 AIM Total Protein 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101210318 09/02/2012<br />
AKL<br />
20101210317 09/02/2012<br />
AKL<br />
20102210327 09/02/2012<br />
Troponin T STAT Elecsys and cobas<br />
e analyzer<br />
Troponin T STAT CalSet Elecsys<br />
and cobas e analyzer<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
ARCHITECT c16000 System and<br />
Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20209210672 05/03/2012 SELECTOGEN 3 + 1 % ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
AKL<br />
20209210671 05/03/2012 ORTHO BLISS<br />
AKL<br />
10101210464 20/02/2012<br />
AKL<br />
20306210593 28/02/2012<br />
AKL<br />
10101210703 09/03/2012<br />
ARCHITECT Active-B12<br />
(Holotranscobalamin) Controls<br />
ABBOTT RealTime ms9 Colerectal<br />
ancer Reagent Kit<br />
ORTHO-CLINICAL DIAGNOSTIC INC.,<br />
JAKARTA<br />
AXIS SHIELD DIAGNOSTICS LIMITED., UK<br />
untuk ABBOTT GMBH & CO. KG., GERMANY<br />
ABBOTT MOLECULAR INC., USA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
TOSOH AIA-PACK Cystatin C<br />
Control Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101210579 28/02/2012 TOSOH Multi-Control Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101210578 28/02/2012 TOSOH ST AIA-PACK C-Peptide TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10204210577 28/02/2012<br />
AKL<br />
10204210576 28/02/2012<br />
TOSOH AIA-PACK Folate<br />
Pretreatment Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK B12<br />
Pretreatment Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101210575 28/02/2012 TOSOH ST AIA-PACK HGH TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101210574 28/02/2012 TOSOH ST AIA-PACK IRI TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305210429 17/02/2012<br />
AKL<br />
20101210426 17/02/2012<br />
AKL<br />
20101210427 17/02/2012<br />
AKL<br />
20305210428 17/02/2012<br />
AKL<br />
10208210704 09/03/2012<br />
TOSOH AIA-PACK HBsAg<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK Intact PTH<br />
calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK C-Peptide<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK HBsAb<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK Cystatin C<br />
Sample Diluting Solution TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305210702 09/03/2012 TOSOH ST AIA-PACK HBsAb TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101210696 09/03/2012<br />
AKL<br />
20101210697 09/03/2012<br />
AKL<br />
20101210698 09/03/2012<br />
AKL<br />
20101210699 09/03/2012<br />
AKL<br />
20101210695 09/03/2012<br />
TOSOH ST AIA-PACK CORT<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK FER<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK HGH<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK IRI Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK ACTH<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101210451 20/02/2012 SYVA Nitrite Validity Test<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210455 20/02/2012 SYVA Nitrite Validity Calibrator 200<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210454 20/02/2012 SYVA Nitrite Validity Calibrator 500<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210453 20/02/2012 SYVA Nitrite Validity Calibrator 1000<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210452 20/02/2012<br />
SYVA Validity Negative<br />
Calibrator/Control<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103210450 20/02/2012 SYVA pH Validity Calibrator 11.0<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA
AKL<br />
20103210449 20/02/2012 SYVA pH Validity Calibrator 12.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20206801412 28/02/2012 HUMAN Hexagon OBTI HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10208210705 09/03/2012<br />
AKL<br />
20306210700 09/03/2012<br />
Hemolyzing Reagent Gen. 2 COBAS<br />
INTEGRA<br />
COBAS 4800 HPV Amplification /<br />
Detection Kit<br />
AKL<br />
20306210701 09/03/2012 COBAS 4800 HPV Controls Kit<br />
AKL<br />
20304210461 20/02/2012<br />
AKL<br />
20304210462 20/02/2012<br />
VENTANA UltraVIEW SISH DNP<br />
Detection Kit<br />
VENTANA UltraVIEW Red ISH DIG<br />
Detection Kit<br />
AKL<br />
10203210693 09/03/2012 PATHTEZT Pap Test Collection Kit<br />
AKL<br />
10102210694 09/03/2012<br />
AKL<br />
20304210674 05/03/2012<br />
INTHERMA-168 Biochemistry<br />
Analyzer<br />
VENTANA HER2 Dual ISH 3-in-1<br />
Xenograft Slides<br />
AKL<br />
10204210673 05/03/2012 VENTANA HybReady Solution<br />
AKL<br />
10101210460 20/02/2012<br />
VITROS Immunodiagnostic Products<br />
Anemia Controls<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS I<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS I<br />
BIOCYTECH CORPORATION SDN. BHD.,<br />
MALAYSIA<br />
SHENZEN EMPEROR ELECTRONIC<br />
TECHNOLOGY CO. LTD., CHINA<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS I<br />
VENTANA MEDICAL SYSTEMS INC., USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS I<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. CAKRA MEDIKA UTAMA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210675 05/03/2012 ALERE Determine HBsAg ALERE MEDICAL CO. LTD., JAPAN PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
20303210457 20/02/2012<br />
AKL<br />
20303210456 20/02/2012<br />
AKL<br />
20103210458 20/02/2012<br />
TETRAMED Tes Gonorrhea<br />
Cassette Urine / Swab<br />
TETRAMED Tes Syphilis Casstte -<br />
Darah<br />
TETRAMED Multi Drugs 5 rapid test<br />
panel urine (methamphetamine,<br />
Morphine, THC Marijuana, Opium,<br />
Amph<br />
AKL<br />
10303210205 02/02/2012 BIONEXIA Influenza A+B<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., P.R. CHINA<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., P.R. CHINA<br />
GUANGZHOU WONDFO BIOTECH CO.<br />
LTD., P.R. CHINA<br />
BIOMERIEUX (SHANGHAI) BIOTECH CO.<br />
LTD., CHINA untuk BIOMERIEUX SA.,<br />
FRANCE<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. MITRA ABADI <strong>ALKES</strong>INDO<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303210676 05/03/2012 ALERE Determine Syphilis TP ALERE MEDICAL CO. LTD., JAPAN PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
20306210204 02/02/2012 BIONEXIA BTA<br />
AKL<br />
20305210210 02/02/2012 BIONEXIA CRP Plus<br />
AKL<br />
20206210206 02/02/2012 BIONEXIA FOB Plus<br />
AKL<br />
20101210584 28/02/2012<br />
AKL<br />
20101210586 28/02/2012<br />
AKL<br />
20101210587 28/02/2012<br />
AKL<br />
10204210743 09/03/2012<br />
AKL<br />
10203210724 09/03/2012<br />
MINDRAY CREA Creatinine Kit<br />
(Modified Jaffe Method)<br />
MINDRAY GLU Glucose Kit (HK<br />
Method)<br />
MINDRAY GLU Glucose Kit (GOD-<br />
POD Method)<br />
COBAS 4800 System Wash Buffer<br />
Kit<br />
SYSMEX Automated Hematology<br />
Slide Preparation Unit SP-10 and<br />
Accessories<br />
AKL<br />
10208210764 12/03/2012 SYSMEX Lysercell WDF<br />
BIOMERIEUX (SHANGHAI) BIOTECH CO.<br />
LTD., CHINA untuk BIOMERIEUX SA.,<br />
FRANCE<br />
BIOMERIEUX (SHANGHAI) BIOTECH CO.<br />
LTD., CHINA untuk BIOMERIEUX SA.,<br />
FRANCE<br />
BIOMERIEUX (SHANGHAI) BIOTECH CO.<br />
LTD., CHINA untuk BIOMERIEUX SA.,<br />
FRANCE<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
ROCHE MOLECULAR INC., LTD., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208210762 09/03/2012 SYSMEX Lysercell WPC<br />
AKL<br />
10208210761 12/03/2012 SYSMEX Lysercell WNR<br />
AKL<br />
10208210763 12/03/2012 SYSMEX Sulfolyser<br />
AKL<br />
10208210709 09/03/2012 SYSMEX Cellpack DFL<br />
AKL<br />
20101210591 28/02/2012<br />
MINDRAY ALB Albumin Kit<br />
(Bromcresol Green Method)<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
AKL<br />
20101210590 28/02/2012<br />
AKL<br />
20101210589 28/02/2012<br />
AKL<br />
20101210588 28/02/2012<br />
AKL<br />
10101210583 28/02/2012<br />
MINDRAY AST Aspartate<br />
Aminotransferase Kit (IFCC Method)<br />
MINDRAY UREA Kit (Urease-<br />
GLDH, UV Method)<br />
MINDRAY Alkaline Phosphatase Kit<br />
(IFCC Modified Method)<br />
MINDRAY UA Uric Acid Kit (Uricase-<br />
Peroxidase Method)<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
AKL<br />
10101210582 28/02/2012<br />
AKL<br />
20205210723 09/03/2012<br />
MINDRAY ALT Alanine<br />
Aminotransferase Kit (IFCC Method)<br />
SYSMEX Automated Hematology<br />
Analyzer XN Series (XN-10) and<br />
Accessories<br />
AKL<br />
20208210332 09/02/2012 MINDRAY B55 Hematology Control<br />
AKL<br />
20208210333 09/02/2012 MINDRAY B30 Control<br />
AKL<br />
20205210746 09/03/2012 Hemolyzer-5-Diff 5P<br />
AKL<br />
20208210747 09/03/2012 Hemolyzer-5-Control (L,N,H)<br />
AKL<br />
10204210748 09/03/2012 Hemolyzer-3-Cleaner<br />
AKL<br />
10204210749 09/03/2012 Hemolyzer Hypocleaner<br />
AKL<br />
10208210750 09/03/2012 Hemolyzer-5-Lyser<br />
AKL<br />
10208210751 09/03/2012 Hemolyzer Diluent<br />
AKL<br />
20101210744 09/03/2012<br />
AKL<br />
20101210585 28/02/2012<br />
VITROS Immunodiagnostic Products<br />
Vitamin B12 Calibrator<br />
MINDRAY CREA Creatinine Kit<br />
(Sarcosine Oxidase Method)<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD. P.R. CHINA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. DJEMBATAN DUA<br />
PT. DJEMBATAN DUA<br />
PT. DJEMBATAN DUA<br />
PT. DJEMBATAN DUA<br />
PT. DJEMBATAN DUA<br />
PT. DJEMBATAN DUA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
AKL<br />
20209210707 09/03/2012<br />
AKL<br />
20205210722 09/03/2012<br />
AKL<br />
20903210752 12/03/2012<br />
BIO-RAD IH-1000 Fully Automated<br />
System for ID-Cards and Accessories DIAMED GMBH., SWITZERLAND<br />
SYSMEX Automated Hematology<br />
Analyzer XN Series (XN-20) and<br />
Accessories<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. SYSMEX INDONESIA<br />
RENO-S130 Low Temperature<br />
Plasma Gas Sterilizer and<br />
Accessories RENOSEM CO. LTD., KOREA PT. MEGAH <strong>ALKES</strong>INDO<br />
AKL<br />
20101210740 09/03/2012<br />
ARCHITECT ActiveB12<br />
(Holotranscobalamin) Calibrators<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101210739 09/03/2012<br />
AKL<br />
20903210753 12/03/2012<br />
AKL<br />
20903210754 12/03/2012<br />
ARCHITECT ActiveB12<br />
(Holotranscobalamin) Reagent Kit<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
RENO-S30 Low Temperature<br />
Plasma Gas Sterilizer and<br />
Accessories RENOSEM CO. LTD., KOREA PT. MEGAH <strong>ALKES</strong>INDO<br />
RENO-D50 Low Temperature<br />
Plasma Gas Sterilizer and<br />
Accessories RENOSEM CO. LTD., KOREA PT. MEGAH <strong>ALKES</strong>INDO<br />
AKL<br />
20303210738 09/03/2012<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV IgM Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
http://www.pdf4free.com<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA
AKL<br />
20303210737 09/03/2012<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV IgM Reagent<br />
Pack<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
AKL<br />
20305210370 14/02/2012 OnSite HCV Ab Rapid Test CTK BIOTECH INC., USA<br />
AKL<br />
20305210369 14/02/2012 OnSite HAV IgM Rapid Test CTK BIOTECH INC., USA<br />
AKL<br />
10204210805 14/03/2012<br />
COBAS 4800 System Liquid<br />
Cytology Preparation Kit<br />
AKL<br />
10204210804 14/03/2012 ZENIX Concentrated Cleanser<br />
AKL<br />
20101210745 09/03/2012<br />
AKL<br />
20102210760 12/03/2012<br />
AKL<br />
10303210756 12/03/2012<br />
AKL<br />
10303210755 12/03/2012<br />
AKL<br />
10204210706 09/03/2012<br />
AKL<br />
20102210759 12/03/2012<br />
VITROS Immunodiagnostic Products<br />
Red Cell Folate Pack<br />
LABOTRON Auto Chemistry LB-<br />
C4800 and Accessories<br />
ROCHE MOLECULAR INC., LTD., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
SHENZHEN LANDWIND INDUSTRY CO.<br />
LTD., CHINA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. ROCHE INDONESIA<br />
PT. SUMIFIN CITRA ABADI<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
INNOGENETICS INNO-LiPA Rif.<br />
TB INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
INNOGENETICS INNO-LiPA Rif.<br />
TB Amp INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
COBAS 4800 System Sample<br />
Preparation Kit<br />
LABOTRON Auto Chemistry<br />
Analyzer LB-C1700 and Accessories<br />
AKL<br />
10208210803 14/03/2012 ZENIX Lyse<br />
AKL<br />
10302210361 13/02/2012 KHB ST-360 Micro-Plato Reader<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
SHENZHEN LANDWIND INDUSTRY CO.<br />
LTD., CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
SHANGHAI KEHUA LABORATORY SYSTEM<br />
CO. LTD., CHINA<br />
PT. ROCHE INDONESIA<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. SUMIFIN CITRA ABADI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
10304702505 17/02/2012 SEBIA Hydragel 7 HR SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20305702497 17/02/2012 SEBIA Hydragel 2 Bence Jones SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20301210932 22/03/2012 BD PHOENIX 25 PMIC/ID-109<br />
AKL<br />
10301210890 20/03/2012 BD BBL Meuller Hinton II Agar<br />
AKL<br />
20301210933 22/03/2012 BD PHOENIX 25 PMIC/ID-101<br />
AKL<br />
20301210931 22/03/2012 BD PHOENIX 25 NMIC/ID-121<br />
AKL<br />
10204210870 19/03/2012<br />
AKL<br />
10204210871 19/03/2012<br />
BECTON DICKINSON AND COMPANY USA,<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY USA,<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY USA,<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY USA,<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE. LTD., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
SEBIA Decolorant / Destaining<br />
Solution SEBIA, FRANCE PT. RIOCA MEDICA<br />
SEBIA Antisera and Fixative<br />
Immunofixation (IF) SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20305801401 22/03/2012 HUMAN Hexagon IgE HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303801903 22/03/2012<br />
HUMAN VZV (Varicella-Zoster Virus)<br />
IgM ELISA Test HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303802020 22/03/2012 HUMAN Hexagon Syphilis HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10304702504 17/02/2012 SEBIA Hydragel 15 HR SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10204210463 20/02/2012 ZENIX Cleanser<br />
AKL<br />
10204211436 11/05/2012<br />
URIT Detergent Hematology<br />
Analyzer Reagent<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO. LTD., CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
PT. SUMIFIN CITRA ABADI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20101802028 11/05/2012<br />
AKL<br />
10204211437 11/05/2012<br />
URITES Reagent Strip 11G (Nitrite,<br />
pH, Glucose, Protein, Occult Blood,<br />
Ketone, Urobilinogen, Specifi<br />
URIT Probe Detergent Hematology<br />
Analyzer Reagent<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
URIT ELECTRONIC GROUP CO. LTD.,<br />
CHINA<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
PT. JAYAMAS MEDICA INDUSTRI<br />
AKL<br />
20103210961 22/03/2012 ADVIA Centaur PHTN ReadyPack<br />
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
10101804023 22/03/2012 RAJAWALI LDH (Liquid UV Test) HUMAN GMBH., GERMANY PT. RAJAWALI NUSINDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305802057 21/03/2012<br />
CERU Ceruloplasmin COBAS<br />
INTEGRA cobas c system<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20105802717 22/03/2012 ADVIA Centaur aTPO ReadyPack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20105802795 22/03/2012 ADVIA Centaur FER ReadyPack<br />
AKL<br />
10206110229 22/03/2012 CONVERGYS Dil Diff (D50)<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
CONVERGENT TECHNOLOGIES GMBH &<br />
CO. KG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. GRAHA HEALTHCARE<br />
INDONESIA<br />
AKL<br />
20303210878 20/03/2012 SD Bioline Malaria Ag STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303210943 22/03/2012 SD BIOLINE Dengue Duo STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303210942 22/03/2012 SD BIOLINE Dengue IgG/IgM STANDARD DIAGNOSTICS INC., KOREA PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20207210964 22/03/2012 FIBROQUANT System Pack TULIP DIAGNOSTICS (P) LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10303801400 20/03/2012 HUMAN Hexagon Chlamydia HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102801912 09/03/2012<br />
COBAS AMPLICOR and<br />
Accessories<br />
ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20304210812 14/03/2012 AXSYM System and Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10203210810 14/03/2012<br />
AKL<br />
20304210811 14/03/2012<br />
AKL<br />
20305802128 09/03/2012<br />
AKL<br />
10305210741 09/03/2012<br />
AKL<br />
20305210736 09/03/2012<br />
AKL<br />
10101210176 31/01/2012<br />
AKL<br />
10305802052 09/03/2012<br />
CELLDYN Slide Maker Stainer and<br />
Accessories<br />
LEICA BIOSYSTEMS MELBOURNE PTY.<br />
LTD., AUSTRALIA untuk ABBOTT<br />
LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
ARCHITECT i2000 System and<br />
Accessories ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
RF-II Tina-Quant [a] Rheumatoid<br />
Factor II COBAS INTEGRA cobas c<br />
system<br />
LPALX Tina-quant [a] Lipoprotein (a)<br />
(Latex) COBAS INTEGRA cobas c<br />
system<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
C3C-2 Tina-quant [a] Complement ROCHE DIAGNOSTICS GMBH., GERMANY<br />
C3c ver.2 COBAS INTEGRA cobas c melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
system<br />
SWITZERLAND<br />
ONE TOUCH SURESTEP Glucose<br />
Control Solution<br />
LIFESCAN INC., USA<br />
PREA Prealbumin COBAS<br />
INTEGRA cobas c system<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
AKL<br />
10102704926 14/02/2012 LABMATE PZ HTL S.A., POLAND<br />
AKL<br />
10102704927 14/02/2012 DISCOVERY Multicahannel Pipettes PZ HTL S.A., POLAND<br />
AKL<br />
10102704928 14/02/2012 MULTIMATE PZ HTL S.A., POLAND<br />
AKL<br />
10102704925 14/02/2012 CLINIPET PZ HTL S.A., POLAND<br />
AKL<br />
10101210580 28/02/2012 ACCU-CHEK Go Control<br />
AKL<br />
10101210757 12/03/2012<br />
AKL<br />
10101210758 12/03/2012<br />
AKL<br />
20305802131 09/03/2012<br />
AKL<br />
10305801908 17/02/2012<br />
AKL<br />
20305210425 17/02/2012<br />
AKL<br />
20305802130 05/03/2012<br />
PDF Creator - PDF4Free v3.0<br />
LIQUICHEK Cardiac Markers Plus<br />
Control LT Level 3<br />
LIQUICHEK Cardiac Markers Plus<br />
Control LT Trilevel<br />
CRPLX C-Reactive Protein (Latex)<br />
COBAS INTEGRA cobas c system<br />
APO B ver.2 Tina-quant [a]<br />
Roche/Hitachi<br />
APO A-I ver.2 Tina-quant [a]<br />
Roche/Hitachi<br />
HAPT2 Tina-quant [a] Haptoglobin<br />
ver.2 COBAS INTEGRA cobas c<br />
systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. ROCHE INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20305802079 05/03/2012<br />
AKL<br />
10101210592 28/02/2012<br />
AKL<br />
20207210742 05/03/2012<br />
AKL<br />
20102210808 14/03/2012<br />
AKL<br />
20103210892 20/03/2012<br />
TRSF2 Tina-quant [a] Transferin<br />
ver.2 COBAS INTEGRA cobas c<br />
systems<br />
ONE TOUCH ULTRA Control<br />
Solution<br />
FRA Fructosamine COBAS<br />
INTEGRA cobas c systems<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
LIFESCAN INC., USA<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
BIOSYSTEMS Random Access<br />
Analyzer A-25 BIOSYSTEMS S.A., SPAIN PT. ELGA TAMA<br />
QUICKSCREEN Cocaine Cassette<br />
Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10102210941 22/03/2012 Tli Analyzer and accessories HOLOGIC INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20209210889 20/03/2012 ASK POLYBRENE TONYAR BIOTECH INC., TAIWAN PT. MITRASAMAYA SEJATI<br />
AKL<br />
20208210331 09/02/2012 MINDRAY S50 Calibrator<br />
AKL<br />
20208210334 09/02/2012 MINDRAY S30 Calibrator<br />
AKL<br />
20305210364 14/02/2012<br />
ABON HCV Hepatitis C Virus Rapid<br />
Test Device (Serum/Plasma)<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10101210945 22/03/2012 CENTROPATH CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101210944 22/03/2012 CENTROCAL CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
10101210946 22/03/2012 CENTRONORM CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20305210368 14/02/2012<br />
AKL<br />
20101211040 10/04/2012<br />
AKL<br />
20101211041 10/04/2012<br />
AKL<br />
20305210367 14/02/2012<br />
AKL<br />
20303210366 14/02/2012<br />
AKL<br />
20305210365 14/02/2012<br />
AKL<br />
10102210987 27/03/2012<br />
AKL<br />
10302210189 01/02/2012<br />
ABON HBsAb One Step Hepatitis B<br />
Surface Antibody Test Strip<br />
(Serum/Plasma)<br />
ON CALL Advanced Blood Glucose<br />
Monitoring System<br />
ON CALL Advanced Blood Glucose<br />
Test Strip<br />
ABON HBsAb One Step Hepatitis B<br />
Surface Antibody Test Device<br />
(Serum/Plasma)<br />
ABON Dengue Rapid Test Device<br />
(Whole/Serum/Plasma)<br />
ABON HCV Hepatitis C Virus Rapid<br />
Strip (Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
MICRO ELISA Reader 270 and<br />
Accessories BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
MICRO ELISA Washer 470 and<br />
Accessories BIOMERIEUX S.A., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20305210927 22/03/2012 TOSOH AIA PACK TgAb TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305210930 22/03/2012<br />
AKL<br />
20305210928 22/03/2012<br />
TOSOH AIA PACK TgAb Control<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA PACK TgAb Calibrator<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305210929 22/03/2012 TOSOH AIA PACK TgAb Conjugate TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10102210947 21/03/2012 MICROSCAN autoSCAN-4<br />
AKL<br />
20207210960 22/03/2012<br />
A1C-3 Tina-quant [a] Hemoglobin<br />
A1c Gen.3 COBAS INTEGRA cobas<br />
c system<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20301210958 22/03/2012<br />
MICROSCAN Neg Urine Combo<br />
Panel Type 55<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301210959 22/03/2012<br />
MICROSCAN Neg Urine MIC Panel<br />
Type 38<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20303210735 09/03/2012<br />
VITROS Immunodiagnostic Products<br />
Anti-HBc IgM (HBc M) Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101210714 09/03/2012<br />
TOSOH AIA-PACK ACTH Control<br />
Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20301210956 22/03/2012<br />
MICROSCAN Neg Combo Panel<br />
Type 50<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301210957 22/03/2012<br />
MICROSCAN Neg Breakpoint<br />
Combo Panel Type 44<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
AKL<br />
10204211001 30/03/2012 DREW 3-PAC DREW SCIENTIFIC CO. LTD., UK<br />
AKL<br />
20205210459 20/02/2012 SYSMEX XS-500i<br />
AKL<br />
20208210937 22/03/2012 XN CHECK BF<br />
AKL<br />
20208210938 22/03/2012 XN CAL PF<br />
AKL<br />
20208210939 22/03/2012 RANGE CHECK X II<br />
AKL<br />
20208210936 22/03/2012 XN CAL PF<br />
AKL<br />
20208210935 22/03/2012 XN CHECK BF<br />
AKL<br />
10201210913 21/03/2012 FLUROCELL PLT<br />
AKL<br />
10201210909 21/03/2012 FLUROCELL RET<br />
AKL<br />
10201210910 21/03/2012 FLUROCELL WPC<br />
AKL<br />
10201210911 21/03/2012 FLUROCELL WNR<br />
AKL<br />
10201210912 21/03/2012 FLUROCELL WDF<br />
AKL<br />
20205210962 22/03/2012<br />
AKL<br />
20205210963 22/03/2012<br />
AKL<br />
20305210923 22/03/2012<br />
AKL<br />
10208210908 21/03/2012<br />
AKL<br />
10101210713 09/03/2012<br />
SYSMEX CA-620 Fully Automated<br />
Coagulation Analyzer<br />
SYSMEX CA-660 Fully Automated<br />
Coagulation Analyzer<br />
SYSMEX Gene Amplication Detector<br />
RD-100i<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
STRECK INC., USA untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PACIFIC PTE. LTD., SINGAPORE<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA P<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA P<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA P<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA P<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA P<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. AEROCOM JENCO<br />
INDINESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
TOSOH AIA-PACK TPO Ab/TgAb<br />
Sample Diluting Solution TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK Intact PTH<br />
Control Set TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101210716 09/03/2012 TOSOH ST AIA-PACK FT3 TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10208210711 09/03/2012<br />
AKL<br />
10208210710 09/03/2012<br />
AKL<br />
10208210712 09/03/2012<br />
AKL<br />
20101210717 09/03/2012<br />
AKL<br />
TOSOH ST AIA-PACK E2 Sample<br />
Diluting Solution TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK Prog Sample<br />
Diluting Solution TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK CA 15-3<br />
Sample Diluting Solution TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK Cystatin C<br />
Calibrator Set TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
20101210715 09/03/2012 TOSOH ST AIA-PACK Cystatin C TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10208210880 20/03/2012 ADVIA Centaur VB12 Diluent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210881 20/03/2012<br />
ADVIA Centaur VB12<br />
DTT/Releasing Agent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210917 21/03/2012 SYVA Ph Validity Calibrator 2.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210914 21/03/2012 SYVA Ph Validity Calibrator 3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210915 21/03/2012 SYVA Ph Validity Calibrator 4.5<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101210916 21/03/2012 SYVA Ph Validity Calibrator 9.0<br />
AKL<br />
20305210951 22/03/2012 SIEMENS N Latex Ferritin<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY<br />
AKL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
20305210950 22/03/2012 SIEMENS N Latex B2 - Microglobulin PRODUCTS GMBH., GERMANY<br />
AKL<br />
20305210949 22/03/2012<br />
AKL<br />
20305210948 22/03/2012<br />
SIEMENS N Antiserum to Human a2<br />
- Microglobulin<br />
SIEMENS N Antiserum to Human<br />
Haptoglobin<br />
AKL<br />
10305210952 22/03/2012 SIEMENS N a1 - Microglobulin<br />
AKL<br />
10203211203 18/04/2012<br />
SURGIPATH Cyto Jar Specimen<br />
Transport<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY<br />
LEICA BIOSYSTEMS RICHMOND INC., USA<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
20305211280 01/05/2012 TOSOH ST AIA-PACK HBcAb TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305211281 01/05/2012 TOSOH ST AIA-PACK HBeAg TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305211282 01/05/2012 TOSOH ST AIA-PACK HBeAb TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305211283 01/05/2012<br />
AKL<br />
20305211284 01/05/2012<br />
AKL<br />
10101211327 07/05/2012<br />
AKL<br />
20102211321 07/05/2012<br />
AKL<br />
10102801698 30/04/2012<br />
AKL<br />
10303801402 20/03/2012<br />
TOSOH AIA-PACK HBV Antibody<br />
CONTROL SET TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK HBV Antigen<br />
CONTROL SET TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
HUMAN Total Iron Binding Capacity<br />
(TIBC) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN HUMALYTE and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN Humapette 8-Channel<br />
Pipettes HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN EBV (Epstein-Barr-Virus)<br />
IgG ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801643 20/03/2012 HUMAN Lipoprotein (a), Lp (a) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101801641 20/03/2012 HUMAN Cholesterol HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801640 22/03/2012 HUMAN Immunoglobulins IgG Test HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801693 22/03/2012 HUMAN CRP-hs HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305801415 20/03/2012 HUMAN Total IgE ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101801419 20/03/2012 HUMAN Humapreg Urine HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20303801421 20/03/2012 HUMAN Humatex Febrile Antigens HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10102210877 20/03/2012<br />
AKL<br />
20305801642 22/03/2012<br />
HUMAREADER PLUS and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
HUMAN Rheumatoid Factors (RF)<br />
Immunoturbidimetric Test for the<br />
Quantitative Determination HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305210885 20/03/2012 HUMAN CRP HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10204211324 07/05/2012 SYSMEX CELLCLEAN CL-50<br />
PDF Creator - PDF4Free v3.0<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
http://www.pdf4free.com<br />
PT. SYSMEX INDONESIA
AKL<br />
20103211286 01/05/2012<br />
ARCHITECT Sirolimus Whole Blood<br />
Precipitation Reagent<br />
FUJIREBIO DIAGNOSTICS, USA untuk<br />
ABBOTT LABORATORIES, USA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20208211325 07/05/2012 ABX Pentra N Control HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101211332 07/05/2012 DIALINE Pancreatic Amylase<br />
AKL<br />
20101211334 07/05/2012 DIALINE Calcium<br />
AKL<br />
20101211335 07/05/2012 DIALINE Creatinine<br />
AKL<br />
20101211333 07/05/2012 DIALINE LDH DGKC<br />
AKL<br />
20102211322 07/05/2012 ELECSYS 2010 and Accessories<br />
AKL<br />
20101211240 30/04/2012<br />
AKL<br />
10208211250 30/04/2012<br />
APOTEK GENERIK One-Step<br />
Pregnancy Test Strip<br />
ARCHITECT Testosterone Manual<br />
Diluent<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEM PTE. LTD., SINGAPORE<br />
HITACHI HIGH TECHNOLOGIES CORP.,<br />
JAPAN untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY melalui ROCHE DIAGNOST<br />
W.H.P.M BIORESEARCH & TECHNOLOGY<br />
CO. LTD., P.R. CHINA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
AKL<br />
20209210888 20/03/2012 NOVOmatic and Accessories LMB TECHNOLOGIE GMBH., GERMANY<br />
AKL<br />
10208210901 21/03/2012 LABNOVATION Diluent CA<br />
AKL<br />
10208211323 07/05/2012<br />
AKL<br />
20305211331 07/05/2012<br />
AKL<br />
20305211330 07/05/2012<br />
AKL<br />
20305211329 07/05/2012<br />
AKL<br />
20101211328 07/05/2012<br />
AKL<br />
20305211384 10/05/2012<br />
AKL<br />
20305211381 10/05/2012<br />
AMPLICOR HCV Specimen<br />
Preparation Kit, version 2.0<br />
LABNOVATION TECHNOLOGIES INC., P.R.<br />
CHINA<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. DIATRON PROMEDIKA<br />
PT. ROCHE INDONESIA<br />
PT. MANDIRI NUGRAHA<br />
AJITUNNGAL<br />
PT. ABBOTT INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. ROCHE INDONESIA<br />
TOSOH AIA-PACK HBcAb<br />
Calibrator Set TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK HBeAb<br />
Calibrator Set TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK HBeAg<br />
Calibrator Set TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK FT3 Calibrator<br />
Set TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
ABBOTT RealTime CMV Calibrator<br />
Kit<br />
ABBOTT RealTime CMV<br />
Amplification Reagent Kit<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20305211382 10/05/2012 ABBOTT RealTime CMV Control Kit ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10101211680 22/05/2012<br />
IMMULITE 2000 Systems BP3<br />
IGFBP-3<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20205211285 01/05/2012 CELLDYN 3200 and Accessories ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
10101211679 22/05/2012 IMMULITE 2000 System IG1 IGF-1<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20303211692 22/05/2012 ABBOTT Rubella IgG Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20303211691 22/05/2012 ABBOTT Rubella IgG Calibrator ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10206211380 10/05/2012 DREW Hematology Sheath DREW SCIENTIFIC CO. LTD., UK<br />
AKL<br />
10204211379 10/05/2012 DREW Hematology Clean EX-ZYME DREW SCIENTIFIC CO. LTD., UK<br />
AKL<br />
10208211378 10/05/2012 DREW Hematology Lyse EX-LYSE DREW SCIENTIFIC CO. LTD., UK<br />
AKL<br />
10208211377 10/05/2012 DREW Hematology Diluent EX-ISO DREW SCIENTIFIC CO. LTD., UK<br />
PT. AEROCOM JENCO<br />
INDINESIA<br />
PT. AEROCOM JENCO<br />
INDINESIA<br />
PT. AEROCOM JENCO<br />
INDINESIA<br />
PT. AEROCOM JENCO<br />
INDINESIA<br />
AKL<br />
20303210884 20/03/2012 AIM Chikungunya IgM Rapid Test TULIP DIAGNOSTICS (P) LTD., INDIA PT. AKURAT INTAN MADYA<br />
AKL<br />
20303210883 20/03/2012 AIM Leptospira IgM Rapid Test TULIP DIAGNOSTICS (P) LTD., INDIA PT. AKURAT INTAN MADYA<br />
AKL<br />
20303210882 20/03/2012<br />
AKL<br />
AIM MALARIA Duo Rapid Test<br />
(PAN/P, Falcifarum) TULIP DIAGNOSTICS (P) LTD., INDIA PT. AKURAT INTAN MADYA<br />
20305211119 16/04/2012 INNO-LIPA HLA-C INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302211120 16/04/2012 INNO-LIPA Mycobacteria V2 Amp INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
20102211383 10/05/2012<br />
PRECISION C200i Auto Chemistry<br />
Analyzer and Accessories<br />
SHENZHEN LANDWIND INDUSTRY CO.<br />
LTD., CHINA<br />
PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
10203210677 06/03/2012 CELLPREP PLUS and Accessories BIODYNE CO. LTD., KOREA PT. BIOZATIX INDONESIA<br />
AKL<br />
20101211288 02/05/2012 DIALINE Total Protein Buiret-Method<br />
AKL<br />
20103211274 01/05/2012<br />
AKL<br />
20103211275 01/05/2012<br />
AKL<br />
20209211261 30/04/2012<br />
AKL<br />
20209211260 30/04/2012<br />
FOKUS 3 in 1 Panel DOA Cassette<br />
(Amphetamine, Morphine,<br />
Marijuana/THC)<br />
FOKUS 5 in 1 Panel DOA Cassette<br />
(Amphetamine, Benzodiazepine,<br />
Marijuana/THC, Methamphetamine,<br />
Morphine)<br />
HELMER DH4 Plasma Thawer and<br />
Accessories<br />
HELMER DH8 Plasma Thawer and<br />
Accessories<br />
DIASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY melalui DIALINE DIAGNOSTIC<br />
SYSTEMS PTE. LTD., SINGAPORE<br />
PULSE SCIENTIFIC INC., CANADA<br />
PULSE SCIENTIFIC INC., CANADA<br />
HELMER INC., USA<br />
HELMER INC., USA<br />
PT. DIATRON PROMEDIKA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20209211262 30/04/2012<br />
HELMER HPF120 Plasma Storage<br />
freezer and accessories<br />
HELMER INC., USA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20209211263 30/04/2012<br />
AKL<br />
20209211264 30/04/2012<br />
AKL<br />
20209211265 30/04/2012<br />
HELMER HPF125 Plasma storage<br />
freezer and accessories<br />
HELMER HLR125 Laboratory<br />
Refrigerator and accessories<br />
HELMER HLR111 Laboratory<br />
Refrigerator and accessories<br />
AKL<br />
20303211683 22/05/2012 VIROTEC Dengue IgG/IgM XP<br />
HELMER INC., USA<br />
HELMER INC., USA<br />
HELMER INC., USA<br />
MED CAN TECHNOLOGY (BEIJING) CO.<br />
LTD., CHINA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
PT. INDEC DIAGNOSTICS<br />
AKL<br />
10101211253 30/04/2012<br />
AKL<br />
10204211252 30/04/2012<br />
AKL<br />
20305211290 02/05/2012<br />
AKL<br />
20305211289 02/05/2012<br />
CLINIQA Liquid QC Cardiac Marker<br />
Control VS<br />
VITROS Immunodiagnostic Products<br />
Maintenance Pack<br />
VITROS Immunodiagnostic Products<br />
Anti-Hbe Reagent Pack<br />
VITROS Immunodiagnostic Products<br />
HBeAg Reagent Pack<br />
CLINIQA CORPORATION, USA untuk<br />
ORTHO-CLINICAL DIAGNOSTICS INC., USA<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305211291 02/05/2012<br />
VITROS Immunodiagnostic Products<br />
HBsAg ES Confirmatory Kit<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101211669 22/05/2012<br />
VITROS Immunodiagnostic Products<br />
NT-proBNP Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101211668 22/05/2012<br />
VITROS Immunodiagnostic Products<br />
Cortisol Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303211684 22/05/2012<br />
VITROS Immunodiagnostics<br />
Products Syphilis TPA Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101211687 22/05/2012<br />
VITROS Immunodiagnostics<br />
Products T3 Uptake Calibrators<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101211685 22/05/2012<br />
VITROS Immunodiagnostics<br />
Products NT-proBNP Calibrators<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303211688 22/05/2012<br />
VITROS Immunodiagnostics<br />
Products Syphilis TPA Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303211694 22/05/2012<br />
VITROS Immunodiagnostics<br />
Products Syphilis TPA Control<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101211686 22/05/2012<br />
VITROS Immunodiagnostics<br />
Products T3 Uptake Reagent Pack<br />
AKL<br />
10201211395 11/05/2012 MINDRAY M-68FN Dye<br />
AKL<br />
10201211394 11/05/2012 MINDRAY M-68FD Dye<br />
AKL<br />
10208211396 11/05/2012 MINDRAY M-68LH Lyse<br />
AKL<br />
10204211397 11/05/2012 MINDRAY Probe Cleanser<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10201211398 11/05/2012 MINDRAY M-68FR Dye<br />
AKL<br />
10208211406 11/05/2012 MINDRAY M-68FR Diluent<br />
AKL<br />
10208211407 11/05/2012 MINDRAY M-68LN Lyse<br />
AKL<br />
10208211405 11/05/2012 MINDRAY M-68DS Diluent<br />
AKL<br />
10101211404 11/05/2012<br />
AKL<br />
10101211403 11/05/2012<br />
AKL<br />
10101211402 11/05/2012<br />
AKL<br />
10101211401 11/05/2012<br />
AKL<br />
10101211444 11/05/2012<br />
MINDRAY TG Triglycerides Kit<br />
(GPO-POD Method)<br />
MINDRAY TC Total Cholesterol Kit<br />
(CHOD-POD Method)<br />
MINDRAY LDL-C LDL-Cholesterol<br />
Kit (Direct Method)<br />
MINDRAY HDL-C HDL-Cholesterol<br />
Kit (Direct Method)<br />
MINDRAY TP Total Cholesterol Kit<br />
(Biuret Method)<br />
AKL<br />
10208211408 11/05/2012 MINDRAY M-68LD Lyse<br />
AKL<br />
10208211409 11/05/2012 MINDRAY M-68LB Lyse<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
AKL<br />
10204211386 10/05/2012 SEBIA Tampon Tris-Barbital SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10101211385 10/05/2012 SEBIA Hydrogel HR K20 SEBIA, FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10204211387 10/05/2012<br />
AKL<br />
10101211717 30/05/2012<br />
SEBIA Solution de Lavage / Wash<br />
Solution SEBIA, FRANCE PT. RIOCA MEDICA<br />
PRECICONTROL TSH Elecsys and<br />
cobas e analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20101211254 30/04/2012<br />
DIMENSION EZCR Enzymatic<br />
Creatinine Flex Reagent Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101211255 30/04/2012 DIMENSION ENZ II CAL<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101211256 30/04/2012 DIMENSION ALTI<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302211677 22/05/2012<br />
MICROSCAN Synergies Plus Pos<br />
Broth<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302211676 22/05/2012<br />
AKL<br />
20304211258 30/04/2012<br />
AKL<br />
20306211259 30/04/2012<br />
AKL<br />
20101211302 02/05/2012<br />
MICROSCAN Rapid Pos ID Panel<br />
Type 2<br />
ABBOTT Vysis ALK Break Apart<br />
FISH Probe Kit<br />
ABBOTT RealTime mS9 Colorectal<br />
Cancer Control Kit<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., GERMANY<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
MINICOLLECT Tube 9NC<br />
Coagulation 3.2% GREINER BIO-ONE GMBH., AUSTRIA PT. WIDYA MITRA PERSADA<br />
AKL<br />
20101210726 09/03/2012 AIM Micro-Albumin Rapid Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20103210728 09/03/2012<br />
AIM Drugtest Uri MULTI PANEL A<br />
(AMP-MET-BZO-MOP-THC) VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20303210729 09/03/2012 AIM TPHA Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20306210725 09/03/2012 AIM CA 125-Q Rapid Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20103210727 09/03/2012<br />
AIM Drugtest Uri MULTI (MET-<br />
MOP-THC) VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20305211002 02/04/2012 i-CHROMARF (IgM) / CRP BODITECH MED INC., KOREA PT. CAKRA MEDIKA UTAMA<br />
AKL<br />
10208210924 22/03/2012 BIOMERIEUX Serum Free BIOMERIEUX SA., FRANCE PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
20303211069 11/04/2012<br />
FOKUS DIAGNOSTIC Malaria<br />
Pan/Pf Cassette<br />
CORE DIAGNOSTICS LTD., CHINA<br />
AKL<br />
20101211058 11/04/2012 FOKUS Amphetamine Strip PULSE SCIENTIFIC INC.,CHINA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
20101210875 20/03/2012<br />
PDF Creator - PDF4Free v3.0<br />
VITROS Immunodiagnostic products<br />
Folate Pack 1/2 Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
http://www.pdf4free.com<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA
AKL<br />
20101210719 09/03/2012<br />
VITROS Immunodiagnostic products<br />
Folate (Fol) Calibrator<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101210718 09/03/2012<br />
VITROS Immunodiagnostic products<br />
Ferritin (Ferr) Calibrator<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210720 09/03/2012<br />
VITROS Immunodiagnostic products<br />
HBeAg Calibrator<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210721 09/03/2012<br />
VITROS Immunodiagnostic products<br />
Anti-Hbe (aHBe) Calibrator<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303210731 09/03/2012<br />
AKL<br />
20305210906 21/03/2012<br />
AKL<br />
20102211007 04/04/2012<br />
VITROS Immunodiagnostic products<br />
Anti-HAV Total (HAV T) Controls<br />
VITROS Immunodiagnostic products<br />
Hbe Control<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
BIOTECNICA BT 3500 Random<br />
Access Chemistry Analyzer and<br />
Accessories BIOTECNICA INSTRUMENTS SpA., ITALY PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305210940 22/03/2012 NOVA Lite ANCA Kit INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20101210581 28/02/2012<br />
BILD2 cobas c III Direct Bilirubin<br />
Gen.2<br />
AKL<br />
10208210904 21/03/2012 LABNOVATION RT-Diluent<br />
AKL<br />
10204210899 21/03/2012 LABNOVATION Cleaner CA<br />
AKL<br />
10208210902 21/03/2012 LABNOVATION RT-Lyse<br />
AKL<br />
10208210900 21/03/2012 LABNOVATION Lyse CA<br />
AKL<br />
10204210903 21/03/2012 LABNOVATION RT-Cleaner<br />
AKL<br />
20101210954 22/03/2012<br />
MICROWELL ELISA NTSH<br />
(Neonatal TSH)<br />
AKL<br />
20305210953 22/03/2012 MICROWELL ELISA Ferritin<br />
AKL<br />
20101210955 22/03/2012 MICROWELL ELISA Free T3<br />
ROCHE DIAGNOSTIC GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
LABNOVATION TECHNOLOGIES INC., P.R.<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC., P.R.<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC., P.R.<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC., P.R.<br />
CHINA<br />
LABNOVATION TECHNOLOGIES INC., P.R.<br />
CHINA<br />
DIAGNOSTIC AUTOMATION/ CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTIC AUTOMATION/ CORTEZ<br />
DIAGNOSTICS INC., USA<br />
DIAGNOSTIC AUTOMATION/ CORTEZ<br />
DIAGNOSTICS INC., USA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. ROCHE INDONESIA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SARANA MAJU SEJAHTERA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103210891 20/03/2012 QUICKSCREEN Cocaine Dipstic Kit PHAMATECH INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20102210874 20/03/2012<br />
IMMULITE 2000XPI and<br />
Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301210925 22/03/2012<br />
MICROSCAN WalkAway -40 Plus<br />
Instrument and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301210926 22/03/2012<br />
MICROSCAN WalkAway -96 Plus<br />
Instrument and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211038 09/04/2012<br />
SYVA Chromin (VI) Validity<br />
Calibrator 50<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211039 09/04/2012 SYVA Ph Validity System<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211037 09/04/2012<br />
SYVA EMIT Ethyl Alcohol 100 mg/dl<br />
Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211035 09/04/2012<br />
SYVA CHromin (VI) Validity<br />
Calibrator/CONTROL 100<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211036 09/04/2012 SYVA EMIT Ethyl Alcohol Negative<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101211900 15/06/2012 AIM Calcium (Ca) 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
10101211868 14/06/2012 AIM GGT 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
10101211869 14/06/2012 AIM Magnesium (Mg) 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101211803 08/06/2012 ABX Minotrol Retic HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305211753 05/06/2012<br />
AKL<br />
20305211754 05/06/2012<br />
AKL<br />
10204211681 22/05/2012<br />
AKL<br />
20205211806 08/06/2012<br />
AKL<br />
20102211805 08/06/2012<br />
AKL<br />
20205211804 08/06/2012<br />
VITROS Immunodiagnostic Products<br />
Myoglobin Calibrators<br />
VITROS Immunodiagnostic Products<br />
Anti - HBc Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS., UK<br />
ORTHO-CLINICAL DIAGNOSTICS., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
TOSOH ST-AIA-PACK HbA1c<br />
Pretreatment Solution TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
MINDRAY BC-3600 AUTO<br />
Hematology Analyzer and<br />
Accessories<br />
MINDRAY BS-800M Chemistry<br />
Analyzer and Accessories<br />
MINDRAY BC-6800 Auto<br />
Hematology Analyzer and<br />
Accessories<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R. CHINA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
AKL<br />
20103211742 04/06/2012<br />
VCARE Multi-Drug One Step Multi-<br />
Line 3 Drug Screen Test Device<br />
(Urine)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20103211741 04/06/2012<br />
VCARE Multi-Drug One Step Multi-<br />
Line 6 Drug Screen Test Panel with<br />
Integrated Cup (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20103211743 04/06/2012<br />
VCARE Multi-Drug One Step 6 Drug<br />
Screen Test Panel (Urine) (AMP,<br />
MOP, THC, MET, COC, BZO)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20103211744 04/06/2012<br />
VCARE Multi-Drug One Step 3 Drug<br />
Screen Test Panel (Urine) (AMP,<br />
MOP, THC)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. CHINA melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20305211719 31/05/2012 DIALAB RF Control DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20305210730 09/03/2012<br />
AKL<br />
10102211772 06/06/2012<br />
DIALAB CRP Control Set (High<br />
Sens, Range) DIALAB GMBH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
CYAN Pipettes Multi-Channel-<br />
Variable Volume-Pipettes CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
20209211769 06/06/2012 CYAN CL009 Hematocrit Centrifuge CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
10102211761 06/06/2012 CYAN Strip Urine Strip Reader CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
20102211762 06/06/2012<br />
AKL<br />
20205211759 06/06/2012<br />
AKL<br />
10102211763 06/06/2012<br />
AKL<br />
20205211764 06/06/2012<br />
CYANPro Automatic Biochemistry<br />
Analyzer CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
CYANHemato Automatic Hematology<br />
Analyzer CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
CYANStart Semi-Automatic<br />
Biochemistry Analyzer CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
CYPRESS CL004 Blood Cell<br />
Counter with 9 Windows CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
20209211768 06/06/2012 CYAN CL008 Table Top Centrifuge CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
10206211766 06/06/2012 CYPRESS CL006 Haemocytometer CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
20903211760 06/06/2012 CYAN CL012 Dry Autoclave CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
10203211767 06/06/2012 CYANScope CM001 CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
AKL<br />
20207211765 06/06/2012<br />
AKL<br />
20101211750 05/06/2012<br />
AKL<br />
20207211750 05/06/2012<br />
AKL<br />
10204211867 14/06/2012<br />
AKL<br />
20306211749 05/06/2012 LYNOAMP BC<br />
CYPRESS CL005 Sahli<br />
Haemometer CYPRESS DIAGNOSTICS, BELGIUM PT. RINDANG BUMI UTAMA<br />
BENECHECK Blood Glucose Test<br />
Strip<br />
BENECHECK Hb Hemoglobin Test<br />
Strip<br />
AKL<br />
20206211703 25/05/2012 INNOVANCE VWF Ac<br />
AKL<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN<br />
GENERAL LIFE BIOTECHNOLOGY CO.<br />
LTD., TAIWAN<br />
PT. SAMUDIA BAHTERA<br />
PT. SAMUDIA BAHTERA<br />
SEKISUI HbA1c Blank Solution for<br />
NORUDIA N HbA1c SEKISUI MEDICAL CO., LTD., JAPAN PT SUMBERMITRA AGUNGJAYA<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION, JAPAN melalui SYSMEX<br />
ASIA PA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE., LTD., SINGA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
30305211864 14/06/2012 HUMAN Hexagon HIV HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303211796 08/06/2012 HUMAN Hexagon H. Pylori HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10204211678 22/05/2012 LYNORHAG<br />
AKL<br />
10303211820 12/06/2012 ASO Latex Autom<br />
AKL<br />
10208211809 08/06/2012 AIM Diluent Reagent<br />
AKL<br />
10208211810 08/06/2012 AIM Lyse Reagent<br />
AKL<br />
10204211808 08/06/2012 AIM Detergent Reagent<br />
SYSMEX INTERNATIONAL REAGENTS CO.<br />
LTD., JAPAN untuk SYSMEX<br />
CORPORATION., JAPAN melalui SYSMEX<br />
ASIA P<br />
SENTINEL CH., S.P.A., ITALY untuk<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERM<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO., LTD., P.R. CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO., LTD., P.R. CHINA<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO., LTD., P.R. CHINA<br />
PT. SYSMEX INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. AKURAT INTAN MADYA<br />
PT. AKURAT INTAN MADYA<br />
PT. AKURAT INTAN MADYA<br />
AKL<br />
20301211798 08/06/2012<br />
MICROSCAN Pos MIC Panel Type<br />
29<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301211799 08/06/2012<br />
MICROSCAN Pos Combo Panel<br />
Type 33<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211800 08/06/2012<br />
SYVA Specific Gravity Validity<br />
Calibrator 1.0200<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211801 08/06/2012<br />
SYVA Specific Gravity Validity<br />
Calibrator 1.0030<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20103211802 08/06/2012 SYVA Specific Gravity Validity Test<br />
AKL<br />
20101211797 08/06/2012<br />
AKL<br />
20305211930 19/06/2012<br />
AKL<br />
20305211929 19/06/2012<br />
AKL<br />
20208211926 19/06/2012<br />
AKL<br />
20303211956 20/06/2012<br />
ASTL Aspartate Aminotransferase<br />
COBAS INTEGRA cobas c systems<br />
PreciControl Anti-HBc Elecsys and<br />
Cobas e Analyzers<br />
PreciControl Anti-HBc IgM Elecsys<br />
and Cobas e Analyzers<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
TOSOH ST AIA-PACK HbA1c<br />
Calibrator Set TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
OnSite Toxoplasma IgG/IgM Rapid<br />
Test<br />
CTK BIOTECH INC., USA<br />
AKL<br />
20303211954 20/06/2012 OnSite Malaria Pf/Pv Ag Rapid Test CTK BIOTECH INC., USA<br />
AKL<br />
20303211955 20/06/2012<br />
OnSite TB IgG/IgM Combo Rapid<br />
Test<br />
CTK BIOTECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10204211936 19/06/2012<br />
RAPIDPOINT 400/405 Series<br />
Wash/Waste Cartridge<br />
AKL<br />
10101211928 19/06/2012 DIASYS UIBC FS<br />
AKL<br />
20102211690 22/05/2012 ILAB ARIES and Accessories<br />
AKL<br />
20101211689 22/05/2012<br />
AKL<br />
20101211444 11/05/2012<br />
AKL<br />
20305211735 04/06/2012<br />
AKL<br />
20305211733 04/06/2012<br />
AKL<br />
20101211732 04/06/2012<br />
AKL<br />
20101211731 04/06/2012<br />
GEM PREMIER 400 and<br />
Accessories<br />
MINDRAY TP Total Protein Kit<br />
(Biuret Method)<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
DYASYS DIAGNOSTIC SYSTEMS GMBH.,<br />
GERMANY<br />
INSTRUMENTATION LABORATORY SPA,<br />
Italy<br />
INSTRUMENTATION LABORATORY CO.,<br />
USA melalui INSTRUMETATION<br />
LABORATORY SPA, Italy<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD, P.R. China<br />
PT. SIEMENS INDONESIA<br />
PT. MULTIREDJEKI KITA<br />
PT. MENDJANGAN<br />
PT. MENDJANGAN<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
TOSOH ST AIA-PACK Myoglobin<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK TPOAb<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH ST AIA-PACK cTnl 3 Gen<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
TOSOH AIA-PACK CK-MB<br />
Calibrator Set TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20103211746 04/06/2012<br />
VCARE MOP One Step Morphine<br />
Test Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. China melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103211745 04/06/2012<br />
VCARE THC One Step Marijuana<br />
Test Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. China melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20103211747 04/06/2012<br />
AKL<br />
20101211738 04/06/2012<br />
AKL<br />
20206211737 04/06/2012<br />
VCARE AMP One Step<br />
Amphetamine Test Device (Urine)<br />
ON-CALL Platinum Blood Glucose<br />
Monitoring System<br />
MISSION Plus Hb Hemoglobin Test<br />
Stips<br />
AKL<br />
21604211736 04/06/2012 ON-CALL Platinum Blood Lancet<br />
AKL<br />
20205211739 04/062012<br />
AKL<br />
20101211740 04/06/2012<br />
MISSION Plus Hb Hemoglobin<br />
Testing System<br />
ON-CALL Platinum Blood Glucose<br />
Test Strip<br />
ABON BIOPHARM (HANGZHOU) CO. LTD.,<br />
P. R. China melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSU<br />
ACON BIOTECH (HANGZHOU) CO. LTD., P.<br />
R. China<br />
ACON BIOTECH (HANGZHOU) CO. LTD., P.<br />
R. China<br />
ACON BIOTECH (HANGZHOU) CO. LTD., P.<br />
R. China<br />
ACON BIOTECH (HANGZHOU) CO. LTD., P.<br />
R. China<br />
ACON BIOTECH (HANGZHOU) CO. LTD., P.<br />
R. China<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
10203211720 31/05/2012 INTELLIPATH FLX BIOCARE MEDICAL LLC., USA PT. BIOZATIX INDONESIA<br />
AKL<br />
20303211782 07/06/2012<br />
AKL<br />
10101211729 04/06/2012 COBAS BM-Control-Lactate<br />
AKL<br />
10208211730 04/06/2012<br />
ADVANTAGE Chikungunya IgM<br />
Card J. MITRA & CO., PVT., LTD., INDIA PT. ABHIMATA MANUNGGAL<br />
Diluents Universal Elecsys and<br />
cobas e analyzers<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20102211326 07/05/2012 ABX Pentra 400 and Accessories HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20102211693 22/05/2012<br />
ADVIA CENTAUR CP and<br />
Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101211932 19/06/2012<br />
IMMULITE 2000 System TPI<br />
Troponin I<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101211933 19/06/2012<br />
IMMULITE 2000 System HCV<br />
Homocysteine<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20102211748 04/06/2012 SELECTRA XL VITAL SCIENTIFIC BV., NETHERLAND PT. MRK DIAGNOSTICS<br />
AKL<br />
20303211785 07/06/2012<br />
ADVIA CENTAUR HAV IgM Ready<br />
Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305211757 05/06/2012 ABX Pentra Ferritin Cal HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305211758 05/06/2012 ABX Pentra Low CRP Control HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10102210934 22/03/2012<br />
AKL<br />
10102211981 22/06/2012<br />
ACURA Electron Electronic<br />
Micropipettes<br />
SOCOREX ISBA SA, Switzerland<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
TC-URITEK 101 Urine Strip Reader<br />
and Accessories TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
10102211982 22/06/2012<br />
TC-3300 Semi-Automatic Chemistry<br />
Analyzer and Accessories TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
10102211980 22/06/2012 TC-96+ ELISA Microplate Reader TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
30305210976 26/03/2012 ALERE Determine HIV-1/2 ALERE MEDICAL CO. LTD., Japan PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
10102211938 19/06/2012<br />
AKL<br />
20102210573 28/02/2012<br />
AKL<br />
10102211978 22/06/2012<br />
AKL<br />
10101212095 04/07/2012<br />
AKL<br />
20306212096 04/07/2012<br />
LEICA ASP6025 High Performance<br />
Tissue Processor and Accessories<br />
LEICA BIOSYSTEMS NUSSLOCH GmbH.,<br />
Germany melalui LEICA MICROSYSTEMS<br />
(SEA) PTE., LTD., Singapore<br />
PT. BIOGEN SCIENTIFIC<br />
METROLAB 2300 Plus Clinical<br />
Analyzer UV-VIS METROLAB S.A., Argentina PT. CITRAMED INDONESIA<br />
SCREEN POINT Blochemistry<br />
Analyzer HOSPITEX DIAGNOSTICX S.r.l., Italy PT. DEMKA SAKTI<br />
LIQUICHEK Cardiac Markers Plus<br />
Control (Level 1, 2 & 3)<br />
LYPHOCHEK Tumor Marker Plus<br />
Control (Level 1, 2 &3)<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE., LTD.,<br />
Singapore<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE., LTD.,<br />
Singapore<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305212094 04/07/2012<br />
LYPHOCHEK Immunology Plus<br />
Control Bilevel<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE., LTD.,<br />
Singapore<br />
AKL<br />
INFOPIA CO., LTD., Korea untuk ENTRA<br />
10101211984 22/06/2012 MYGLUCOHEALTH Control Solution HEALTH SYSTEMS., USA<br />
AKL<br />
20101212101 06/07/2012<br />
AKL<br />
10208211991 26/06/2012<br />
MYGLUCOHEALTH Blood Glucose<br />
Monitoring System<br />
INFOPIA CO., LTD., Korea untuk ENTRA<br />
HEALTH SYSTEMS LLC., USA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
MYTHIC 18 Cyanide Free Lytic<br />
Solution ORPHEE SA., Switzerland PT. INDOLABTEK DINAMIKA<br />
AKL<br />
10208211990 26/06/2012 MYTHIC 18 Diluent ORPHEE SA., Switzerland PT. INDOLABTEK DINAMIKA<br />
AKL<br />
20303212093 04/07/2012 DALF AL Dengue NS1 Ag Test<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO,<br />
LTD, China Dikemas Ulang Oleh PT. JAFAREL<br />
MEDIATICS, Jakarta<br />
AKL<br />
10208211979 22/06/2012 0.8 % SURGISCREEN ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
AKL<br />
10204211977 22/06/2012 ORTHO 20X Wash Buffer ORTHO-CLINICAL DIAGNOSTIC INC., USA<br />
AKL<br />
20305210921 21/03/2012<br />
VITROS Immunodiagnostic Products<br />
Anti-HBc Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JAFAREL MEDIATICS<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210922 21/03/2012<br />
VITROS Immunodiagnostic Products<br />
Myoglobin Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTIC INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303210734 09/03/2012<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV Total (HAV T)<br />
Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210876 20/03/2012<br />
VITROS Immunodiagnostics<br />
Products Ferrittin Reagent Pack<br />
ORTHO-CLINICAL DIAGNOSTICS, INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303210732 09/03/2012<br />
VITROS Immunodiagnostic Products<br />
Anti-HAV IgM (HAV M) Controls<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210733 09/03/2012<br />
VITROS Immunodiagnostics<br />
Products Anti-HBc (aHBc) Controls<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210919 21/03/2012<br />
VITROS Immunodiagnostics<br />
Products Anti-HAV Total Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210920 21/03/2012<br />
VITROS Immunodiagnostics<br />
Products Anti-HBc IgM Calibrator<br />
ORTHO-CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20305210907 21/03/2012<br />
VITROS Immunodiagnostic Prosucts<br />
Anti-HBc IgM Controls<br />
ORTHO-CLINICAL DIAGNOSTIC, INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20105210919 21/03/2012<br />
VITROS Immunodiagnostic Products<br />
Anti-HAV Total Calibrator<br />
ORTHO-CLINICAL DIAGNOSTIC, INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101210905 21/03/2012<br />
AKL<br />
20105210920 21/03/2012<br />
AKL<br />
20101210918 21/03/2012<br />
VITROS Immunodiagnostic Produtcs<br />
Vitamin B12 Reagent Pack 1/2<br />
VITROS Immunodiagnostic Produtcs<br />
Anti-HBc IgM Calibrator<br />
VITROS Immunodiagnostics<br />
Produtcs Metabolism Controls<br />
ORTHO-CLINICAL DIAGNOSTIC, INC., UK<br />
ORTHA-CLINICAL DIAGNOSTIC. INC., UK<br />
ORTHA-CLINICAL DIAGNOSTIC. INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20101211778 07/06/2012 TOSOH ST AIA-PACK cTnl 3rd Gen TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305211776 07/06/2012 TOSOH ST AIA-PACK Myoglobin TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305211777 07/06/2012 TOSOH AIA-PACK TPOAb TOSOH CORPORATION, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20208211776 07/06/2012<br />
TOSOH AIA-PACK HbA1c Control<br />
Set TOSOH CORPORATION,, Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20101211780 07/06/2012 TOSOH ST AIA-PACK CK-MB TOSOH CORPORATION., Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
10101212000 28/06/2012<br />
AKL<br />
10101211999 28/06/2012<br />
AKL<br />
20303211961 21/06/2012<br />
AKL<br />
20303211960 21/06/2012<br />
AKL<br />
10303211784 07/06/2012<br />
WOMAN CHOICE Menopause Test<br />
Medstream IND DIAGNOSTIC INC., Canada PT. LANIROS DIAN PHARMA<br />
WOMAN CHOICE Menopause Test<br />
Strip IND DIAGNOSTIC INC., Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST Malaria Antibody<br />
Test Cassette IND DIAGNOSTIC INC., Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST Malaria Antigen Test<br />
Cassette IND DIAGNOSTIC INC., Canada PT. LANIROS DIAN PHARMA<br />
SPEEDYTEST Tuberculosis<br />
Cassette Test<br />
AIDE DIAGNOSTIC., China untuk IND<br />
DIAGNOSTIC INC., Canada<br />
PT. LANIROS DIAN PHARMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20305211942 19/06/2012<br />
ABON HBsAg One Step Hepatitis B<br />
Surface Antigen Test Strip<br />
(Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20101211940 19/06/2012<br />
ABON Hcg One Step Pregnancy<br />
Test Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303211939 19/06/2012<br />
ABON Oxy One Step Oxycodone<br />
Test Device<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305211941 19/06/2012<br />
ABON HBsAg One Step Hepatitis B<br />
Surface Antigen Test Device<br />
(Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303211943 19/06/2012<br />
ABON Malaria p.f Rapid Test Device<br />
(Whole Blood)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303211944 19/06/2012<br />
ABON Torch Antibodies Combo<br />
Rapid Test Device<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303211945 19/06/2012<br />
ABON Gonorrhea and Chlamydia<br />
Combo Test (Swab)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303211946 19/06/2012<br />
ABON Syphilis Ultra Rapid Test<br />
Device (Whole Blood/Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20103211947 19/06/2012<br />
ABON Multi-Drug One step 4 Drug<br />
Screen Test Panel (Urine)<br />
(AMP/MOP/THC/KET)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20103211950 19/06/2012<br />
ABON COT One Step Cotinine Test<br />
Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20101211949 19/06/2012<br />
ABON cTnl One Step Troponin I<br />
Test Device (Whole<br />
Blood/Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20103211951 19/06/2012<br />
ABON KET One Step Ketamine Test<br />
Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20103211948 19/06/2012<br />
ABON MDMA One Step Ecstasy<br />
Test Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303210788 14/03/2012<br />
ONSITE Syphilis Ab Rapid Test -<br />
Dip Strip<br />
CTK BOITECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20303210789 14/03/2012<br />
ONSITE Syphilis Ab Rapid Test -<br />
Cassette<br />
CTK BITECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305210790 14/03/2012<br />
ONSITE HBsAg Rapid Test -<br />
Cassette<br />
CTK BITECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305210791 14/03/2012<br />
ONSITE HBsAg Rapid Test -Dip<br />
Strip<br />
CTK BITECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20305210792 14/03/2012<br />
ONSITE HCV Ab Rapid Test -<br />
Cassette<br />
CTK BITECH INC., USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10303210794 14/03/2012<br />
ABON One Step H. Pylori Antigen<br />
Test Device (Feses)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10303210796 14/03/2012<br />
ABON One Step H. Pylori Antigen<br />
Test Device (Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSULTING<br />
SERVICE CO. LTD., CHINA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10303210797 14/03/2012<br />
ABON One Step Rotavirus and<br />
Adenovirus Combo Test Device<br />
(Feses)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
10303210795 14/03/2012<br />
ABON Teberculosis Rapid Test<br />
Devices (Whole Blood/<br />
Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20304210793 14/03/2012<br />
AKL<br />
20303211781 07/06/2012<br />
ABON Syphilis Ultra Rapid Test Strip<br />
(Whole Blood/ Serum/Plasma)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
SD BIOLINE Malaria Ag P.f/Pan<br />
POCT STANDARD DIAGNOSTICS INC., Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20303210897 21/03/2012 SD BIOLINE Leptospira IgM STANDARD DIAGNOSTICS INC., Korea PT. MEGA MEDIKA MANDIRI<br />
AKL<br />
20101211833 13/06/2012<br />
V CARE HCG One Step Pregnancy<br />
Test Device (Urine/Serum)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20103211836 13/06/2012<br />
V CARE Multi-Drug One Step 5<br />
Drug Screen Test Panel<br />
(Urine)(AMP, MOP, THC, MET,<br />
COC)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20101211835 13/06/2012<br />
V CARE HCG One Step Pregnancy<br />
Test Strip (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20101211834 13/06/2012<br />
V CARE HCG One Step Pregnancy<br />
Test Device (Urine)<br />
ABON BIOPHARM (HANGZHOU) CO, LTD.,<br />
P.R, China Melalui INVERNESS MEDICAL<br />
INNOVATION (HANGZHOU) CONSUL<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
AKL<br />
20102211992 26/06/2012 SELECTRA E VITAL SCIENTIFIC BV., Netherland PT. MRK DIAGNOSTICS<br />
AKL<br />
10101211827 13/06/2012 PROLINE - b Trulab N<br />
AKL<br />
10101211826 13/06/2012 PROLINE - b Trulab P<br />
AKL<br />
20101212002 28/06/2012 PROLINE - b TruCal U<br />
AKL<br />
20101212005 28/06/2012 PROLINE - a Albumin FS<br />
AKL<br />
10101211829 13/06/2012<br />
AKL<br />
10101211828 13/06/2012<br />
PROLINE -a ALAT (GPT) FS (IFCC<br />
mod.)<br />
PROLINE -a Gamma-GT FS (Szasz<br />
mod./ IFCC stand.)<br />
AKL<br />
20101212001 28/06/2012 PROLINE - a Total Protein FS<br />
AKL<br />
10101211830 13/06/2012 PROLINE - a Uric Acid FS TBHBA<br />
AKL<br />
20101212092 04/07/2012<br />
AKL<br />
20305210887 20/03/2012<br />
EASYTEST Alat Tes Kehamilan<br />
Pribadi<br />
Anti HCV II Elecsys and cobas e<br />
analyzers<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, Cikarang<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, Cikarang<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, Cikarang<br />
DIASYS DIAGNOSTIC SYSTEM GmbH.,<br />
Germany dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, Cikarang<br />
SHANGHAI CHEMTRON BIOTECH CO.<br />
LTD., P.R. China<br />
ROCHE DIAGNOSTICS GmbH., Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PUSPA PHARMA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20304210893 20/03/2012<br />
VENTANA INFORM HER 2 Dual<br />
ISH DNA Probe Cocktail<br />
VENTANA MEDICAL SYSTEMS INC, USA<br />
untuk ROCHE DIAGNOSTICS GmbH,<br />
Germany melalui ROCHE DIAGNOSTICS INT<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20306210894 21/03/2012<br />
AKL<br />
10204210708 09/03/2012 HIT-D Hitergent<br />
VENTANA anti-HER2/new (4B5)<br />
Rabbit Monoclonal Primary Antibody<br />
VENTANA MEDICAL SYSTEMS INC, USA<br />
untuk ROCHE DIAGNOSTICS GmbH,<br />
Germany melalui ROCHE DIAGNOSTICS INT<br />
ROCHE DIAGNOSTICS GmbH., Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10101211983 22/06/2012 SERODOS Plus HUMAN GmbH, Germany PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101211866 14/06/2012 BENECHECK Uric Acid Test Strip<br />
AKL<br />
10101211865 14/06/2012<br />
BENECHECK Total Cholesterol Test<br />
Strip<br />
GENERAL LIFE BIOTECHNOLOGY CO.,<br />
LTD., Taiwan<br />
GENERAL LIFE BIOTECHNOLOGY CO.,<br />
LTD., Taiwan<br />
PT. SAMUDIA BAHTERA<br />
PT. SAMUDIA BAHTERA<br />
AKL<br />
20207210886 20/03/2012 FAST CORMAY HbA1c Control PZ Cormay S.A, Poland PT. SAPTA LARONA MUDA<br />
AKL<br />
10302211995 28/06/2012 LIOFILCHEM Mac Conkey Agar LIOFILCHEM s.r.l., Italy PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10302211994 28/06/2012 LIOFILCHEM Blood Agar Base LIOFILCHEM s.r.l., Italy PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20301211998 28/06/2012<br />
AKL<br />
20301211997 28/06/2012<br />
AKL<br />
20301211996 28/06/2012<br />
MICROSCAN Rapid Neg Urine<br />
Combo Panel Type 1<br />
MICROSCAN Synergies Plus<br />
Neg/Urine Combo Panel Type 5<br />
MICROSCAN Rapid Neg ID Panel<br />
Type 4<br />
AKL<br />
20101210879 20/03/2012 ADVIA Centaur SHBG Calibrator<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
INC, USA melalui SIEMENS AG., Germany<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101212098 04/07/2012 NORUDIA Cystatin C SEKISUI MEDICAL CO. LTD., Japan PT SUMBERMITRA AGUNGJAYA<br />
AKL<br />
20208212097 04/07/2012<br />
AKL<br />
10204211927 19/06/2012<br />
AKL<br />
20101211756 05/06/2012<br />
AKL<br />
20101211755 05/06/2012<br />
SEKISUI HbA1c Calibrator for<br />
NORUDIA N HbA1c SEKISUI MEDICAL CO. LTD., Japan PT SUMBERMITRA AGUNGJAYA<br />
SEKISUI HbA1c Pretreatment<br />
Solution for NORUDIA N HbA1c SEKISUI MEDICAL CO. LTD., China PT SUMBERMITRA AGUNGJAYA<br />
VACUETTE NH Trace Elements<br />
Sodium Heparin GREINER BIO-ONE GmbH., Autria PT. WIDYA MITRA PERSADA<br />
VACUETTE Z Trace Elements<br />
Serum Clot Activator GREINER BIO-ONE GmbH., Autria PT. WIDYA MITRA PERSADA<br />
AKL<br />
20205212213 13/07/2012<br />
SYSMEX Automated Hematology<br />
Analyzer KX-21N and Accessories<br />
SYSMEX CORPORATION, Japan melalui<br />
SYSMEX ASIA PACIFIC PTE.LTD., Singapore<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
20305211779 07/06/2012 TOSOH ST AIA-PACK Myoglobin TOSOH CORPORATION,Japan PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305212235 13/07/2012 LYPHOCHEK Immunology Control<br />
AKL<br />
10101212236 13/07/2012<br />
LYPHOCHEK Immunoassay Plus<br />
Control<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
AKL<br />
20101212115 06/07/2012 HUMAN Hexagon Troponin HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306212110 06/07/2012<br />
LYPOCHECK Tumor Marker Plus<br />
Control Trilevel Minipak<br />
AKL<br />
20208212309 13/07/2012 Precinorm HBA1C Roche Systems<br />
AKL<br />
10102212189 11/07/2012<br />
AKL<br />
20204212366 26/07/2012<br />
AKL<br />
20204212256 13/07/2012<br />
AKL<br />
20305212208 13/07/2012<br />
AKL<br />
20304212249 13/07/2012<br />
BIORAD LABORATORIES INC., USA melalui<br />
BIORAD LABORATORIES PTE. LTD.,<br />
SINGAPORE<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL. LTD.,<br />
SWITZERLAND<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ROCHE INDONESIA<br />
HUMAN Combilyzer and<br />
Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
ABBOTT Vysis MultiVysion PGT<br />
Multi-Colar Probes<br />
ABBOTT Vysis Pariffin Pretreatment<br />
IV & Post-Hybridization Wash Buffer<br />
Kit<br />
ABBOTT RealTime HIV-1<br />
Qualtitative Control Kit<br />
VYSIS MET SpectrumRed FISH<br />
Probe Kit<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10204212430 02/08/2012 ABBOTT MOLECULAR NP-40 ABBOTT MOLECULAR INC., USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
20204212365 26/07/2012<br />
AKL<br />
20304212353 25/07/2012<br />
ABBOTT Vysis CEP 16 (D16Z3)<br />
(Satelite II) SpectrumGreen Probe<br />
ABBOTT Vysis LSI MYC<br />
SpectrumGold Probe<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10204212429 02/08/2012 DAPI II CounterStain ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304212354 25/07/2012<br />
AKL<br />
10101212348 25/07/2012<br />
ABBOTT Vysis LSI D13S319<br />
SpectrumGrange Probe<br />
PROBECHECK ALK Positive<br />
Control Slides<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20304212265 13/07/2012<br />
AKL<br />
10101212347 25/07/2012<br />
AKL<br />
20304212260 13/07/2012<br />
AKL<br />
20304212273 13/07/2012<br />
VYSIS LSI BCR/ABL Dual Color,<br />
Dual Fusion Translocation Probe Kit<br />
PROBECHEK ALK Negative Control<br />
Slides<br />
VYSIS LSI 21 Spectrum Orange<br />
Probe<br />
VYSIS LSI RB1 (13q14) Spectrum<br />
Orange Probe<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
20304212272 13/07/2012<br />
VYSIS LSI PML/RARA Dual Color<br />
Eusion Translocation Probe Set<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10204212431 02/08/2012 ABBOTT MOLECULAR 20X SSC ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20101212255 13/07/2012<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
DIAZYME Homocysteine Smart<br />
Enzymatic Assay DIAZYMED LABORATORIES., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101212254 13/07/2012 DIAZYME Cystatin C Smart Assay DIAZYMED LABORATORIES., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20206212284 13/07/2012<br />
DADE PFA Collagen/EPI Test<br />
Cartridge<br />
AKL<br />
20206212344 25/07/2012 INNOVANCE PFA P2Y<br />
AKL<br />
20206212283 13/07/2012<br />
DADE PFA Collagen/ADP Test<br />
Catridge<br />
AKL<br />
20205212282 13/07/2012 INNOVANCE PFA-200 System<br />
AKL<br />
20206212433 02/08/2012 DADE PFA Trigger Solution<br />
AKL<br />
20303212352 25/07/2012<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE atas penunjukan SYSMEX ASIA<br />
CORPORATION, JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE atas penunjukan SYSMEX ASIA<br />
CORPORATION, JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE atas penunjukan SYSMEX ASIA<br />
CORPORATION, JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE atas penunjukan SYSMEX ASIA<br />
CORPORATION, JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS GMBH., GERMANY melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE atas penunjukan SYSMEX ASIA<br />
CORPORATION, JAPAN<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
REMEL Salmonella H Rapid<br />
Diagnostic 1 REMEL EUROPE LTD, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303212351 25/07/2012 REMEL Salmonella H (i) Antisera REMEL EUROPE LTD, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20103212741 15/08/2012<br />
AKL<br />
20103212743 15/08/2012<br />
AKL<br />
20103212742 15/08/2012<br />
AKL<br />
20103212740 15/08/2012<br />
RIGHTSIGN THC Rapid Test Strip<br />
(Urine)<br />
RIGHTSIGN AMP Rapid Test Strip<br />
(Urine)<br />
RIGHTSIGN AMP Rapid Test<br />
Cassette (Urine)<br />
RIGHTSIGN THC Rapid Test<br />
Cassette (Urine)<br />
AKL<br />
20101212744 15/08/2012 SEIMITSU Creatinine<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R., CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R., CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R., CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R., CHINA<br />
FUREIYA CO. LTD., JAPAN melalui YUSHI-<br />
SEIHIN CO. LTD., JAPAN<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. SEIMITSU DIAGNOSTICS<br />
AKL<br />
10208212726 15/08/2012 DIAGON DIAPACK M DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20303213051 23/08/2012 AIM Rubella IgM ELISA Test VEDA.LAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20303213050 23/08/2012 AIM Rubella IgG ELISA Test VEDA.LAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20303213049 23/08/2012 AIM Toxo IgM ELISA Test VEDA.LAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20303213048 23/08/2012 AIM Toxo IgG ELISA Test VEDA.LAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20102213052 23/08/2012 SELECTRA Pro S<br />
VITAL SCIENTIFIC BV., THE<br />
NETHERLANDS<br />
AKL<br />
20101213044 23/08/2012 VACUTEST Plast Clot Activator VACUTEST KIMA S.r.l., ITALY<br />
AKL<br />
20101213043 23/08/2012 VACUTEST Plast K3 EDTA 5.4 mg VACUTEST KIMA S.r.l., ITALY<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com<br />
PT. MRK DIAGNOSTICS<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA
AKL<br />
20101213042 23/08/2012<br />
VACUTEST Plast Na Citrate 9NC<br />
(3.8%) 0.2 ml VACUTEST KIMA S.r.l., ITALY<br />
AKL<br />
20101213041 23/08/2012 STANBIO Albumin Liquicolor STANBIO LABORATORY, USA<br />
AKL<br />
10101213040 23/08/2012 STANBIO HDL Cholesterol STANBIO LABORATORY, USA<br />
AKL<br />
20101213039 23/08/2012<br />
AKL<br />
20101213046 23/08/2012<br />
AKL<br />
20101213045 23/08/2012<br />
STANBIO Bilirubin Liquicolor Total<br />
and Direct<br />
BD Vacutainer Sodium Flouride<br />
Potassium Oxilate<br />
BD Vacutainer Serum Blood<br />
Collection Tube<br />
AKL<br />
10101212725 15/08/2012 SYSMEX CK Isozyme Control<br />
AKL<br />
20305212739 15/08/2012<br />
RIGHTSIGN HBsAg Rapid Test<br />
Cassette<br />
STANBIO LABORATORY, USA<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDING PTE.<br />
LTD., SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE., LTD.,<br />
SINGAPORE<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R., CHINA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. MITRA BAHAGIA CITRA<br />
MEDIKA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SYSMEX INDONESIA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
AKL<br />
20205212732 15/08/2012 HUMACLOT DUO and Accessories HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10204212729 15/08/2012<br />
AKL<br />
10204212730 15/08/2012<br />
AKL<br />
20103212735 15/08/2012<br />
AKL<br />
10101212736 15/08/2012<br />
AKL<br />
20305212733 15/08/2012<br />
AMPLICOR CT/NG Specimen<br />
Preparation Kit (CT/NG PREP)<br />
AMPLICOR Respiratory Specimen<br />
Preparation Kit<br />
IMMULITE / IMMULITE 1000<br />
Systems THE Theophylline<br />
IMMULITE 2000 Systems FE3<br />
Uncojugated Estriol<br />
IMMULITE / IMMULITE 1000<br />
Systems IL-10<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101212633 15/08/2012 ABX Pentra CK Control HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305212639 15/08/2012<br />
ADVIA Chemistry Inorganic<br />
Phosphorus Reagents<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101212648 15/08/2012 ABX Pentra Uric Acid CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101212647 15/08/2012 ABX Pentra ALT CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212004 28/06/2012 PROLINE - a Creatinine FS<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
PT. PRODIA DIAGNOSTIC LINE<br />
AKL<br />
10101212645 15/08/2012 ABX Pentra GGT CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
10101212644 15/08/2012 ABX Pentra Triglycerides CP HORIBA ABX SAS., FRANCE PT. DOS NI ROHA<br />
AKL<br />
20306212290 13/07/2012<br />
TOSOH ST AIA-PACK Free PSA<br />
CALIBRATOR SET TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20306212270 13/07/2012 TOSOH ST AIA-PACK Free PSA TOSOH CORPORATION., JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20801212435 02/08/2012 JMS Single Patient Dialysis System<br />
AKL<br />
20305212114 06/07/2012 DALF AI Hepatitis A IgM Drop Test<br />
AKL<br />
20101212237 13/07/2012 GP Vacu Tube 3.2 % Sodium Citrate<br />
AKL<br />
20101212238 13/07/2012 GP Vacu Tube EDTA. K2<br />
AKL<br />
20101212239 13/07/2012 GP Vacu Tube EDTA. K3<br />
AKL<br />
20101212240 13/07/2012 GP Vacu Tube Clot Activator<br />
AKL<br />
20301212212 13/07/2012 ALFRED 60<br />
MED-TECH INC., JAPAN untuk JMS CO.,<br />
LTD., JAPAN melalui JMS SINGAPORE PTE.,<br />
LTD., SINGAPORE<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO.<br />
LTD., CHINA dikemas ulang oleh PT.<br />
JAFAREL MEDIATICS, JAKARTA<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
SIRE ANALYTICAL SYSTEMS S.r.l., melalui<br />
ALIFAX S.P.A., ITALY<br />
PT. KARINDO <strong>ALKES</strong>TRON<br />
PT. JAFAREL MEDIATICS<br />
PT. GOLDEN PRATAMA<br />
PT. GOLDEN PRATAMA<br />
PT. GOLDEN PRATAMA<br />
PT. GOLDEN PRATAMA<br />
PT. MULTISERA INDOSA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103212278 13/07/2012<br />
AKL<br />
20103212277 13/07/2012<br />
AKL<br />
20103212281 13/07/2012<br />
AKL<br />
20103212280 13/07/2012<br />
AKL<br />
20103212279 13/07/2012<br />
NAL VON MINDEN Drug Screen<br />
Multi Saliva 5 (advanced) COC20,<br />
THC12, MET50, OPI40, AMP50 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
NAL VON MINDEN Drug Screen<br />
Multi Saliva 6GA (Integrated) COC20,<br />
THC12, MET50, MTD30, OPI40,<br />
AMP50 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
NAL VON MINDEN Drug Screen<br />
Multi Saliva 6GD (Integrated) COC20,<br />
MET50, BZD10, MTD30, OPI40,<br />
AMP50 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
NAL VON MINDEN Drug Screen<br />
Multi Saliva 6CA (Classic) COC20,<br />
THC12, MET50, MTD30, OPI40,<br />
AMP50 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
NAL VON MINDEN Drug Screen<br />
Multi Saliva 6CB (Classic) COC20,<br />
THC12, MET50, OPI40, AMP50,<br />
PCP10 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
AKL<br />
20207212423 31/07/2012 NORUDIA N HbA1c SEKISUI MEDICAL CO., LTD., JAPAN PT SUMBERMITRA AGUNGJAYA<br />
AKL<br />
20208212229 13/07/2012<br />
AKL<br />
20101212231 13/07/2012<br />
AKL<br />
10101212230 13/07/2012<br />
SEKISUI HbA1c Control for<br />
NORUDIA N HbA1c SEKISUI MEDICAL CO., LTD., JAPAN PT SUMBERMITRA AGUNGJAYA<br />
SEKISUI Cystatin C Calibrator for<br />
NORUDIA Cystatin C SEKISUI MEDICAL CO., LTD., JAPAN PT. SUMBERMITRA AGUNGJAYA<br />
SEKISUI Cystatin C Control for<br />
NORUDIA Cystatin C SEKISUI MEDICAL CO., LTD., JAPAN PT. SUMBERMITRA AGUNGJAYA<br />
AKL<br />
10101212262 13/07/2012 CHOLESTEST Control 1 & 2 SEKISUI MEDICAL CO., LTD., JAPAN<br />
AKL<br />
20102212432 02/08/2012<br />
AKL<br />
20305212276 13/07/2012<br />
AKL<br />
20101212003 28/06/2012<br />
AKL<br />
20101212292 13/07/2012<br />
IMMULITE 1000 Analyzer and<br />
accessories<br />
COBAS AmpliPrep/COBAS TaqMan<br />
HCV Quantitative Test V2.0<br />
(HCVQTV2)<br />
PROLINE - a ASAT (GOT) FS<br />
(IFCC mod.)<br />
PROLINE - a Bilirubin Auto Direct<br />
FS<br />
AKL<br />
20101212293 13/07/2012 PROLINE - a Creatinine PAP FS<br />
AKL<br />
20101212291 13/07/2012 PROLINE - a Bilirubin Auto Total FS<br />
AKL<br />
20101212296 13/07/2012 PROLINE - a Urea FS<br />
AKL<br />
10101212023 28/06/2012 PROLINE - a Cholesterol FS 10<br />
AKL<br />
20101212295 13/07/2012<br />
PROLINE - a Alkaline Phosphatase<br />
FS IFCC 37C<br />
AKL<br />
20101212294 13/07/2012 PROLINE - a Glucose GOD FS 10<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
DIASYS DIAGNOSTIC SYSTEM GMBH.,<br />
GERMANY dikemas ulang oleh PT. PRODIA<br />
DIAGNOSTIC LINE, CIKARANG<br />
PT. SUMBER MITRA AGUNG<br />
JAYA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
PT. PRODIA DIAGNOSTIC LINE<br />
AKL<br />
20101212731 15/08/2012 DIAGON Glucose (Hexokinase) DIAGON KFT., HUNGARY PT. INTI DIAGONTAMA SELARAS<br />
AKL<br />
20305212207 11/07/2012<br />
Ferritin CalSet Elecsys and cobas e<br />
analyzer<br />
AKL<br />
10208212119 06/07/2012 M-58LBA Lyse<br />
AKL<br />
10208212118 06/07/2012 M-58LEO (II) Lyse<br />
AKL<br />
20201212185 11/07/2012<br />
PDF Creator - PDF4Free v3.0<br />
CHROMOPLEX"! 1 Dual Detection<br />
for Bond<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS INTL., LTD.,<br />
SWITZERLAND<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
LEICA BIOSYSTEMS NEWCASTLE LTD.,<br />
United Kingdom melalui LEICA<br />
MICROSYSTEMS (SEA) PTE. LTD.,<br />
Singapore<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. BIOGEN SCIENTIFIC
AKL<br />
10302212188 11/07/2012<br />
AKL<br />
10102212187 11/07/2012<br />
MINDRAY MW-12A Microplate<br />
Washer and Accessories<br />
MINDRAY UA-600T Urine Analyzer<br />
and Accessories<br />
AKL<br />
10208212120 06/07/2012 M-58LEO (I) Lyse<br />
AKL<br />
10208212117 06/07/2012 M-58LH Lyse<br />
AKL<br />
10208212116 06/07/2012 M-58D Diluent<br />
AKL<br />
20101212202 11/07/2012<br />
DIMENSION® CSA Flex® Reagent<br />
Cartridge<br />
AKL<br />
10204212199 11/07/2012 IMMULITE Probe Cleaning Kit<br />
AKL<br />
10204212200 11/07/2012<br />
AKL<br />
10208212201 11/07/2012<br />
AKL<br />
10208212186 11/07/2012<br />
AKL<br />
10204212197 11/07/2012<br />
IMMULITE® 2000/ IMMULITE®<br />
2500 Probe Wash Module<br />
ADVIA CENTAUR® VITAMIN D<br />
Total Diluent<br />
DIMENSION® CHK Flex® Reagent<br />
Cartridge<br />
IMMULITE 2000/ IMMULITE® 2500<br />
Probe Cleaning Kit<br />
AKL<br />
10204212198 11/07/2012 IMMULITE® Probe Wash Module<br />
AKL<br />
20305212192 11/07/2012 DIMENSION® MALB Calibrator<br />
AKL<br />
20305212191 11/07/2012<br />
AKL<br />
20305212193 11/07/2012<br />
AKL<br />
20101212220 13/07/2012<br />
AKL<br />
20205212308 13/07/2012<br />
AKL<br />
20101212306 13/07/2012<br />
AKL<br />
10101212307 13/07/2012<br />
DIMENSION® TRNF Flex® Reagent<br />
Cartridge<br />
DIMENSION® MALB Flex® Reagent<br />
Cartridge<br />
AMYL Roche/Hitachi ± -Amylase<br />
Liquid acc.to IFCC Amylase test<br />
system<br />
BENECHECK"! Hb Hemoglobin Test<br />
System<br />
BENECHECK Dual Monitoring<br />
System (CHOL.GLU meter)<br />
BENECHECK Uric Acid Monitoring<br />
System<br />
AKL<br />
20101212218 13/07/2012 DIMENSION® ECO2 Calibrator<br />
AKL<br />
20305212214 13/07/2012 DIMENSION FERR Calibrator<br />
AKL<br />
20305212215 13/07/2012<br />
AKL<br />
20305212216 13/07/2012<br />
AKL<br />
20101212217 13/07/2012<br />
AKL<br />
20101212248 13/07/2012<br />
DIMENSION® C4 Flex® Reagent<br />
Cartridge<br />
DIMENSION® C3 Flex® Reagent<br />
Cartridge<br />
DIMENSION® ECO2 Flex® Reagent<br />
Cartridge<br />
iBGSTAR Blood Glucose Monitoring<br />
System<br />
AKL<br />
20101212247 13/07/2012 BGSTAR Blood Glucose Test Strips<br />
AKL<br />
20101212246 13/07/2012<br />
AKL<br />
20209212210 11/07/2012<br />
AKL<br />
20209212209 11/07/2012<br />
AKL<br />
20209212211 11/07/2012<br />
AKL<br />
20209212208 11/07/2012<br />
BGSTAR Blood Glucose Monitoring<br />
System<br />
DALF BG Blood Grouping Anti - B<br />
Monoclonal<br />
DALF BG Blood Grouping Anti - AB<br />
Monoclonal<br />
DALF BG Blood Grouping Anti - D<br />
Monoclonal<br />
DALF BG Blood Grouping Anti - A<br />
Monoclonal<br />
AKL<br />
20303212302 13/07/2012 NOVALISA"! Rubella Virus IgG<br />
AKL<br />
20303212305 13/07/2012 NOVALISA Toxoplasma Gondii IgG<br />
AKL<br />
20303212304 13/07/2012<br />
NOVALISA Cytomegalovirus (CMV)<br />
IgG<br />
AKL<br />
20303212303 13/07/2012 NOVALISA"! Measles Virus IgG<br />
AKL<br />
GEA Medical HCG Pregnancy Test<br />
20101212264 13/07/2012 (Strip)<br />
PDF Creator - PDF4Free v3.0<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO, LTD, P.R China<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melali SIEMENS AG., Germany<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
GENERAL LIFE BIOTECHNOLOGY CO,<br />
LTD., Taiwan<br />
GENERAL LIFE BIOTECHNOLOGY CO,<br />
LTD., TAIWAN<br />
GENERAL LIFE BIOTECHNOLOGY CO,<br />
LTD., TAIWAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
AML MEDICAL DEVICE LTD., China untuk<br />
AGAMATRIX, INC., USA<br />
I-SENSINO, Republik of Korea untuk<br />
AGAMATRIX INC., USA<br />
AML MEDICAL DEVICE LTD., China untuk<br />
AGAMATRIX, INC., USA<br />
CINNAGEN CO, Iran dikemas ulang oleh PT.<br />
JAFAREL MEDIATICS, Jakarta<br />
CINNAGEN CO, Iran dikemas ulang oleh PT.<br />
JAFAREL MEDIATICS, Jakarta<br />
CINNAGEN CO, Iran dikemas ulang oleh PT.<br />
JAFAREL MEDIATICS, Jakarta<br />
CINNAGEN CO, Iran dikemas ulang oleh PT.<br />
JAFAREL MEDIATICS, Jakarta<br />
NOVATEC IMMUNDIAGNOSTICA GmbH.,<br />
Germany<br />
NOVATEC IMMUNDIAGNOSTICA GmbH.,<br />
Germany<br />
NOVATEC IMMUNDIAGNOSTICA GmbH.,<br />
Germany<br />
NOVATEC IMMUNDIAGNOSTICA GmbH.,<br />
Germany<br />
BLUE CROSS BIO-MEDICAL (Beijing) CO.,<br />
LTD., China<br />
http://www.pdf4free.com<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SAMUDIA BAHTERA<br />
PT. SAMUDIA BAHTERA<br />
PT. SAMUDIA BAHTERA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. AVENTIS PHARMA<br />
PT. AVENTIS PHARMA<br />
PT. AVENTIS PHARMA<br />
PT. JAFAREL MEDIATICS<br />
PT. JAFAREL MEDIATICS<br />
PT. JAFAREL MEDIATICS<br />
PT. JAFAREL MEDIATICS<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. BESTARI SUKSES MAKMUR<br />
PT. MEGA PRATAMA<br />
MEDICALINDO
3822.00.9000 13/07/2012<br />
AKL<br />
20101212261 13/07/2012<br />
AKL<br />
20303212321 13/07/2012<br />
AKL<br />
20303212319 13/07/2012<br />
AKL<br />
20303212322 13/07/2012<br />
VYSIS LSI 21 Spectrum Orange<br />
Probe<br />
ADVIA CENTAUR® Systems<br />
Calibrator 80<br />
ADVIA® Centaur Systems SYPH<br />
QC<br />
IMMULITE® 2000 Systems Rub<br />
Rubella Quantitative IgG<br />
ADVIA® Centaur SYPH Ready<br />
Pack®<br />
AKL<br />
20101212285 13/07/2012 ADVIA® Chemistry A1c_3 Calibrator<br />
ABBOTT MOLECULAR INC, USA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., Germany<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101212263 13/07/2012 AIM UTSH Elisa Test TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
20103212317 13/07/2012 FOKUS Cocaine Strip PULSE SCIENTIFIC INC, Canada<br />
AKL<br />
20103212318 13/07/2012 FOKUS Methamphetamine Strip PULSE SCIENTIFIC INC, Canada<br />
AKL<br />
20103212316 13/07/2012 FOKUS Benzodiazepine (BZD) Strip PULSE SCIENTIFIC INC, Canada<br />
AKL<br />
20103212315 13/07/2012 FOKUS Morphine Strip PULSE SCIENTIFIC INC, Canada<br />
AKL<br />
20103212314 13/07/2012 FOKUS Marijuana (THC) Strip PULSE SCIENTIFIC INC, Canada<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
PT. FOKUS DIAGNOSTIC<br />
INDONESIA<br />
AKL<br />
20305212274 13/07/2012 AIM Rheumatoid Factor Reagent Set TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
20303212268 13/07/2012 DIASOURCE Syphilis IgM ELISA<br />
AKL<br />
20303212267 13/07/2012 DIASOURCE Syphilis IgG ELISA<br />
AKL<br />
20208212205 11/07/2012<br />
AKL<br />
20208212206 11/07/2012<br />
AKL<br />
20305212082 04/07/2012<br />
AKL<br />
20101212310 13/07/2012<br />
RAYTO Hematology Control Model<br />
Q3<br />
RAYTO Hematology Calibrator Model<br />
Q3<br />
COBAS AmpliPrep/COBAS TaqMan<br />
HBV Test V2.0<br />
COMBUR 10 Test® UX (Specific<br />
Gravity, Ph, Leukocytes, Nitrite,<br />
Protein, Glucose, Ketones,<br />
Urobilinogen, Bilirubin, Blood)<br />
AKL<br />
20207212227 13/07/2012 D-D12 Tina-quant (a) Roche/Hitachi<br />
AKL<br />
20207212226 13/07/2012 D-D12 Tina-quant (a) cobas c 111<br />
AKL<br />
20102212234 13/07/2012<br />
AKL<br />
20205212233 13/07/2012<br />
DIASOURCE IMMUNO ASSAYS S.A.,<br />
Belgium<br />
DIASOURCE IMMUNO ASSAYS S.A.,<br />
Belgium<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO., LTD., China<br />
RAYTO LIFE AND ANALYTICAL SCIENCES<br />
CO., LTD., China<br />
ROCHE DIAGNOSTICS SYSTEMS INC.,<br />
USA melalui ROCHE DIAGNOSTICS INTL.,<br />
LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ABHIMATA MANUNGGAL<br />
PT. SUMIFIN CITRA ABADI<br />
PT. SUMIFIN CITRA ABADI<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
BM 240S Auto-Chemistry Analyzer<br />
and Accessories DIRUI INDUSTRIAL CO, LTD, China PT. BUANA MITRA SUKSES<br />
BM-H3000S Hematology Analyzer<br />
and Accessories DIRUI INDUSTRIAL CO, LTD, China PT. BUANA MITRA SUKSES<br />
AKL<br />
10101212106 06/07/2012 INDEC BIO Hormolisa Progesterone<br />
AKL<br />
10101212107 06/07/2012 INDEC BIO Fertilisa Prolactin<br />
AKL<br />
20305212108 06/07/2012 INDEC BIO Hormolisa Ferritin<br />
AKL<br />
20101212109 06/07/2012 Indec BIO Thyrolisa U-TSH<br />
AKL<br />
10101212105 06/07/2012 Indec BIO Hormolisa test system<br />
AKL<br />
10208212102 06/07/2012<br />
AKL<br />
10208212100 06/07/2012<br />
AKL<br />
10208212101 06/07/2012<br />
AKL<br />
10208212251 13/07/2012<br />
PDF Creator - PDF4Free v3.0<br />
IMMULITE® 2000 Systems ID1<br />
IgG/IgM Sample Diluent<br />
IMMULITE® 2000 Systems OV OM-<br />
MA Sample Diluent<br />
IMMULITE® 2000 Systems FSH<br />
Sample Diluent<br />
IMMULITE® 2000 Systems FVB<br />
Vitamin B12/Folic Acid Sample<br />
Diluent<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, Jakarta<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, Jakarta<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, Jakarta<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, Jakarta<br />
BIOCHECK INC., USA dikemas ulang oleh PT.<br />
INDEC DIAGNOSTICS, Jakarta<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUTS LTD., UK melalui SIEMENS AG.,<br />
Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUTS LTD., UK melalui SIEMENS AG.,<br />
Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUTS LTD., UK melalui SIEMENS AG.,<br />
Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUTS LTD., UK melalui SIEMENS AG.,<br />
Germany<br />
http://www.pdf4free.com<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. INDEC DIAGNOSTICS<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA
AKL<br />
20207212252 13/07/2012<br />
AKL<br />
20305212250 13/07/2012<br />
AKL<br />
20207212266 13/07/2012<br />
AKL<br />
10204212186 11/07/2012<br />
IMMULITE® 2000 Systems EPN<br />
EPO<br />
IMMULITE® 2000 Systems BMG<br />
Beta-2 Microglobulin<br />
ADVIA® Chemistry Hemoglobin<br />
A1c_3 Reagents (A1c_3)<br />
DIMENSION ®CHK Flex® Reagent<br />
Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUTS LTD., UK melali SIEMENS AG.,<br />
Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUTS LTD., UK melalui SIEMENS AG.,<br />
Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., Germany<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalui SIEMENS AG., Germany<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301212243 13/07/2012 MUELLER Hinton Agar LIOFILCHEM S.R.L., Italy PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20102212259 13/07/2012 BIOLYZER® 600 and Accessories<br />
FURUNO ELECTRIC CO. LTD., Japan untuk<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
Germany<br />
PT. DJEMBATAN DUA<br />
AKL<br />
10302212203 11/07/2012<br />
AKL<br />
10302212368 26/07/2012<br />
INNOGENETICS® INNO-LiPA<br />
Mycobacteria V2 Line Probe Assay INNOGENETICS N.V., Belgium PT. BEHRINDO NUSA PERKASA<br />
ORTHO BioVUE® System Heat<br />
Block-32<br />
SERVICIO DE INSTRUMENTACION<br />
HOSPITALARIA S.L., Spain untuk ORTHO-<br />
CLINICAL DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10102212232 13/07/2012<br />
BIOHIT Proline Single-Channel Fixed<br />
Volume Pipettes and Accessories BIOHIT OYJ., Finland PT. MULTI SARANA MEDIKA<br />
AKL<br />
20209211775 07/06/2012<br />
SIFIN Monoclonal Test Reagents<br />
Anti-B<br />
SIFIN INSTITUTE FUR IMMUNPRAPARATE<br />
UND NAHRMEDIEN GmbH BERLIN, Germany<br />
PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20209211774 07/06/2012<br />
SIFIN Monoclonal Test Reagents<br />
Anti-A<br />
SIFIN INSTITUTE FUR IMMUNPRAPARATE<br />
UND NAHRMEDIEN GmbH BERLIN, Germany<br />
PT. ABHIMATA MANUNGGAL<br />
AKL<br />
20303212356 25/07/2012 VEDA. LAB, France VEDA. LAB, France PT. AKURAT INTAN MADYA<br />
AKL<br />
20303212357 25/07/2012 AIM HSV-2 IgG Elisa Test VEDA. LAB, France PT. AKURAT INTAN MADYA<br />
AKL<br />
20303212355 25/07/2012 AIM HSV-1 IgM Elisa Test VEDA. LAB, France PT. AKURAT INTAN MADYA<br />
AKL<br />
20301212357 25/07/2012 AIM HSV-1 IgG Elisa Test VEDA. LAB, France PT. AKURAT INTAN MADYA<br />
AKL<br />
10102212373 26/07/2012 HEMOCUEHB - 301 Microcuvette HEMOCUE AB, Sweden PT. PUTERA SEGARA ANAKAN<br />
AKL<br />
10102212369 26/07/2012<br />
AKL<br />
10204212345 25/07/2012<br />
ORTHO BioVUE® System<br />
Centrifuge<br />
AKL<br />
20101212360 26/07/2012 DALF AI Troponin - I Drop Test<br />
AKL<br />
20202212346 25/07/2012<br />
FELTRON ELEKTRONIK-ZEISSLER & CO.<br />
GmbH., Germany untuk ORTHO-CLINICAL<br />
DIAGNOSTICS INC., UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
MYTHIC 18-22 Enzymatic Cleaning<br />
Solution ORPHEE, S.A., Switzerland PT. INDOLABTEK DINAMIKA<br />
PREPSTAIN® GYN Cytology Test<br />
Kit<br />
ATLAS LINK (BEIJING) TECHNOLOGY CO,<br />
LTD, China dikemas ulang oleh PT. JAFAREL<br />
MEDIATICS, Jakarta<br />
TRIPATH IMAGING INC., USA melalui<br />
BECTON DICKINSON HOLDING PTE. LTD.,<br />
Singapore<br />
PT. JAFAREL MEDIATICS<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101212349 25/07/2012 AIM T3 ELISA Test TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
20101212350 25/07/2012 AIM fT3 ELISA Test TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
10204212363 26/07/2012 ISE Deproteinizer Cobas c 111<br />
AKL<br />
10204212364 26/07/2012 ISE Diluent Gen.2 Roche/Hitachi<br />
AKL<br />
20101212362 26/07/2012<br />
AKL<br />
20101212361 26/07/2012<br />
ISE Reference Electrolyte<br />
Roche/Hitachi<br />
ISE Internal Standard Gen.2<br />
Roche/Hitachi<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
ROCHE DIAGNOSTICS GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
Switzerland<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20209212607 14/08/2012<br />
SCINOMED Plasma Separator for<br />
Single Use<br />
ZHENGYUAN TECHNOLOGY LTD., CHINA<br />
melalui SCINOMED LTD., UNITED KINGDOM<br />
AKL<br />
10204212428 02/08/2012 VYSIS LSI/WCP Hybridization Buffer ABBOTT MOLECULAR INC., USA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20301807871 03/10/2012 OXOID Cefuroxime CXM 30 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303212184 11/07/2012 OXOID Legionella Latex Test OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20205213232 21/09/2012 FRESENIUS KABI ACD-A Solution<br />
FRESENIUS KABI NORGE AS., NORWAY<br />
untuk FRESENIUS KABI AG., GERMANY<br />
PT. FRESENIUS KABI<br />
INDONESIA<br />
AKL<br />
20209212407 31/07/2012<br />
FENWAL CPD/ADSOL Blood Pack<br />
Unit: Integral Sepacell Flex-Excel Red FENWAL INTERNATIONAL INC., PUERTO<br />
Cell Leukocyte Reduction Filter RICO untuk FENWAL INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209212406 31/07/2012<br />
FENWAL CPD/ADSOL Blood Pack<br />
Unit: Integral Sepacell Flex-Excel Red FENWAL INTERNATIONAL INC., PUERTO<br />
Cell Leukocyte Reduction Filter RICO untuk FENWAL INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209212408 31/07/2012<br />
AKL<br />
20209212078 03/07/2012<br />
AKL<br />
20209211500 11/05/2012<br />
AKL<br />
20304213733 31/10/2012<br />
FENWAL CPD/ADSOL Blood Pack<br />
Unit: Integral Sepacell Flex-Excel Red FENWAL INTERNATIONAL INC., PUERTO<br />
Cell Leukocyte Reduction Filter RICO untuk FENWAL INC., USA<br />
AMICUS Apheresis Kit Single<br />
Needle<br />
AMICUS Apheresis Kit Double<br />
Needle with Platelet Additive Solution<br />
(PAS) Connector and Inlet Line<br />
Sampling<br />
VYSIS MultiVysion PB Multi-Color<br />
Probes<br />
FENWAL INTERNATIONAL INC., PUERTO<br />
RICO untuk FENWAL INC., USA<br />
FENWAL INTERNATIONAL INC., PUERTO<br />
RICO untuk FENWAL INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304213732 31/10/2012 VYSIS LSI IGF1R Spectrum Probes ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20101213101 27/08/2012 CALIBZYME CK (MB)<br />
AKL<br />
10102213078 24/08/2012<br />
EPPENDORF Research Plus<br />
(Adjustable Volume) Pipette<br />
SYSMEX INTERNASIONAL REAGENTS CO,<br />
LTD JAPAN Melalui SYSMEX ASIA PACIFIC<br />
PTE, LTD, SINGAPORE<br />
EPPENDORF AG., GERMANY melalui<br />
EPPENDORF ASIA PACIFIC SDN. BHD.,<br />
MALAYSIA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SYSMEX INDONESIA<br />
PT. BAVARIA COMBININDO<br />
AKL<br />
20205212367 26/07/2012 HEMOCUE Hb 301 Analyzer HOMOCUE AB, SWEEDEN PT. PUTERA SEGARA ANAKAN<br />
AKL<br />
20102212081 04/07/2012<br />
IMMULITE 2000 Immunoassay<br />
System And Accessories<br />
AKL<br />
20303213417 04/10/2012 BIORAD Dengeu NS1 Ag Strip<br />
AKL<br />
20207213423 04/10/2012<br />
VARIANT II Turbo Hemoglobin<br />
Testing System<br />
AKL<br />
20303213419 04/10/2012 PASTOREX Meningitis<br />
AKL<br />
20306212225 13/07/2012<br />
AFP CalSet II Elecsys and cobas e<br />
analyzers<br />
AKL<br />
20205010283 15/08/2012 IMMULITE 2000 Systems ECP<br />
AKL<br />
10204212244 13/07/2012 NaOH-D / Basic Roche/Hitachi<br />
AKL<br />
20305212224 13/07/2012<br />
AKL<br />
20305212313 13/07/2012<br />
AKL<br />
20205212286 13/07/2012<br />
AKL<br />
20208212204 11/07/2012<br />
AKL<br />
20305213289 01/10/2012<br />
PDF Creator - PDF4Free v3.0<br />
PreciControl Anti-CCP Elecsys and<br />
Cobas e Analyzer<br />
IMMULITEC Syetem CRP C -<br />
Reactive Protein Control<br />
ADVIA 120 ADVIA 2120i CN-Free<br />
CBC Timepac<br />
Precipath Fructosamine Roche<br />
Systems<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LIMITED, UK melalui SIEMENS<br />
AG, GERMANY<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE, LTD.,<br />
SINGAPORE<br />
OEM SYSTEM CO. LTD., JAPAN untuk BIO-<br />
RAD LABORATORIES INC., USA melalui BIO-<br />
RAD LABORATORIES (SINGAPORE) PTE,<br />
LTD., SINGAPORE<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE, LTD.,<br />
SINGAPORE<br />
ROCHE DIAGNOSTICS GmbH, GERMANY<br />
malalui ROCHE DIAGNOSTICS, LTD,<br />
SWIZERLAND<br />
SIEMENS HELATHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
SIEMENS HEALTH DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG,<br />
GERMANY<br />
SIEMENS HEALTH DIAGNOSTICS<br />
PRODUCTS INC., USA melalui SIEMENS AG,<br />
GERMANY<br />
ROCHE DIAGNOSTIC GmbH, Germany<br />
melalui ROCHE DIAGNOSTICS INTL, LTD,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
INNOGENETIC INNO-LIPA HLA-A<br />
Multiplex INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
http://www.pdf4free.com
AKL<br />
10102213338 02/10/2012<br />
RAMP Reader System and<br />
Accessories<br />
AKL<br />
10102213337 02/10/2012 RAMP 200 System and Accessories<br />
AKL<br />
20305213283 01/10/2012<br />
ROCHE MOLECULAR SYSTEMS<br />
INC., USA melalui ROCHE<br />
DIAGNOSTICS INTERNASIONAL<br />
LTD., SWITZERLAND<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNASIONAL LTD., SWITZERLAND<br />
PT. SETIA ANUGRAH MEDIKA<br />
PT. SETIA ANUGRAH MEDIKA<br />
PT. ROCHE INDOENSIA<br />
AKL<br />
20103212301 13/07/2012<br />
AKL<br />
20102212622 15/08/2012<br />
AKL<br />
10302801672 13/07/2012<br />
AKL<br />
20102212432 02/08/2012<br />
SPEEDYTEST-DOA 5 Drugs Panel<br />
Test<br />
(MOP/COC/THC/AMP/BZO/MDMA) IND DIAGNOSTIC., CANADA PT. LANIROS DIAN PHARMA<br />
PALIO 300 Clinical Chemistry<br />
Analyzer and Accessories<br />
ISE S.r.l., ITALY untuk SCLAVO<br />
DIAGNOSTICS INTERNASIONAL S.r.L.,<br />
ITALY<br />
PT. BUANA MITRA SUKSES<br />
OXOID Mac Conkey Agar and CLED<br />
Medium Dip Slides OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
IMMULITE 1000 Analyzer and<br />
Accessories<br />
SIEMENS HEALHCARE DIAGNOSTICS INC,<br />
USA Melalui SIEMENS AG, GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10102212245 13/07/2012 TC-94+ Microplate/strip washer TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
20101212253 13/07/2012 AIM TSH ELISA Test TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
20209212632 18/10/2012<br />
HELMER HB120 Blood Bank<br />
Refrigerators and Accessories<br />
HELMER INC., USA<br />
PT. FRISMED HOSLAB<br />
INDONESIA<br />
AKL<br />
20102212621 15/08/2012<br />
20102212734 15/08/2012<br />
PALIO 200 Clinical Chemistry<br />
Analyzer And Accessories<br />
ISE S.r.l., ITALY untuk SCLAVO<br />
DIAGNOSTICS INTERNASIONAL S.r.l., ITALY PT. BUANA MITRA SUKSES<br />
VEGASYS Automated Chemistry<br />
Analyzer ANALYZER MEDICAL SYSTEM S.r.l, ITALY PT. BAVARIA COMBININDO<br />
AKL<br />
20101212323 13/07/2012<br />
AKL<br />
20102213627 29/10/2012<br />
RAPIDLAB 1200 Blood GAS<br />
Analyzer and Accessories<br />
SAT-450 Clinical Chemistry Analyzer<br />
and Accessories<br />
AKL<br />
20102213495 16/10/2012 ALGERIA and Accessories<br />
AKL<br />
10204213496 16/10/2012 ECO-D Roche/Hitachi<br />
AKL<br />
20205213364 02/10/2012<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD., UK untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS INC., USA<br />
melalui SIEMENS AG., GERMANY<br />
AMS Sri ANALYZER MEDICAL SYSTEM,<br />
ITALY<br />
BIT ANALYTICAL INSTRUMENT GmbH,<br />
GERMANY untuk ORGENTEC DIAGNOSTIKA<br />
GmbH, GERMANY<br />
ROCHE DIAGNOSTICS GmbH., GERMANY<br />
melalui ROCHE DIAGNOSTICS Intl., LTD.,<br />
SWITZERLAND<br />
ROTEM Delta Whole Blood TEM INNOVATIONS GmbH., GERMANY<br />
HaemostasisSystem and Accessories melalui ALBIAN LIMITED., HONGKONG<br />
PT. SIEMENS INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. NELTA MULTI GRACIA<br />
PT. ROCHE INDONESIA<br />
PT. STARINDO MEDICAL<br />
SYSTEM<br />
AKL<br />
20301213285 01/10/2012 OXOID Tetracycline TE 50 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10102806793 28/06/2012<br />
AKL<br />
10102806793 28/06/2012<br />
AKL<br />
10102806793 28/06/2012<br />
AKL<br />
10102806791 28/06/2012<br />
KUBOTA Table Top Micro<br />
Refrigerator Centrifuge 3500 adn<br />
Accessories<br />
KUBOTA Table Top Micro<br />
Refrigerator Centrifuge 3500 adn<br />
Accessories<br />
KUBOTA Table Top Micro<br />
Refrigerator Centrifuge 3500 adn<br />
Accessories<br />
KUBOTA Compact Table Top<br />
Refrigator Centrifuge 2800 and<br />
Accessories<br />
AKL<br />
20205212426 02/08/2012 JT. Baker Diluid 100 plus Azide Free<br />
AKL<br />
20306212637 15/08/2012 ARCHITECT CA 125 II Reagent Kit<br />
AKL<br />
10102804284 10/07/2012<br />
AKL<br />
20209806038 13/07/2012<br />
PDF Creator - PDF4Free v3.0<br />
ADVIA WORK CELL CDX<br />
Automation Solution and Accessories<br />
KUBOTA Compact High Speed<br />
Refrigerated Centrifuge 6200 and<br />
Accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NOREHERLAND melalui AVANTOR<br />
PERFORMANCE MATERIAL SDN., BHD.,<br />
MALAYSIA<br />
FUJIREBIO DIAGNOSTIC INC., USA untuk<br />
ABBOTT LABORATORIES., USA<br />
ATS AUTOMATION TOOLING SYSTEMS,<br />
INC., CANADA untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS INC., USA<br />
melalui SIEMENS AG., GERMANY<br />
KUBITA MANUFACTURING<br />
CORPORATION, JAPAN<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. MULTIREDJEKI<br />
PT. ABBOTT INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. DEMKA SAKTI<br />
http://www.pdf4free.com
AKL<br />
20205213336 02/10/2012<br />
AKL<br />
20305213282 22/10/2012<br />
AKL<br />
20305213355 02/10/2012<br />
UNICEL DxH 800 COULTER BECKMAN COULTER INC, USA meLalui<br />
CELLULAR ANALSIS SYSTEM AND BECKMAN COULTER SINGAPORE PTE,<br />
ACCESSORIES<br />
LTD, SINGAPORE<br />
COBAS DNA SAMPLE<br />
PREPATION KIT (DNA SP)<br />
Anti-IgG ORTHO BioVue System<br />
Cassatte<br />
ROCHE MOLECULAR SYSTEMS INC., USA<br />
melalui ROCHE DIAGNOSTIC<br />
INTERNASIONAL LTD., SWITZERLAND<br />
ORTHO CLINICAL DIAGNOSTIC INC, USA<br />
untuk ORTHO CLINICAL DIAGNOSTIC, UK<br />
PT. BEHRINDO NUSAPERKASA<br />
PT. ROCHE INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10102806794 28/06/2012<br />
KUBOTA Micro Refrigator Centrifuge<br />
3794 and accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
PT. DEMKA SAKTI<br />
AKL<br />
10102806795 30/06/2012<br />
KUBOTA Micro Refrigator Centrifuge<br />
3780 and Accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
PT. DEMKA SAKTI<br />
AKL<br />
20209806044 13/06/2012<br />
AKL<br />
20209806040 13/06/2012<br />
AKL<br />
20303212728 15/08/2012<br />
AKL<br />
10204211682 22/05/2012<br />
KUBOTA High Capacity Table Top<br />
Centrifuge 8420 and Accessories<br />
KUBOTA Plate Centrifuge PlateSpin<br />
II and Accessories<br />
COBAS AmpliPrep/COBAS TaqMan<br />
CMW Test (CMVCAP)<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
ROCHE MOLECULAR SYSTEMS, INC., USA<br />
melalui ROCHE DIAGNOSTICS INTL., LTD<br />
SWITZERLAND<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. ROCHE INDONESIA<br />
TOSOH AIA-PACK TPO Ab<br />
Conjugate TOSOH CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
20101210073 16/01/2012 GLUCOCARD X-mini<br />
AKL<br />
20102212300 13/07/2012<br />
AKL<br />
20209806042 02/08/2012<br />
AKL<br />
20209806043 02/08/2012<br />
AKL<br />
20209806039 02/08/2012<br />
ADVIA 1200 Chemistry System and<br />
Accessories<br />
KUBOTA Table Top Centrifuge 4200<br />
and Accessories<br />
KUBOTA Centrifuge for<br />
Immunohematology KA 2200<br />
KUBOTA High Speed General<br />
Purpose Refrigerator Centrifuge 5930<br />
adn Accessories<br />
ARKAY GLOBAL BUSINESS INC, JAPAN A<br />
subsidiary company of ARKRAY, JAPAN<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC, USA melalu SIEMENS AG, GERMANY<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION<br />
PT. ABADINUSA<br />
USAHASEMESTA<br />
PT. SIEMENS INDONESIA<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
AKL<br />
20209806037 02/08/2012<br />
KUBOTA TAbale Top Refrigerator<br />
Centrifuge 5500 and accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION<br />
PT. DEMKA SAKTI<br />
AKL<br />
20209806041 13/07/2012<br />
AKL<br />
20209806036 02/08/2012<br />
AKL<br />
20209806035 13/07/2012<br />
AKL<br />
30305213088 24/08/2012<br />
AKL<br />
30305213089 24/08/2012<br />
KUBOTA High Capacity Refrigerator<br />
Centrifuge 7780 and Accessories<br />
KUBOTA High Speed General<br />
Purpose Refrigerator Centrifuge 5922<br />
adn Accessories<br />
KUBOTA Compact High Speed<br />
Refrigerator Centrifuge 6500 and<br />
Accessories<br />
COBAS TaqMan HIV-1 Test v2.0<br />
For Use With The High Pure System<br />
( HIVHP2)<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
ROCHE MOLECULAR SYSTEM INC, USA<br />
Melalui ROCHE DIAGNOSTICS<br />
INTERNASIONAL LTD, SWITZERLAND<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. ROCHE INDONESIA<br />
ALERE Determine HIV-1/2 Ag/Ab<br />
Combo ALERE MEDICAL CO, LTD., JAPAN PT. ARYASA TEKAD PRATAMA<br />
AKL<br />
1080521537 13/08/2012 A Adult Diapers PT. ARKSTARINDO ARTHA MAKMUR -<br />
AKL<br />
10102806790 28/06/2012<br />
KUBOTA Micro Refrigerator<br />
Centrifuge 3700 adn Accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
PT. DEMKA SAKTI<br />
AKL<br />
20101212631 15/08/2012 ABX Pentra Amylase CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10102806789 28/06/2012<br />
AKL<br />
10102211870 14/06/2012<br />
KUBOTA Compact Table Top Micro<br />
Centrifuge 3300 and Accessories<br />
KUBOTA MANUFACTURING<br />
CORPORATION, JAPAN<br />
PT. DEMKA SAKTI<br />
DIRUI BM-SP7000 S Semi-<br />
Automatic Chemistry Analyzer and<br />
Accessories DIRUI INDUSTRIAL CP., LTD., P.R.CHINA PT. BUANA MITRA SUKSES<br />
AKL<br />
20101212627 15/08/2012 ABX PENTRA UREA CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL ABX<br />
PENTRA<br />
AMYLASE CP 15/08/2012 ABX Pentra Amylase CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212630 15/08/2012 ABX Pentra Glucise HK CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101212629 15/08/2012 ABX Pentra Glucise PAP CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212628 15/08/2012 ABX Pentra Multical HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212626 15/08/2012 ABX Pentra Total Protein CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212625 15/08/2012 ABX Pentra CK NAC CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212623 15/08/2012 ABX Pentra Bilirubin, Direct CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101212624 15/08/2012 ABX Pentra AST CP HORIBA ABX S.A.S, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101213095 24/08/2012 AIM T4 ELISA Test TECO DIAGNOSTICS, USA PT. AKURAT INYAN MADYA<br />
AKL<br />
20101213096 24/08/2012 AIM FT4 ELISA TEST TECO DIAGNOSTICS, USA PT. AKURAT INYAN MADYA<br />
AKL<br />
10302213102 27/08/2012 LIOFILCHEM SS Agar (Modified) LIOFILCHEM s.r.l., ITALY PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
10102804452 26/06/2012<br />
FREEDOM EVOlyzer and<br />
Accessories TECAN SCHWEIZ AG., SWITZERLAND PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20101212737 15/08/2012 GlucoDr Blood Glucose Test Strip ALLMEDICUS CO., LTD., KOREA PT. MEDISINDO BAHANA<br />
AKL<br />
20101212738 15/08/2012 GlucoDr Super Sensor Test Strip ALLMEDICUS CO., LTD., KOREA PT. MEDISINDO BAHANA<br />
AKL<br />
20101212636 15/08/2012<br />
AKL<br />
10101212634 15/08/2012 OVUSAL<br />
AKL<br />
10102212727 15/08/2012 BOECO Centrifuge S-8<br />
AKL<br />
10102213075 24/08/2012 BOECO Centrifuge C-28A<br />
AKL<br />
10205213074 24/08/2012<br />
AKL<br />
20306804691 15/08/2012<br />
AKL<br />
20306804697 15/08/2012<br />
GLUCODR Super Sensor Blood<br />
Glucose Monitoring System ALLMEDICUS CO, LTD., KOREA PT. MEDISINDO BAHANA<br />
BOECO Hematocrit Centrifyge H-<br />
240<br />
IMMULITE / IMMULITE 1000<br />
System OV OM-MA (CA 125)<br />
IMMULITE System TMC Tumor<br />
Marker Controls<br />
AKL<br />
20207804539 15/08/2012 DCA Syatems Hemoglobin A1c<br />
AKL<br />
20306804971 15/08/2012 IMMULITE 2000 Systems AF AFP<br />
SUZHOU HEALTH PLASTIC PRODUCTS<br />
CO. LTD., P.R CHINA untuk FERTILITY TECH<br />
INC., USA<br />
ANDREA HETTICH GmbH & CO. KG.,<br />
GERMANY untuk BOECKEL + CO. (GmbH +<br />
CO) KG, GERMANY<br />
ANDREA HETTICH GmbH & CO. KG.,<br />
GERMANY untuk BOECKEL + CO. (GmbH +<br />
CO) KG, GERMANY<br />
ANDREA HETTICH GmbH & CO. KG.,<br />
GERMANY untuk BOECKEL + CO. (GmbH +<br />
CO) KG, GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG,<br />
GERMANY<br />
AKL<br />
20209212427 02/08/2012 SURGISCREEN ORTHO-CLINICAL DIAGNOSTICS INS., USA<br />
AKL<br />
20209212425 02/08/2012 0.8% SELECTOGEN ORTHO-CLINICAL DIAGNOSTICS INS., USA<br />
AKL<br />
20303211818 12/06/2012 JE-Dengeu IgM Combo ELISA<br />
AKL<br />
10208213077 24/08/2012 HEMOLYZER-3-Lyser<br />
AKL<br />
202029213452 05/10/2012<br />
AKL<br />
10102213459 05/10/2012<br />
Anti-D/ Anti-C/ Anti-E/ Anti-c/ Control<br />
ORTHO BioVue System Cassette<br />
(Rh-hr Cassette)<br />
MAP-LAB Plus Semi Automated<br />
Analyzers and Accessories<br />
INVERNESS MEDICAL INNOVATION<br />
AUSTRALIA PTY., LTD., AUSTRALIA<br />
ANALYTICON BIOTECHNOLOGIES AG.,<br />
GERMANY<br />
ORTHO-CLINICAL DIAGNOSTICS INS., USA<br />
untuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
BIOCHEMICAL SYSTEMS INTERNASIONAL,<br />
Srl, ITALY<br />
PT. PHAROS INDONESIA<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. BAVARIA COMBININDO<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. DJEMBATAN DUA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. AKURAT INYAN MADYA<br />
AKL<br />
20101213408 02/10/2012 AIM Creatinine 5 Reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
AKL<br />
20101213410 03/10/2012 AIM Bilirubin Direct 5 Reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
AKL<br />
20208213356 02/10/2012<br />
ANTI-C/ ANTI-E/ ANTI-c/ANTI-e/<br />
ANTI K/ Control<br />
ORTHO CLINICAL DIAGNOSTICS INC, untuk<br />
ORTHO CLINICAL DIAGNOSTICS, UK<br />
PT. JONHSON & JOHNSON<br />
AKL<br />
20209213357 02/10/2012<br />
PDF Creator - PDF4Free v3.0<br />
Neutral ORTHO BiovUE System<br />
Cassette<br />
ORTHO CLINICAL DIAGNOSTICS INC, untuk<br />
ORTHO CLINICAL DIAGNOSTICS, UK PT. JONHSON & JOHNSON<br />
http://www.pdf4free.com
AKL<br />
20206213290 01/10/2012<br />
AKL<br />
20103213362 02/10/2012<br />
AKL<br />
20103213361 02/10/2012<br />
AKL<br />
20103213360 02/10/2012<br />
AKL<br />
20103213359 02/10/2012<br />
AKL<br />
20103213363 02/10/2012<br />
AKL<br />
10102213242 28/09/2012<br />
AKL<br />
10101213437 04/10/2012<br />
FORTEL (EZ DETECT) COLON<br />
DISEASE SCREANING TEST BIOMERICA INC, USA PT. MITRA ASA PRATAMA<br />
RIGHTSIGN COC Rapid Test<br />
(Urine)<br />
RIGHTSIGN COC Rapid Test<br />
Cassette (Urine)<br />
RIGHTSIGN MOPRapid Test Strip<br />
(Urine)<br />
RIGHTSIGN MOP Rapid Test<br />
Cassette (Urine)<br />
RIGHTSIGN HBsAg Rapid Test<br />
Strip (Serum/Plasma)<br />
ALERE Cholestech LDX System<br />
Accessory Kit<br />
MORE Diagnostics Rap/Tac/CsA<br />
COntrol<br />
HANGZOU BIOTEST BIOTECH. LTH., PR,<br />
CHINA<br />
HANGZOU BIOTEST BIOTECH. LTH., PR,<br />
CHINA<br />
HANGZOU BIOTEST BIOTECH. LTH., PR,<br />
CHINA<br />
HANGZOU BIOTEST BIOTECH. LTH., PR,<br />
CHINA<br />
HANGZOU BIOTEST BIOTECH. LTH., PR,<br />
CHINA<br />
ALLERE SAN DIEGO INC, USA melalui<br />
ALERE NORTH AMERICA INC. USA<br />
MORE DIAGNOSTICS, USA melalui<br />
SIEMENS AG., GERMANY atas penunjukan<br />
SIEMENS HEALHCARE DIAGNOSTICS INC.,<br />
USA<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20209213453 05/10/2012<br />
Anti-A/Anti-B/Anti-C/Anti-D/Anti-<br />
CDE/ Control/ ORTHO BioVue<br />
System Cassette (ABO Rh Cassette)<br />
ORTHO CLINICAL DIAGNOSTICS INC, untuk<br />
ORTHO CLINICAL DIAGNOSTICS, UK<br />
PT. JONHSON & JOHNSON<br />
AKL<br />
20101213448 05/10/2012 AIM Albumin 5 Reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
AKL<br />
20101213450 05/10/2012 AIM SGOT (AST) 5 Reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
AKL<br />
20101213444 04/10/2012 SCLAVO Glucose<br />
AKL<br />
20101213344 02/10/2012 SCLAVO Total Protein<br />
AKL<br />
20101213346 02/10/2012 SCLAVO Chloride<br />
AKL<br />
10101213335 31/10/2012 SCLAVO Cholesterol<br />
AKL<br />
10101213334 02/10/2012 SCLAVO HDL Cholestrol<br />
AKL<br />
10101213333 02/10/2012 SCLAVO LDL Cholesterol<br />
AKL<br />
1012213399 03/10/2012<br />
AKL<br />
10102213397 03/10/2012<br />
AKL<br />
10302213400 03/10/2012<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
LAB-MATE Adjustable Pipette 100<br />
uL - 1000 uL TECO DIAGNOSTICS, USA PT. AKURAT INYAN MADYA<br />
LAB-MATE Adjustable Pipette 0,5 uL<br />
- 10 uL TECO DIAGNOSTICS, USA PT. AKURAT INYAN MADYA<br />
iWO Autobio Microplate Washer<br />
With Four Liquid Lines<br />
AKL<br />
20101213434 04/10/2012 RAMP Cardiac Calver Controls<br />
AKL<br />
20306213421 04/10/2012 ARCHITECT AFP Calibrators<br />
AUTOBIO LABTEC INSTRIMENT CO., LTD.,<br />
CHINA<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, SLIGO - IRELAND<br />
PT. ABHIMATA MANUNGGAL<br />
PT. SETIA ANUGRAH MEDIKA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10101213467 05/10/2012 ELITECH Gamma-GT Plus SL SEPPIM S.A.S, FRANCE PT. MRK DIAGNOSTICS<br />
AKL<br />
20303213451 05/10/2012 DALF CR Salmonella IgM Drop Test CORE DIAGNOSTICS LTD, UK PT. JAFAREL MEDIATICS<br />
AKL<br />
10101213455 05/10/2012 AIM Prolactin - Q Rapid Test VEDA LAB, FRANCE PT. AKURAT INYAN MADYA<br />
AKL<br />
20205213432 04/10/2012<br />
COULTER A T Diff Hematology<br />
Analyzer and Accessories<br />
AKL<br />
20305213438 04/10/2012 RAMP Myoglobin Assay<br />
AKL<br />
10101213404 03/10/2012 SCLAVO Phosphorus<br />
AKL<br />
10101213405 03/10/2012 SCLAVO Triglycerides<br />
AKL<br />
10101213406 03/10/2012 SCLAVO Uric Acid<br />
AKL<br />
20101213439 04/10/2012 RAMP Troponin I Assay<br />
AKL<br />
20101213440 04/10/2012 RAMP CK-MB Assay<br />
BECKMAN COULTER, inc, IRELAND melalui<br />
BECKMAN COULTER SINGAPORE, Pte,<br />
LTD, SINGAPORE<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l .,ITALY<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. SETIA ANUGRAH MEDIKA<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. SETIA ANUGRAH MEDIKA<br />
PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20303213435 04/10/2012 DENGUE Virus IgG DxSelect FOCUS DIAGNOSTICS, INC, USA PT. NELTA MULTI GRACIA<br />
AKL<br />
20102213277 01/10/2012<br />
HUMALYTE PLUS 5 AND<br />
ACCESSORIES HUMAN GmbH, GERMANY PT. SALI POLAPA BERSAMA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20102213276 01/10/2012<br />
AKL<br />
20306213411 03/10/2012 ARCHITECT AFP Controls<br />
HUMALYTE PLUS 3 AND<br />
ACCESSORIES HUMAN GmbH, GERMANY PT. SALI POLAPA BERSAMA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, SLIGO - IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20102213278 01/10/2012 ELISYS DUO and Accessories HUMAN GmbH, GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101213454 05/10/2012 AIM SGPT (ALT) 5 Reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
AKL<br />
20101213449 05/10/2012<br />
AKL<br />
20306213412 03/10/2012 ARCHITECT AFP Reagent Kit<br />
AKL<br />
20101213441 04/10/2012 RAMP NT-proBNP Assay<br />
AKL<br />
10102213398 03/10/2012<br />
AKL<br />
20101213416 03/10/2012<br />
AKL<br />
20305213422 04/10/2012<br />
AKL<br />
20305213420 04/10/2012<br />
AIM Alkaline Phosphatase (ALP) 5<br />
reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, SLIGO - IRELAND<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
PT. ABBOTT INDONESIA<br />
PT. SETIA ANUGRAH MEDIKA<br />
LAB-MATE Adjustable Pipette 20 uL -<br />
200 uL TECO DIAGNOSTICS, USA PT. AKURAT INYAN MADYA<br />
ABBOTT Clinical Chemistry albumin<br />
BCG ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
BLUE CROSS One Step HBsAg<br />
Test Cassette<br />
BLUE CROSS BIO-MEDICAL (Beijing) CO.<br />
LTD., CHINA<br />
PT. ABHIMATA MANUNGGAL<br />
INNOGENETICS INNO-LiPA HBV<br />
Genotyping INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
10101213407 03/10/2012 AIM Triglseride 5 Reagent Kit CENTRONIC GmbH, GERMANY PT. AKURAT INYAN MADYA<br />
AKL<br />
10101213402 03/10/2012 SENTINEL Clin Chem Control 1<br />
AKL<br />
10101213403 03/10/2012 SENTINEL Clin Chem Control 2<br />
SIEMENS CH, SpA., ITALY untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS, USA melalui<br />
SIEMENS AG., GERMANY<br />
SIEMENS CH, SpA., ITALY untuk SIEMENS<br />
HEALTHCARE DIAGNOSTICS, USA melalui<br />
SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101806507 05/10/2012<br />
AKL<br />
10103213468 05/10/2012<br />
ADVIA Chemistry LDL - Direct LDL<br />
Cholesterol Reagents<br />
BECKMAN TER COULTER<br />
Cholinesterase<br />
AKL<br />
10101213470 05/10/2012 BECKMAN COULTER Cholestrol<br />
RANDOX LABORATORIES LTD., UK untuk<br />
SIEMENS HEALTHCARE DIAGNOSTIC INC.,<br />
USA melalui SIEMENS AG, GERMANY<br />
BECKMAN COULTER, inc, IRELAND melalui<br />
BECKMAN COULTER SINGAPORE, Pte,<br />
LTD, SINGAPORE<br />
BECKMAN COULTER, inc, IRELAND melalui<br />
BECKMAN COULTER SINGAPORE, Pte,<br />
LTD, SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
10102213460 05/10/2012<br />
GESAN Chem 100 Semi Automatic<br />
Biochemistry Analyzer<br />
GESAN PRODUCTION Srl, ITALY<br />
PT. SUNRISE INTERMEDICA<br />
INDO<br />
AKL<br />
20103213456 05/10/2012 AIM Drugtest Uri Morphine Strip VEDA LAB, FRANCE PT. AKURAT INYAN MADYA<br />
AKL<br />
20103213457 05/10/2012<br />
AKL<br />
20103213458 05/10/2012<br />
AIM Drugtest Uri Benzodiazepines<br />
(BZD) Strip VEDA LAB, FRANCE PT. AKURAT INYAN MADYA<br />
AIM Drugtest Uri Marijuana (THC)<br />
Strip VEDA LAB, FRANCE PT. AKURAT INYAN MADYA<br />
AKL<br />
20205213477 05/10/2012 DREW3 HEMATOLOGY Analyzer<br />
AKL<br />
10101213293 01/10/2012 SEIMITSU Cholestrol HDL<br />
AKL<br />
10101213294` 01/10/2012 SEIMITSU Cholestrol<br />
AKL<br />
20101213284 01/10/2012<br />
C2 DIAGNOSTICS, FRANCE untuk DREW<br />
SCIENTIFIC CO. LIMITED., UK<br />
FUREIYA CO, LTD, Japan melalui YUSHI-<br />
SEIHIN CO, LTD, Japan<br />
FUREIYA CO, LTD, Japan melalui YUSHI-<br />
SEIHIN CO, LTD, Japan<br />
PT. AEROCOM JENCO<br />
INDONESIA<br />
PT. SEIMITSU DIAGNOSTICS<br />
PT. SEIMITSU DIAGNOSTICS<br />
GlucoDR Plus AGM-4000 Blood<br />
Glucose Monitoring ALLMEDICUS C0, LTD., KOREA PT. MEDISINDO BAHANA<br />
AKL<br />
10102213331 02/10/2012 TC 84 Semi Automatic Photometer TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
10102213332 02/10/2012 TC-URITEK 720 Plus Urine Analyzer TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
AKL<br />
10102213330 02/10/2012<br />
AKL<br />
10102213426 04/10/2012<br />
TC 84 Plus Spectrophotometer<br />
Analyzer TECO DIAGNOSTICS, USA PT. AKURAT INTAN MADYA<br />
RADIPCHEM 744 Analyzer and<br />
Accessories<br />
AKL<br />
10103807486 04/10/2012 Total MPA Controls Roche Systems<br />
MEDICA CORPORATION, USA untuk<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SIEMENS AG, GERMANY<br />
ROCHE DIAGNOSTICS GmbH., GERMANY,<br />
melalui ROCHE DIAGNOSTIC, INTL, LTD,<br />
SWITZERLAND<br />
PT. SIEMENS INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
20205802071 02/10/2012<br />
POINTCARE NOW HEMATOLOGY<br />
ANALYZER AND ACCESSORIES POINTCARE TECHNOLOGIES., USA PT. MEGA UTAMA MEDICA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20102213279 01/10/2012<br />
AKL<br />
20102213395 03/10/2012<br />
AKL<br />
20102213396 03/10/2012<br />
AKL<br />
20707806715 23/10/2012<br />
ACCESS 2 Immunoassay System<br />
and Accessories<br />
BECKMAN COULTER AU 480<br />
Chemistry Analyzer and Accessories<br />
BECKMAN COULTER AU<br />
680Chemistry Analyzer and<br />
Accessories<br />
DCA Vantage Analyzer &<br />
Accessories<br />
BECKMAN COULTER IRELAND, INC, Ireland<br />
melalui BECKMAN COULTER SINGAPORE,<br />
Pte, Ltd, SINGAPORE<br />
BECMAN COULTER MISHIMA K.K, JAPAN<br />
untuk BECMAN COULTER INC,USA melalui<br />
BECMAN COULTER SINGAPORE PTE. LTD.,<br />
SINGAPORE<br />
BECMAN COULTER MISHIMA K.K, JAPAN<br />
untuk BECMAN COULTER INC,USA melalui<br />
BECMAN COULTER SINGAPORE PTE. LTD.,<br />
SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING. LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101213556 22/10/2012 FORTEL Ovulation Prediction Test BIOMERICA INC, USA PT. MITRA ASA PRATAMA<br />
AKL<br />
20205213594 23/10/2012<br />
C2000-4 Semi-Automatic Blood<br />
Coagulation Analyzer<br />
BEIJING PRECIL INSTRUMENT CO, LTD.,<br />
P.R, CHINA<br />
PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20102213582 23/10/2012<br />
FULLY SMART Automated Clinical<br />
Chemistry Analyzer and Accessories<br />
AKL<br />
20305213581 23/10/2012 ARCHITECT HBsag reagent Kit<br />
BIOCHEMICAL SYSTEM INTERNASIONAL,<br />
Srl, ITALY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, SLIGO - IRELAND<br />
PT. AKURAT INTAN MADYA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101213583 23/10/2012<br />
ADVIA Centaur System B.R.A.H.M.S<br />
Procalcitonin (PCT) QC<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101213584 23/10/2012<br />
AKL<br />
20101213284 01/10/2012<br />
AKL<br />
20305213585 23/10/2012<br />
ADVIA Centaur System B.R.A.H.M.S<br />
Procalcitonin (PCT) Ready Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
GlucoDr Auto Blood Glucose<br />
Monitoring Systems ALLMEDICUS CO, LTD, KOREA PT. MEDISINDO BAHANA<br />
VERSANT HCV Control 2.0 Kit<br />
(LiPA)<br />
AKL<br />
20101213603 24/10/2012 SCLAVO Bilirubin Direct<br />
AKL<br />
20101213600 24/10/2012<br />
SCLAVO Alkaline Phospatase<br />
Methode IFCC<br />
AKL<br />
20101213602 24/10/2012 SCLAVO AST-GOT<br />
AKL<br />
20101213601 24/10/2012 SCLAVO BCG Albumin<br />
AKL<br />
20208213558 22/10/2012 J.T Baker K-Diff Control Normal<br />
AKL<br />
20208213557 22/10/2012 J.T Baker K-Diff COntrol Low<br />
AKL<br />
20101213343 02/10/2012 SCLAVO Urea UV<br />
AKL<br />
20101213443 04/10/2012 SCLAVO Creatinine<br />
AKL<br />
20101213442 04/10/2012<br />
SCLAVO CK NAC Method IFGG -<br />
DGKC<br />
AKL<br />
20101213345 02/10/2012 SCLAVO Total Bilirubin<br />
AKL<br />
20101213445 04/10/2012<br />
SCLAVO CK - MB Method IFCC -<br />
DGKC<br />
AKL<br />
10208213590 23/10/2012 J.T BAKER CyMet KX<br />
AKL<br />
20208213589 23/10/2012 J.T BAKER Parameter Control High<br />
AKL<br />
20208213588 23/10/2012 J.T BAKER Parameter Control Low<br />
PDF Creator - PDF4Free v3.0<br />
INNOGENETICS N.V, BELGIUM untuk<br />
SIEMENS HEALTHCARE DIAGNOSTICS,<br />
USA melalui SEIEMSNS AG, GERMANY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
R.r>l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
R.r>l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
R.r>l., ITALY<br />
AVANTOR PERFORMANCE MATERIAL B.V.,<br />
Netherlands melalui AVANTOR<br />
PERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
AVNTOR PERFORMANCE MATERIAL B.V.,<br />
NETHERLANDS melalui AVANTOR<br />
OERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
SCLAVO DIAGNOSTICS INTERNASIONAL,<br />
S.r.l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL,<br />
S.r.l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL,<br />
S.r.l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL,<br />
S.r.l., ITALY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL,<br />
S.r.l., ITALY<br />
AVNTOR PERFORMANCE MATERIAL B.V.,<br />
NETHERLANDS melalui AVANTOR<br />
OERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
AVNTOR PERFORMANCE MATERIAL B.V.,<br />
NETHERLANDS melalui AVANTOR<br />
OERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
AVNTOR PERFORMANCE MATERIAL B.V.,<br />
NETHERLANDS melalui AVANTOR<br />
OERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. BUANA MITRA SUKSES<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA
AKL<br />
20208213587 23/10/2012<br />
J.T BAKER Parameter control<br />
Normal<br />
AKL<br />
20208213586 23/10/2012 J.T Baker K-Diff Control High<br />
AKL<br />
20305213591 23/10/2012<br />
AKL<br />
20101213593 23/10/2012<br />
AKL<br />
20101213592 23/10/2012<br />
IMMULITE/IMMULITE 1000<br />
Systems TOP AlaTop Allegry Screen<br />
IMMULITE 2000 Systems FBC Free<br />
Beta HCG<br />
IMMULITE/IMMULITE 1000<br />
Systems ECP<br />
AKL<br />
10209804261 02/10/2012 HETTICH Floorstanding Centrifuge<br />
AKL<br />
10209804258 02/10/2012 HETTICH Hematocrit Centrifuge<br />
AKL<br />
10209804260 02/10/2012 HETTICH Benchtop Centrifuge<br />
AVNTOR PERFORMANCE MATERIAL B.V.,<br />
NETHERLANDS melalui AVANTOR<br />
OERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
AVNTOR PERFORMANCE MATERIAL B.V.,<br />
NETHERLANDS melalui AVANTOR<br />
OERFORMANCE MATERIALS Sdn. Bhd.,<br />
MALAYSIA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD, UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD, UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD, UK melalui SIEMENS AG.,<br />
GERMANY<br />
ANDREAS HETTICH GmbH & CO. KG.,<br />
GERMANY melalui HETTICH ASIA PACIFIC<br />
PTE. LTD., SINGAPORE<br />
ANDREAS HETTICH GmbH & CO. KG.,<br />
GERMANY melalui HETTICH ASIA PACIFIC<br />
PTE. LTD., SINGAPORE<br />
ANDREAS HETTICH GmbH & CO. KG.,<br />
GERMANY melalui HETTICH ASIA PACIFIC<br />
PTE. LTD., SINGAPORE<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
AKL<br />
10203213415 03/10/2012 PATHOS DELTA MILESTONE S.r.l, ITALY PT. INDO CLARO SEJAHTERA<br />
AKL<br />
20303213419 04/10/2012 PASTOREX Meningitis<br />
AKL<br />
20207213418 04/10/2012 VARIANT II TURBO HbA1c - 2.0<br />
AKL<br />
20303213417 04/10/2012 BIORAD Dengeu NS! Ag Strip<br />
AKL<br />
20207213423 04/10/2012<br />
VARIANT II Turbo Hemoglobin<br />
Testing System<br />
AKL<br />
20207213348 02/10/2012 SEIMITSU HbA1c<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
BIO-RAD LABORATORIES INC., USA melalui<br />
BIO-RAD LABORATORIES (SINGAPORE)<br />
PTE. LTD., SINGAPORE<br />
BIO-RAD, FRANCE melalui BIO-RAD<br />
LABORATORIES (SINGAPORE) PTE. LTD.,<br />
SINGAPORE<br />
OEM SYSTEM CO. LTD., JAPAN melalui BIO-<br />
RAD LABORATORIES INC., USA melalui BIO-<br />
RAD LABORATORIES (SINGAPORE( PTE.<br />
LTD., SINGAPORE<br />
FUREIYA CO, LTD, JAPAN melalui YUSHI-<br />
SEIHIN CO, LTD., JAPAN<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. SEIMITSU DIAGNOSTICS<br />
AKL<br />
10303213350 02/10/2012 DIALAB ASO ( Anti-Streptolysin O) DIALAB GmbH, AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
10101213237 28/09/2012<br />
AKL<br />
20101213239 28/09/2012<br />
ALERE Cholestech LDX Multianalyte<br />
Control<br />
ALERE Cholestech LDX Calibration<br />
Verification<br />
ALLERE SAN DIEGO INC, USA melalui<br />
ALERE NORTH AMERICA INC., USA<br />
ALLERE SAN DIEGO INC, USA melalui<br />
ALERE NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20301213286 01/10/2012 OXOID Nitrofuration F 100 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
akl<br />
10101213329 02/10/2012 SCLAVO ALT/GPT<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l., ITALY<br />
PT. BUANA MITRA SUKSES<br />
AKL<br />
20301213287 01/10/2012 AXOID Piperacilin PRL 30 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301213288 01/10/2012 OXOID Cefuroxime Sodium CXM 5 OXOID LIMITED, UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101213349 02/10/2012<br />
FORTEL Menopause Midstream<br />
Test BIOMERICA INC, USA PT. MITRA ASA PRATAMA<br />
AKL<br />
10203213328 02/10/2012 RAFFAELO Automatic Stainer DIAPATH S.p.A., ITALY PT. INDO CLARO SEJAHTERA<br />
AKL<br />
10101213325 02/10/2012 PROLINE - b HDL - C Immuno FS<br />
AKL<br />
10101213326 02/10/2012 PROLINE - b LDL - C Select FS<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH,<br />
GERMANY<br />
DIASYS DIAGNOSTICS SYSTEMS GmbH,<br />
GERMANY<br />
PT. PRODIA DIAGNOSTICS LINE<br />
PT. PRODIA DIAGNOSTICS LINE<br />
AKL<br />
10203213353 02/10/2012 HISTOPOT SEROSEP LIMITED, IRELAND PT. GRACIA VISI PRATAMA<br />
AKL<br />
10102213352 02/10/2012<br />
SMARTLYTE Na/K/Cl/Ca/Li<br />
Analyzer DIAMOND DIAGNOSTICS INC., USA PT. MULTISERA INDOSA<br />
AKL<br />
20306213186 04/09/2012 TOSON ST AIA-PACK PAP TOSON CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20305213185 04/09/2012<br />
TOSON AIA-PACK TPOAb Control<br />
set TOSON CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20207213184 04/09/2012 TOSON ST AIA-PACK HbA1c TOSON CORPORATION, JAPAN PT. KARINDO <strong>ALKES</strong>TRON<br />
AKL<br />
20303213342 02/10/2012 AIM Salmonella IgM Rapid Test TULIP DIAGNOSTICS (P) LTD., INDIA PT. AKURAT INTAN MADYA<br />
AKL<br />
20101213340 02/10/2012 AIM Potassium (K) Reagent Kit CENTRONG GmbH, GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101213339 02/10/2012 AIM Chloride (CI) 5 Reagent Kit CENTRONG GmbH, GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101213341 02/10/2012 AIM Sodium (Na) 5 Reagent Kit CENTRONG GmbH, GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101213281 01/10/2012<br />
GlucoDR Plus AGM-3000 Blood<br />
Glucose Test Meter ALLMEDICUS CO, LTD., KOREA PT. MEDISINDO BAHANA<br />
AKL<br />
10101213347 02/10/2012 AIM Cholesterol 5 Reagent Kit CENTRONG GmbH, GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
10101213351 02/10/2012 RAMP Cardiac controls<br />
RESPONSE BIOMEDICAL CORPORATION,<br />
CANADA<br />
PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20101213280 01/10/2012 GlucoDR Auto Test Strips ALLMEDICUS CO, LTD., KOREA PT. MEDISINDO BAHANA<br />
AKL<br />
10101213238 28/09/2012 ALERE Cholestech LDX Lipid Profile<br />
AKL<br />
20101213240 28/09/2012<br />
ALERE Cholesterol LDX ALT.AST<br />
(GPT.GOT)<br />
ALLERE SAN DIEGO INC, USA melalui<br />
ALERE NORTH AMERICA INC., USA<br />
ALERE SAN DIEGO INC., USA melalui<br />
ALERE NORTH AMERICA INC., USA<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209213446 04/10/2012<br />
Anti-A/ ANti-B/ Anti-A,B/ Anti-D/<br />
Contro;/ Anti-IgG ORTHO BioVue<br />
System Cassete (Newborn Cassette)<br />
AKL<br />
10101213291 01/10/2012 SEIMITSU triglycerides<br />
AKL<br />
10101213292 01/10/2012 SEIMITSU Cholesterol LDL<br />
AKL<br />
20101213241 28/09/2012<br />
ALERE Cholesterol LDX Lipid Profile<br />
GLU<br />
ORTHO-CLINICAL DIAGNOSTICS INC. USA<br />
intuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
FUREIYA CO, LTD, JAPAN melalui YUSHI-<br />
SEIHIN CO, LTD, JAPAN<br />
FUREIYA CO, LTD, JAPAN melalui YUSHI-<br />
SEIHIN CO, LTD, JAPAN<br />
ALERE SAN DIEGO INC., USA melalui<br />
ALERE NORTH AMERICA INC., USA<br />
PT. JOHNSON & JOHNSON<br />
PT. SEIMITSU DIAGNOSTICS<br />
PT. SEIMITSU DIAGNOSTICS<br />
PT. MEDQUEST JAYA GLOBAL<br />
AKL<br />
20209213447 04/10/2012<br />
Anti-A/ ANti-B/ Anti-D/ Anti-D/ Anti-<br />
K/ Control ORTHO BioVue System<br />
Cassete (Newborn Cassette)<br />
AKL<br />
10204213428 04/10/2012 RAPIDCHEM 744 Daily Cleaner Kit<br />
AKL<br />
20205213433 04/10/2012<br />
AKL<br />
10101213425 04/10/2012<br />
ORTHO-CLINICAL DIAGNOSTICS INC. USA<br />
intuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
MEDICA CORPORATION, USA untuk<br />
SIEMENS HEALTCARE DIAGNOSTICS., USA<br />
melalui SIEMENS AG., GERMANY<br />
PT. JOHNSON & JOHNSON<br />
PT. SIEMENS INDONESIA<br />
TEG 5000 Hemostasis analyzer<br />
system and accessories HAEMOSCOPE CORPORATION, USA PT. TRANSMEDIC INDONESIA<br />
BECKMAN COULTER CK-MB<br />
Control Serum Level 1<br />
AKL<br />
20305213466 11/05/2012 VIDAS Anti-HCV<br />
BECKMAN COULTER IRELAND INC,<br />
IRELAND untuk BECKMAN COULTER INC,<br />
USA melalui BECKMAN SINGAPORE Pte,<br />
LTD, SINGAPORE<br />
BIOMERIEUX INC, USA melalui<br />
BIOMERIEUX., FRANCE<br />
PT. BEHRINDO NUSAPERKASA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
AKL<br />
10101013413 03/10/2012 AIM Phosporus (P) 5 reagent Kit CENTRONG GmbH, GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
10101213414 03/10/2012<br />
AKL<br />
20205213471 05/10/2012<br />
AKL<br />
20303213436 04/10/2012<br />
AIM LDL CHOLESTEROL 5<br />
Reagent Kit CENTRONG GmbH, GERMANY PT. AKURAT INTAN MADYA<br />
COULTER LH 750 series system<br />
and accessories<br />
BECKMAN COULTER INC, USA melalui<br />
BECKMAN COULTER (SINGAPORE) PTE.<br />
LTD., SINGAPORE<br />
PT. BEHRINDO NUSAPERKASA<br />
DENGEU Virus IgM Capture<br />
DxSelect FOCUS DIAGNOSTICS, INC, USA PT. NELTA MULTI GRACIA<br />
AKL<br />
10102213401 03/10/2012 PHOMO Autobio Microplate Reader<br />
AKL<br />
20301213464 05/10/2012 VITEK 2 AST-GN53<br />
AKL<br />
20301213463 05/10/2012 BacT/ALERT FA Plus<br />
AKL<br />
20301213462 05/10/2012 BacT/ALERT FN Plus<br />
AKL<br />
20301213461 05/10/2012 BacT/ALERT PF Plus<br />
AKL<br />
20101212103 06/07/2012<br />
AKL<br />
20305213476 05/10/2012<br />
AKL<br />
20305213475 05/10/2012<br />
MYGLUCOHEALTH Blood Glucose<br />
Test Strip<br />
AUTOBIO LABTEC INSTRUMENTS CO.,<br />
LTD., CHINA<br />
BIOMERIEUX INC., USA melalui<br />
BIOMERIEUX SA., FRANCE<br />
BIOMERIEUX INC., USA melalui<br />
BIOMERIEUX SA., FRANCE<br />
BIOMERIEUX INC., USA melalui<br />
BIOMERIEUX SA., FRANCE<br />
BIOMERIEUX INC., USA melalui<br />
BIOMERIEUX SA., FRANCE<br />
INFOPIA CO., LTD., KOREA untuk ENTRA<br />
HEALTH SYSTEMS LLC., USA<br />
PT. ABHIMATA MANUNGGAL<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. ENSEVAL MEDIKA PRIMA<br />
ARCHITECH HIV Ag/Ab Combo<br />
Calibrator ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ARCHITECH HIV Ag/Ab Combo<br />
Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101213611 24/10/2012<br />
AKL<br />
20101213604 24/10/2012<br />
AKL<br />
20304213730 31/10/2012<br />
DIRUI A10 Reagent Strip for<br />
Urinalysis DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
DIRUI H11 Reagent Strips for<br />
Urinalysis DIRUI INDUSTRIAL CO., LTD., P.R. CHINA PT. ENSEVAL MEDIKA PRIMA<br />
VYSIS LSI KRAS SpectrumGold<br />
Probe<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304213729 31/10/2012 VYSIS LSI KIT SpectrumRed Probe ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304213731 31/10/2012<br />
AKL<br />
20304213763 01/11/2012<br />
AKL<br />
20304213761 01/11/2012<br />
AKL<br />
20304213764 01/11/2012<br />
AKL<br />
20304213762 01/11/2012<br />
AKL<br />
20304213760 01/11/2012<br />
AKL<br />
20304213769 01/11/2012<br />
AKL<br />
20304213767 01/11/2012<br />
AKL<br />
20304213768 01/11/2012<br />
AKL<br />
20304213765 01/11/2012<br />
AKL<br />
20304213766 01/11/2012<br />
AKL<br />
20101213097 24/08/2012<br />
VYSIS To TelVysion Multi-Color<br />
FISH Probes<br />
VYSIS LSI ZNF217 SpectrumGold<br />
Probe<br />
VYSIS LSI D5S23, D5S721<br />
SpectrumGreen Probe<br />
VYSIS LSI RREB1 (6p25)<br />
SpectrumRed Probe<br />
VYSIS LSI EGFR SpectrumRed<br />
Probe<br />
VYSIS LSI TOP2A 17q21-22<br />
SpectrumOrange Probe<br />
VYSIS LSI CCND1 SpectrumGreen<br />
Probe<br />
VYSIS LSI MAPT 17q21<br />
SpectrumGreen Probe<br />
VYSIS LSI ERBB2 (17q12)<br />
SpectrumGreen Probe<br />
VYSIS LSI p16 (9p21) SpectrumRed<br />
Probe<br />
VYSIS LS1 MYC (8q24)<br />
SpectrumAqua Probe<br />
ESCHWEILER Blood Gases Plus<br />
Electrolytes BGA + ISE Combiline<br />
and Accessories<br />
AKL<br />
30305211057 11/04/2012 VISITEC HIV TRIO<br />
AKL<br />
20101212320 13/07/2012<br />
AKL<br />
20103212195 11/07/2012<br />
AKL<br />
20103212194 11/07/2012<br />
RAPIDPOINT 500 System and<br />
Accessories<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ESCHWEILER GMBH & CO., KG.,<br />
GERMANY<br />
QUALPRO DIAGNOSTICS, INDIA melalui<br />
ZEPHYR BIOMEDICALS, INDIA<br />
SIEMENS HEALTHCARE DIAGNOSTIC<br />
MANUFACTURING LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. BAVARIA COMBININDO<br />
PT. INDEC DIAGNOSTICS<br />
PT. SIEMENS INDONESIA<br />
NAL VON MINDEN Drug-Screen<br />
Multi Saliva 3CA (Classic) COC,<br />
THC12, AMP50 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
NAL VON MINDEN Drug-Screen<br />
Multi Saliva 3CB (Classic) COC20,<br />
THC12, MET50 NAL VON MINDEN GMBH., GERMANY PT. MARADA PHARMA MEDICA<br />
AKL<br />
20305212417 31/07/2012 Rapid fFN Control Kit HOLOGIC INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20305212416 31/07/2012 Rapid fFN Cassette Kit HOLOGIC INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20205212275 13/07/2012<br />
AKL<br />
10203213354 02/10/2012<br />
DREW XL 2280 Hematology<br />
Analyzer<br />
DREW SCIENTIFIC CO. LIMITED., UK<br />
PT. AEROCOM JENCO<br />
INDINESIA<br />
CANOVA Embedding Module and<br />
Accessories DIAPATH S.p.A., ITALY PT. INDO CLARO SEJAHTERA<br />
AKL<br />
20207213471 05/10/2012 AIM Hemoglobin 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20101213472 05/10/2012 AIM rea (BUN) UV 5 Reagent Kit CENTRONIC GMBH., GERMANY PT. AKURAT INTAN MADYA<br />
AKL<br />
20207213474 05/10/2012 DIALAB Thromboplastin Liquid (Lyo) DIALAB GMBH, AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20301213465 05/10/2012 VITEK 2 AST-N156<br />
AKL<br />
10201213777 01/11/2012<br />
J.T. Baker Papanicolaou Solution 2A<br />
Orange G Solution OG6<br />
AKL<br />
10201213780 01/11/2012 J.T. Baker UltraKitt<br />
AKL<br />
10204213779 01/11/2012 J.T. Baker UltraClear<br />
BIOMERIEUX Inc., USA melalui biomerieux<br />
sa., france<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
PT. ENSEVAL MEDIKA PRIMAA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10201213778 01/11/2012<br />
AKL<br />
20304213781 01/11/2012<br />
AKL<br />
20304213782 01/11/2012<br />
AKL<br />
10201213776 01/11/2012<br />
J.T. Baker Papanicolaou Solution 3b,<br />
Polychrome solution EA 50<br />
VYSIS CEP 18 (D18Z1) (a satellite)<br />
SpectrumAqua Probe<br />
VYSIS Prader-Willi/Angelman<br />
Region SNRPN/CEP 15/PML FISH<br />
Probe Kit<br />
J.T. baker Hematoxyline<br />
(Papanicolaou 1)<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
PT. MULTIREDJEKI KITA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. MULTIREDJEKI KITA<br />
AKL<br />
20208213673 31/10/2012<br />
Anti-A/ Anti-B/ Anti-D ORTHO<br />
BioVUE System<br />
ORTHO-CLINICAL DIAGNOSTICS Inc., USA<br />
untuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
10102213785 01/11/2012 EPPENDORF iophotometer Plus EPPENDORF AG., GERMANY PT. BAVARIA COMBININDO<br />
AKL<br />
10101213677 31/10/2012 BECKMAN COULTER ALT<br />
AKL<br />
10302213672 31/10/2012<br />
AIM Incubator Hole Block (Dual Size<br />
Holes)<br />
AKL<br />
20101213678 31/10/2012 BECKMAN COULTER Albumin<br />
AKL<br />
20101213676 31/10/2012 BECKMAN COULTER Creatinine<br />
AKL<br />
10101213773 01/11/2012<br />
AKL<br />
10101213772 01/11/2012<br />
AKL<br />
10101213771 01/11/2012<br />
AKL<br />
10101213770 01/11/2012<br />
KOVA Liqua-Trol Level II Normal<br />
with hCG<br />
KOVA Trol I High Normal with<br />
Urobilinogen<br />
KOVA - Trol II High Normal with<br />
Urobilinogen<br />
KOVA - Trol III High Normal with<br />
Urobilinogen<br />
AKL<br />
10201213658 30/10/2012 J.T. BAKER Cervix Fixative<br />
AKL<br />
10204213660 30/10/2012<br />
AKL<br />
10201213659 30/10/2012<br />
J.T. baker Formalin Solution 10%<br />
(v/v)<br />
J.T. BAKER EOSINE Y Solution<br />
0.2%<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
RAYTO LIFE and ANALYTICAL SCIENCES<br />
CO. LTD., P.R. CHINA<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
BECKMAN COULTER IRELAND Inc,<br />
IRELAND untuk BECKMAN COULTER Inc,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE Pte, Ltd., SINGAPORE<br />
HYCOR BIOMEDICAL Inc., USA untuk TECO<br />
DIAGNOSTICS Inc, USA<br />
HYCOR BIOMEDICAL Inc., USA untuk TECO<br />
DIAGNOSTICS Inc, USA<br />
HYCOR BIOMEDICAL Inc., USA untuk TECO<br />
DIAGNOSTICS Inc, USA<br />
HYCOR BIOMEDICAL Inc., USA untuk TECO<br />
DIAGNOSTICS Inc, USA<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
AVANTOR PERFORMANCE MATERIALS<br />
B.V., NETHERLANDS melalui AVANTOR<br />
PERFOMANCE MATERIAL SDN. BHD.,<br />
MALAYSIA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. AKURAT INTAN MADYA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. AKURAT INTAN MADYA<br />
PT. AKURAT INTAN MADYA<br />
PT. AKURAT INTAN MADYA<br />
PT. AKURAT INTAN MADYA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
PT. MULTIREDJEKI KITA<br />
AKL<br />
20208213675 31/10/2012<br />
Anti-K/ Control ORTHO BioVue<br />
System Cassette<br />
ORTHO-CLINICAL DIAGNOSTICS Inc., USA<br />
untuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20208213674 31/10/2012<br />
AKL<br />
10101213774 01/11/2012<br />
AKL<br />
11603213775 01/11/2012<br />
Anti-K ORTHO BioVue System<br />
Cassette<br />
KOVA Liqua-Trol Level I Abnormal<br />
with hCG<br />
Gluco Dr 200 Sterile Disposable<br />
Lancet<br />
ORTHO-CLINICAL DIAGNOSTICS Inc., USA<br />
untuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
HYCOR BIOMEDICAL Inc., USA untuk TECO<br />
DIAGNOSTICS Inc, USA<br />
SAE HAN MED CORP., KOREA untuk ALL<br />
MEDICUS CO., LTD., KOREA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. AKURAT INTAN MADYA<br />
PT. MEDISINDO BAHANA<br />
AKL<br />
20209213452 05/10/2012<br />
AKL<br />
20101213047 23/08/2012<br />
Anti-D/ Anti-C/ Anti-E/ Anti-c/ Anti-e/<br />
Control ORTHO BioVue System<br />
Cassette (Rh-hr Cassette)<br />
ROCHE/HITACHI CA2 Calcium<br />
Gen.2<br />
ORTHO-CLINICAL DIAGNOSTICS Inc., USA<br />
untuk ORTHO-CLINICAL DIAGNOSTICS, UK<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. ROCHE INDONESIA<br />
AKL<br />
10204213664 30/10/2012 ADVIA 120/2120/2120i EZ WASH<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
http://www.pdf4free.com<br />
PT. SIEMENS INDONESIA
AKL<br />
20305213662 30/10/2012 CRYSTAL HBsAg (Device) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20303213661 30/10/2012 CRYSTAL TP SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20305213661 30/10/2012 CRYSTAL HBsAg (dIPSTICK) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGRAH MEDIKA<br />
AKL<br />
20303213645 30/10/2012 AIM CMV IgM ELISA Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20303213646 30/10/2012 AIM CMV IgG ELISA Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
20103213644 30/10/2012 AIM Drugtest Uri Amphetamine Strip VEDALAB, FRANCE PT. AKURAT INYAN MADYA<br />
AKL<br />
20305213666 30/10/2012 AIM CRP Q Rapid Test VEDALAB, FRANCE PT. AKURAT INTAN MADYA<br />
AKL<br />
10302214061 29/11/2012 MICROSCAN HNID Inoculum Broth<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302214044 29/11/2012 MICROSCAN Alpha Naphthol VP2<br />
AKL<br />
10102804137 14/03/2013<br />
AKL<br />
20103803655 10/01/2013 ROCHE/HITACHI Ethyl Alcohol<br />
AKL<br />
10102803503 14/03/2013<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
EOS BRAVO Clinical Chemistry<br />
Analyzer HOSPITEX DIAGNOSTICS S.R.L., ITALY PT. GRAHA ISMAYA LTD<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
HOSPITEX Screen Master Touch<br />
Biochemistry Analyzer HOSPITEX DIAGNOSTICS S.R.L., ITALY PT. GRAHA ISMAYA LTD<br />
AKL<br />
10102803498 14/03/2013 EOS BRAVO FORTE BENCH TOP HOSPITEX DIAGNOSTICS S.R.L., ITALY PT. GRAHA ISMAYA LTD<br />
AKL<br />
10102803496 14/03/2013 EOS BRAVO PLUS HOSPITEX DIAGNOSTICS S.R.L., ITALY PT. GRAHA ISMAYA LTD<br />
AKL<br />
10202801069 30/01/2013 VITROLIFE Freeze-kit 1 VITROLIFE AB., SWEDEN<br />
AKL<br />
10302310246 31/01/2013 MICROSCAN Inoculum Water<br />
AKL<br />
10101310427 14/03/2013<br />
AKL<br />
10101310428 14/03/2013<br />
AKL<br />
10101310393 06/03/2013<br />
ONETOUCH VERIO Pro + Linearity<br />
Solution Levels 1,2,3,4 and 5<br />
ONETOUCH VERIO Control<br />
Solution Hospital - Levels Low, Mid<br />
and High<br />
MINDRAY HDL & LDL Cholesterol<br />
Control P<br />
AKL<br />
10204310392 06/03/2013 MINDRAY CD80 Detergent<br />
AKL<br />
10101310394 06/03/2013 MINDRAY Lipids Control N<br />
AKL<br />
10101310391 06/03/2013 MINDRAY Multi Control Sera N<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
BIONOSTICS INC., USA untuk LIFESCAN<br />
EUROPE, Division of Cilag GMBH<br />
INTERNATIONAL., SWITZERLAND<br />
BIONOSTICS INC., USA untuk LIFESCAN<br />
EUROPE, Division of Cilag GMBH<br />
INTERNATIONAL., SWITZERLAND<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. SIEMENS INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
AKL<br />
10205803526 14/03/2013 HOSPITEX Ves-Static u 16 HOSPITEX DIAGNOSTICS S.R.L., ITALY PT. GRAHA ISMAYA LTD<br />
AKL<br />
10101310435 15/03/2013<br />
AKL<br />
10203310436 15/03/2013<br />
AKL<br />
20209806235 18/03/2013<br />
IMMULITE / IMMULITE 1000<br />
Systems PRG Progresterone<br />
LEICA RM2265 Fully Motorized,<br />
Rotary Microtome and Accessories<br />
HETTICH ROTOFIX 32A Benchtop<br />
Centrifuge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MICROSYSTEM<br />
(SEA) PTE. LTD., SINGAPORE<br />
ANDREAS HETTICH GMBH & CO. KG.,<br />
GERMANY melalui HETTICH ASIA PASIFIC<br />
PTE. LTD., SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. BIOGEN SCIENTIFIC<br />
PT. PRIMA <strong>ALKES</strong>INDO<br />
NUSANTARA<br />
AKL<br />
10101310430 15/03/2013 HUMAN HDL Cholesterol HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10208310429 15/03/2013<br />
IMMULITE IMMULITE 1000 System<br />
PRL Prolactin Sample Diluent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101310439 15/03/2013<br />
ARCHITECT 2 Generation<br />
Testosterone Controls<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10204310475 21/03/2013 COBAS Eco-D Eco Targent ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10101310449 15/03/2013<br />
ARCHITECT 2 Generation<br />
Testosterone Reagent Kit<br />
AKL<br />
10203310432 15/03/2013 LEICA DM1000 and Accessories<br />
AKL<br />
10203310437 15/03/2013<br />
LEICA CM1520 - Cryostat and<br />
Accessories<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT GMBH & CO. KG., GERMANY<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MICROSYSTEM<br />
(SEA) PTE. LTD., SINGAPORE<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MICROSYSTEM<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. ABBOTT INDONESIA<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10101310431 15/03/2013 TDM Control Set Roche Systems ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20101310474 20/03/2013<br />
CA2 Calcium Gen.2 COBAS<br />
INTEGRA / cobas c system ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20302310390 06/03/2013 VITEK MS System and Accessories<br />
KRATOS ANALYTICAL LTD., UK untuk<br />
BIOMERIEUX SA., FRANCE<br />
AKL<br />
20305310267 31/01/2013 NOVA Lite ANA Plus INOVA DIAGNOSTICS INC., USA<br />
AKL<br />
20305310259 31/01/2013<br />
AKL<br />
20305310260 31/01/2013<br />
BECKMAN COULTER CRP (Latex)<br />
Control Serum<br />
BECKMAN COULTER CRP LATEX<br />
calibrator Normal (N) set<br />
AKL<br />
20305310263 31/01/2013 BECKMAN COULTER APO A1<br />
AKL<br />
20305310258 31/01/2013<br />
BECKMAN COULTER CRP LATEX<br />
calibrator Highly Sensitive (HS) Set<br />
AKL<br />
20305310257 31/01/2013 BECKMAN COULTER APO B<br />
AKL<br />
20102310453 18/03/2013<br />
AKL<br />
20205310318 19/02/2013<br />
AKL<br />
20102310338 26/02/2013<br />
AKL<br />
20102310339 26/02/2013<br />
AKL<br />
20102310384 06/03/2013 ILAB 300 Plus<br />
AKL<br />
20102310385 06/03/2013 ILAB Aries<br />
AKL<br />
20102310336 25/02/2013<br />
COBAS 6000 Analyzer Series and<br />
Accessories<br />
COAG 4D COAGULOMETER and<br />
Accessories<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
PT. ENSEVAL MEDIKA PRIMA<br />
PT. PACIFIC BIOTEKINDO<br />
INTRALAB<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
HITACHI HIGH-TECHNOLOGIES CORP.,<br />
JAPAN untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
PT. ROCHE INDONESIA<br />
PT. DEC HEALTHCARE<br />
DIAGON LTD., HUNGARY<br />
INDONESIA<br />
URISED Fully Automatic Urine<br />
Sediment Analyzer 77 ELEKTRONIKA Kft., HUNGARY PT. RAFA TOPAZ UTAMA<br />
LABUMAT Fully Automated Urine<br />
Chemistry Analyzer 77 ELEKTRONIKA Kft., HUNGARY PT. RAFA TOPAZ UTAMA<br />
AUTOMATED CLINICAL<br />
ANALYZER TMS 50i Superior and<br />
Accessories<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
INSTRUMENTATION LABORATORY SPA.,<br />
ITALY<br />
HIROSE ELECTRONIC SYSTEM CO. LTD.,<br />
JAPAN untuk TOKYO BOEKI MACHINERY<br />
LTD., JAPAN<br />
PT. DEXA ARFINDO PRATAMA<br />
PT. DEXA ARFINDO PRATAMA<br />
PT SUMBERMITRA AGUNGJAYA<br />
AKL<br />
20305310274 31/01/2013<br />
AKL<br />
20305310275 31/01/2013<br />
AKL<br />
10101310251 31/01/2013<br />
AKL<br />
20304310265 31/01/2013<br />
AKL<br />
20304310266 31/01/2013<br />
AKL<br />
20304310264 31/01/2013<br />
AKL<br />
20101310250 31/01/2013<br />
PDF Creator - PDF4Free v3.0<br />
ABBOTT RealTime HCV Genotype II<br />
Amplification Reagent Kit<br />
ABBOTT RealTime HCV Genotype II<br />
Control Kit<br />
BECKMAN COULTER Control<br />
serum 2<br />
VYSIS LSI MYB (6q23)<br />
SpectrumGold Probe<br />
VYSIS CEP 18 (D18Z1) (a satelite)<br />
Spectrum Orange Probe<br />
VYSIS LSI MYC SpectrumGreen<br />
Probe<br />
BECKMAN COULTER APO A1&B<br />
calibrator<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT MOLECULAR INC., USA<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
http://www.pdf4free.com<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. BEHRINDO NUSA PERKASA
AKL<br />
20207310252 31/01/2013 BECKMAN COULTER HbA1c<br />
AKL<br />
20102310279 01/02/2013<br />
AKL<br />
10102701441 14/03/2013<br />
AKL<br />
10302310245 31/01/2013<br />
AKL<br />
20304310253 31/01/2013<br />
BECKMAN COULTER AU 5800<br />
Clinical Chemistry Analyzer and<br />
Accessories<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTERK.K., JAPAN untuk<br />
BECKMAN COULTER INC., USA melalui<br />
BECKMAN COULTER SINGAPORE PTE.,<br />
LTD., SINGAPORE<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
TECO URITEK TC-101 Urine<br />
Analizer and Accessories TECO DIAGNOSTIC., USA PT. RIOCA MEDICA<br />
MICROSCAN Yeast Turbidity<br />
Standard<br />
VYSIS LSI PDGFRB (Tel)<br />
SpectrumGreen Probe<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304310255 31/01/2013 VYSIS SRY/CEP X FISH Probe Kit ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20205310182 30/01/2013 ROTEM ap-tem<br />
AKL<br />
20205310183 30/01/2013 ROTEM hep-tem<br />
AKL<br />
20205310184 30/01/2013 ROTEM star-tem<br />
AKL<br />
20102310130 29/01/2013<br />
PROCLEIX Panther system and<br />
Accessories<br />
AKL<br />
20101310262 31/01/2013 BECKMAN COULTER LDH<br />
AKL<br />
20101310261 31/01/2013 BECKMAN COULTER CK (NAC)<br />
AKL<br />
20304310254 31/01/2013<br />
VYSIS LSI 22 (BCR)<br />
SpectrumGreen Probe<br />
TEM INNOVATIONS GMBH., GERMANY<br />
melalui ALBIAN LIMITED, HONGKONG<br />
TEM INNOVATIONS GMBH., GERMANY<br />
melalui ALBIAN LIMITED, HONGKONG<br />
TEM INNOVATIONS GMBH., GERMANY<br />
melalui ALBIAN LIMITED., HONGKONG<br />
STRATEC BIOMEDICAL AG., GERMANY<br />
untuk GEN-PROBE INC., USA melalui<br />
NOVARTIS VACCINES AND DIAGNOSTICS<br />
INC., USA<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
ABBOTT MOLECULAR INC., USA<br />
PT. SIEMENS INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. STARINDO MEDICAL<br />
SYSTEMS<br />
PT. STARINDO MEDICAL<br />
SYSTEM<br />
PT. STARINDO MEDICAL<br />
SYSTEM<br />
PT. MEDQUEST JAYA GLOBAL<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
AKL<br />
20101310249 31/01/2013 CEDIA Core TDM Multi-Cal ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20304310256 31/01/2013 VYSIS LSI Xq13.2 (XIST) SP Probe ABBOTT MOLECULAR INC., USA<br />
AKL<br />
10101310241 31/01/2013 BECKMAN COULTER Lipase<br />
AKL<br />
20305310240 31/01/2013 BECKMAN COULTER CRP LATEX<br />
AKL<br />
10101310242 31/01/2013 BECKMAN COULTER GGT<br />
AKL<br />
20101310164 29/01/2013 BECKMAN COULTER Glucose<br />
AKL<br />
10101310166 30/01/2013<br />
BECKMAN Coulter CK-MB Control<br />
Serum Level 2<br />
AKL<br />
20303310160 29/01/2013 SD BIOLINE HAV IgG/IgM<br />
AKL<br />
20101310161 29/01/2013 SD BIOLINE Troponin I<br />
AKL<br />
20303310159 29/01/2013 SD BIOLINE Chikungunya IgM<br />
AKL<br />
20306310163 29/01/2013 SD BIOLINE FOB<br />
PDF Creator - PDF4Free v3.0<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
http://www.pdf4free.com
AKL<br />
20303310162 29/01/2013<br />
SD BIOLINE Salmonella Typhi<br />
IgG/IgM<br />
AKL<br />
20306310189 30/01/2013 SD BIOLINE AFP<br />
AKL<br />
10303310191 30/01/2013 SD BIOLINE JEV IgM<br />
AKL<br />
10303310192 30/01/2013 SD JEV IgM Capture ELISA<br />
AKL<br />
10303310193 30/01/2013 SD BIOLINE H. Pylori Ag<br />
AKL<br />
20306310188 30/01/2013 SD BIOLINE CEA<br />
AKL<br />
20303310187 30/01/2013 SD BIOLINE Cholera Ag 01/0139<br />
AKL<br />
20304310168 30/01/2013<br />
VYSIS LSI PDGFRB (Cen)<br />
SpectrumOrange Probe<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304310169 30/01/2013 VYSIS BRAF SpectrumGold Probe ABBOTT MOLECULAR INC., USA<br />
AKL<br />
20304310170 30/01/2013<br />
VYSIS LSI ETV 6 SpectrumOrange<br />
Probe<br />
AKL<br />
20101310129 23/01/2013 SYSMEX D-Bil Reagent A<br />
AKL<br />
10302310247 31/01/2013<br />
MICROSCAN PROMPT Inoculation<br />
System-D<br />
ABBOTT MOLECULAR INC., USA<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. SYSMEX INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302310244 31/01/2013<br />
AKL<br />
10302310248 31/01/2013<br />
MICROSCAN MH Broth with 3%<br />
LHB<br />
MICROSCAN Supplemented Mueller-<br />
Hinton Broth, pH 7.2<br />
AKL<br />
20101310238 31/01/2013 BECKMAN COULTER a-Amylase<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BEHRINDO NUSA PERKASA<br />
AKL<br />
20203807103 31/01/2013 SPAN Stained Salmonella ntigen Set SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20103310272 31/01/2013<br />
AKL<br />
20103310273 31/01/2013<br />
AKL<br />
10302310271 31/01/2013<br />
AKL<br />
20101310268 31/01/2013<br />
AKL<br />
20303700815 30/01/2013<br />
IMMULITE 2000 System DGT<br />
Digitoxin<br />
IMMULITE 2000 Systems DGX<br />
Digokin<br />
MICROSCAN 0,4% Saline with<br />
Pluronic<br />
BECKMAN COULTER Control<br />
Serum 1<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS.,<br />
UK melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. BEHRINDO NUSA PERKASA<br />
REMEL Salmonella Polyvalent (E) for<br />
eh, enx, enz15 REMEL EUROPE LTD., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301700823 31/01/2013 OXOID VORICONAZOLE VOR 1 OXOID LIMITED., UNITED KINGDOM PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10302310269 31/01/2013 MICROSCAN 3 ml Inoculum Saline<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10302310270 31/01/2013<br />
MICROSCAN Inoculum Water with<br />
Pluronic<br />
AKL<br />
10303310133 29/01/2013 SD BIOLINE H. Pylori<br />
AKL<br />
20306310131 29/01/2013 SD BIOLINE PSA<br />
PDF Creator - PDF4Free v3.0<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
PT. SIEMENS INDONESIA<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
http://www.pdf4free.com
AKL<br />
10303310135 29/01/2013 SD BIOLINE Rapid TB<br />
AKL<br />
10303310132 29/01/2013 SD BIOLINE Rotavirus<br />
AKL<br />
10303310134 29/01/2013 SD BIOLINE TB Ag MPt64 Rapid<br />
AKL<br />
20303310190 30/01/2013 SD BIOLINE Leptospira IgM<br />
AKL<br />
20101310151 29/01/2013<br />
AKL<br />
20103310152 29/01/2013<br />
AKL<br />
20101310156 29/01/2013<br />
AKL<br />
20305310158 29/01/2013<br />
AKL<br />
20305310149 29/01/2013<br />
AKL<br />
20103310155 29/01/2013<br />
AKL<br />
20103310154 29/01/2013<br />
AKL<br />
20103310153 29/01/2013<br />
AKL<br />
20101310150 29/01/2013<br />
MS. TELLME hCG Test Cassette<br />
(Urine)<br />
RIGHTSIGN Multi 3 Drugs Rapid<br />
Test Cassette (Urine) (AMP, MOP,<br />
THC)<br />
RIGHTSIGN MET Rapid Test strip<br />
(Urine)<br />
RIGHTSIGN HCV Rapid Test<br />
Cassette<br />
RIGHTSIGN HCV Rapid Test<br />
Dipstick<br />
RIGHTSIGN BZO Rapid Test<br />
Cassette (Urine<br />
RIGHTSIGN MET Rapid Test<br />
Cassette (Urine)<br />
RIGHTSIGN Multi 6 Drugs Rapid<br />
Test Cassette (Urine) (AMP, MOP,<br />
THC, MET, COC, BZO)<br />
MS. TELLME Midstream Pregrancy<br />
Test<br />
AKL<br />
20101310157 29/01/2013 MS. TELLME hCG Test Strip<br />
AKL<br />
20205310185 30/01/2013 ROTEM ROTROL N<br />
AKL<br />
20303310010 03/01/2013 SD BIOLINE Malaraia Ag p.f/Pan<br />
AKL<br />
20303310009 03/01/2013 SD BIOLINE Malaria Ag p.f/P.v<br />
AKL<br />
20303310011 03/01/2013 SD BIOLINE Dengue IgG/IgM<br />
AKL<br />
20303310012 03/01/2013 SD BIOLINE Syphilis 3.0<br />
AKL<br />
20208310023 14/01/2013<br />
MINDRAY BC-RET Hematology<br />
Control (H, N, L)<br />
AKL<br />
20101310027 14/01/2013 MINDRAY Multi Sera Calibrator<br />
AKL<br />
20208310022 14/01/2013<br />
AKL<br />
20208310025 14/01/2013<br />
AKL<br />
20101310026 14/01/2013<br />
MINDRAY BC-NRBC Hematology<br />
Control (H, N, L)<br />
MINDRAY S-C Cal Plus Hematology<br />
calibrator<br />
MINDRAY Bil-D Bilirubin Kit (VOX<br />
Method)<br />
AKL<br />
20101310024 14/01/2013 MINDRAY Multi Control Sera P<br />
AKL<br />
20208310021 14/01/2013<br />
AKL<br />
20305807627 29/01/2013<br />
AKL<br />
20304310165 29/01/2013<br />
AKL<br />
20101807840 10/01/2013<br />
MINDRAY BC-6D Hematology<br />
Control (H, N, L)<br />
ABBOTT ARCHITECT Anti-HBs<br />
Controls<br />
VYSIS LSI RUNX1 SpectrumGreen<br />
Probe<br />
ABBOTT AXSYM System Total B-<br />
hCG Reagent Pack<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
HANGZHOU BIOTEST BIOTECH CO. LTD.,<br />
P.R. CHINA<br />
TEM INNOVATIONS GMBH., GERMANY<br />
melalui ALBIAN LIMITED., HONGKONG<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
STANDARD DIAGNOSTICS INC., KOREA<br />
melalui ALERE INTERNATIONAL LIMITED,<br />
IRELAND<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ABBOTT MOLECULAR INC., USA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. MACROCITRA<br />
ARDANASEJATI<br />
PT. STARINDO MEDICAL<br />
SYSTEM<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. ALERE HEALTH<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20301807797 30/01/2013 OXOID Imipenem (IMP 10) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
10101807814 31/01/2013 ABBOTT Prolactin Controls ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20101310015 10/01/2013<br />
ABBOTT AXSYM System<br />
Cylosporine eagent Pack<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20103807816 10/01/2013<br />
ABBOTT AXSYM System<br />
Cyclosporine Standard Calibrators<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10205310020 14/01/2013 HUMASED 25 and Accessories<br />
SKYKOD POLYSCIENCE SDN. BHD.,<br />
MALAYSIA untuk HUMAN GMBH., GERMANY<br />
PT. SALI POLAPA BERSAMA<br />
AKL<br />
10205310019 14/01/2013 HUMASED 100 and Accessories<br />
AKL<br />
20305807625 29/01/2013<br />
ABBOTT ARCHITECT Anti-HBs<br />
Calibrator<br />
SKYKOD POLYSCIENCE SDN. BHD.,<br />
MALAYSIA untuk HUMAN GMBH., GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. SALI POLAPA BERSAMA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
10304905073 25/02/2013 SEBIA Capillarys CDT Kit SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10204905078 25/02/2013 SEBIA Capiclean SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20103807496 25/02/2013<br />
ONCOPROBE Amphetamine Rapid<br />
Test CDI / BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
20103807497 26/02/2013 ONCOPROBE THC Rapid Test CDI / BIOCHEM CORP., USA PT. ONCOPROBE UTAMA<br />
AKL<br />
10304905077 14/03/2013 SEBIA Capillarys HR Kit SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10304905075 14/03/2013 SEBIA Capillarys Immunotyping SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10304905074 21/03/2013 SEBIA Capillarys and Accessories SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
20101310473 20/03/2013<br />
AKL<br />
10304905072 21/03/2013<br />
MINDRAY BIL-T Bilirubin otal Kit<br />
(VOX Method)<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
SEBIA Capillarys Hemoglobin (E) 6<br />
Kit SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10304905076 14/03/2013 SEBIA Capillarys Hemoglobin (E) Kit SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10101310434 15/03/2013 MINDRAY Lipids Control P<br />
AKL<br />
20305807624 29/01/2013<br />
AKL<br />
20305310454 18/03/2013<br />
ABBOTT ARCHITECT Anti-HBs<br />
Reagent Kit<br />
COBAS TaqMan 48 and<br />
Accessories<br />
AKL<br />
10101310433 15/03/2013 SCLAVO Magnesium<br />
AKL<br />
20303501680 25/02/2013<br />
AKL<br />
20303501679 26/02/2013<br />
AKL<br />
20101807800 10/01/2013<br />
AMPLICOR Internal Control<br />
Detection Kit (IC MWP DK)<br />
AMPLICOR Neisseria Gonorrhoeae<br />
Detection Kit NG MWP DK<br />
ABBOTT AXSYM System Total B-<br />
hCG Standard Calibrators<br />
SHENZHEN MINDRAY BIO-MEDICAL<br />
ELECTRONICS CO. LTD., P.R., CHINA<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
ROCHE MOLECULAR SYSTEMS INC, USA<br />
untuk ROCHE DIAGNOSTICS GMBH.,<br />
GERMANY<br />
SCLAVO DIAGNOSTICS INTERNASIONAL<br />
S.r.l., ITALY<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
ROCHE MOLECULAR SYSTEM INC., USA<br />
untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. MINDRAY MEDICAL<br />
INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. BUANA MITRA SUKSES<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20301807792 30/01/2013 OXOID Norfloxacin - NOR 10 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20103807838 31/01/2013<br />
AKL<br />
10101310237 31/01/2013<br />
AKL<br />
20101010957 29/01/2013<br />
ABBOTT AXSYM System<br />
Cyclosporine Controls<br />
BECKMAN COULTER HDL-<br />
Cholesterol<br />
BD vacutainer SST II Advance Plus<br />
Blood Collection Tubes<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION., IRELAND<br />
BECKMAN COULTER IRELAND INC.,<br />
IRELAND untuk BECKMAN COULTER INC.,<br />
USA melalui BECKMAN COULTER<br />
SINGAPORE PTE. LTD., SINGAPORE<br />
BECTON DICKINSON & COMPANY., USA<br />
melalui BECTON DICKINSON HOLDINGS<br />
PTE., LTD., SINGAPORE<br />
PT. ABBOTT INDONESIA<br />
PT. BEHRINDO NUSA PERKASA<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20208500608 31/01/2013 SEBIA Hb AFSC Control SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500604 31/01/2013 SEBIA Hydragel 30 Protein (E) SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500603 31/01/2013 SEBIA Hydragel 7B1 - B2 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500602 31/01/2013 SEBIA Hydragel 7 Hemoglobin (E) SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500607 01/02/2013 SEBIA Hydrasys and Accessories SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500605 31/01/2013 SEBIA Hydragel 15 B1 - B2 SEBIA., FRANCE PT. RIOCA MEDICA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10102500606 31/01/2013<br />
SEBIA Manual Agarose<br />
Electrophoresis K20 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500609 31/01/2013 SEBIA Hydragel Proteinurine K20 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500600 31/01/2013 SEBIA Hydragel Hemoglobin (E) K20 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500601 31/01/2013 SEBIA Hydragel 7 Protein (E) SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500598 31/01/2013 SEBIA Hydragel 15 Protein (E) SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10101500597 31/01/2013 SEBIA Serum De Controle Normal SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10102500610 31/01/2013 SEBIA Hydragel Protein (E) K20 SEBIA., FRANCE PT. RIOCA MEDICA<br />
AKL<br />
10208310239 31/01/2013<br />
ABBOTT AXSYM system Total B-<br />
hCG Specimen Diluent<br />
ABBOTT IRELAND DIAGNOSTICS<br />
DIVISION, IRELAND<br />
PT. ABBOTT INDONESIA<br />
AKL<br />
20101807810 10/01/2013 ABBOTT Prolactin Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20301807591 31/01/2013 OXOID Ciprofloxacin (CIP5) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807592 30/01/2013 OXOID Azithromycin (AZM15) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301806804 06/03/2013<br />
AKL<br />
20205310017 14/01/2013<br />
BD BBL Sensi-Disc Ceftazidime /<br />
Clavulanic Acid 30/10 ug<br />
AUTOMATED HEMATOLOGY<br />
ANALYZER XP-100 and Accessories<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
AKL<br />
20303310243 31/01/2013 ONEMED Anti Malaria Test IND DIAGNOSTIC INC., CANADA<br />
AKL<br />
20305805763 25/02/2013<br />
VERSANT HBV DNA 3.0 Assay<br />
(bDNA)<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SYSMEX INDONESIA<br />
PT. JAYAMAS MEDICA<br />
INDUSTRIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101805786 18/03/2013 OPTI CCA - TS Analyzer OPTI MEDICAL SYSTEM INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
20301806109 29/01/2013<br />
AKL<br />
20301806202 29/01/2013<br />
BD BBL SENSI-DISC Piperacillin<br />
100 ug<br />
BD BBL SENSI-DISC Polymyxin B<br />
300 IU/IE/UI<br />
AKL<br />
20101806191 29/01/2013 SYSMEX AST Reagent L<br />
AKL<br />
20101806192 29/01/2013 SYSMEX BUN Reagent L<br />
AKL<br />
20101806196 01/02/2013 SYSMEX CRE Reagent LB<br />
BECTON DICKINSONAND AND COMPANY,<br />
USA melalui BECTON DICKINSON<br />
HOLDINGS Pte. Ltd., SINGAPORE<br />
BECTON DICKINSON AND COMPANY, USA<br />
melalui BECTON DICKINSON HOLDINGS Pte.<br />
Ltd., SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION, JAPAN melalui<br />
SYSMEX ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
AKL<br />
10302806405 25/02/2013 BD BBL Crystal E/NF<br />
BECTON DICKINSONAND AND COMPANY,<br />
USA melalui BECTON DICKINSON<br />
HOLDINGS Pte. Ltd., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
BECTON DICKINSONAND AND COMPANY,<br />
AKL<br />
20301806519 06/03/2013<br />
USA melalui BECTON DICKINSON<br />
BD BBL SENSI-DISC Ofloxacin 5 ug HOLDINGS Pte. Ltd., SINGAPORE<br />
PT. ANUGRAH ARGON MEDICA<br />
AKL<br />
20101806660 18/03/2013 OPTI LION Analyzer OPTI MEDICAL SYSTEM INC., USA PT. PELITA SANTOSO JAYA<br />
AKL<br />
10102806749 14/03/2013<br />
AKL<br />
20301806870 06/03/2013<br />
AKL<br />
10302806859 07/01/2013<br />
MODULAR Pre Analytics EVO and<br />
Accessories<br />
BD BBL Sensi-Disc Imipenem IPM-<br />
10<br />
HITACHI HIGH TECHNOLOGIES CORP.,<br />
JAPAN untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY<br />
BECTON DICKINSON AND COMPANY., USA<br />
melalui BECTON DICKINSON HOLDING<br />
PTE., LTD., SINGAPORE<br />
PT. ROCHE INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
MERCK BPLS0-Agar for The<br />
Isolation of Salmonella MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302806857 07/01/2013 MERCK Blood Agar (Base) MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20301806858 08/01/2013 MERCK Mueller - Hinton Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302806860 07/01/2013 MERCK DHL Agar acc. to Sakazaki MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302806861 07/01/2013<br />
MERCK Selenite Cystine Enrichment<br />
Broth MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20303310016 14/01/2013 VISITECT Malaria OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
AKL<br />
20303807080 03/01/2013 IMMUTREP VDRL Antigen OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
AKL<br />
10303310013 07/01/2013 AVITEX ASO OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
AKL<br />
20305807108 30/01/2013<br />
AKL<br />
20303807115 01/02/2013<br />
AKL<br />
20303807114 01/02/2013<br />
AKL<br />
20209807111 30/01/2013<br />
AKL<br />
20303807105 31/01/2013<br />
SPAN CRP LATEX Test C-reactive<br />
Protein Test Kit SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPAN S. Paratyphi "B(H)" (Stained<br />
Salmonella Antigen) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPAN S. Paratyphi "A(H)" (Stained<br />
Salmonella Antigen) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPANCLONE Anti D Rho<br />
Monoclonal IgG + IgM SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPAN S. Paratyphi "B(O)" (Stained<br />
Salminella Antigen) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20207807107 30/01/2013 SPAN G-6 PD Test Kit SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10303310167 30/01/2013<br />
AKL<br />
20303807102 31/01/2013<br />
AKL<br />
20303807106 31/01/2013<br />
AKL<br />
20303807101 31/01/2013<br />
SPAN ASO Latex Test Anti<br />
Streptolysin O Test Kit SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPAN S. Typhi O (Stained<br />
Salmonella Antigen) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPAN S. Paratyphi A(O) (Stained<br />
Salmonella Antigen) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
SPAN S. typi H (Stained Salmonella<br />
Antigen) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20209807100 31/01/2013 SPANCLONE Anti B Monoclonal SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20303807112 03/01/2013<br />
SPAN RPR Test (Rapid Plasma<br />
Reagent) SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101807347 25/02/2013<br />
AKL<br />
10101705687 04/01/2013<br />
AKL<br />
10302807820 07/02/2013<br />
AKL<br />
10302807821 21/03/2013<br />
B.BRAUN Omnitest Plus Blood<br />
Glucose Monitor Set<br />
INFOPIA CO. LTD., KOREA untuk B.BRAUN<br />
MELSUNGEN AG OPM., GERMANY<br />
PT. B.BRAUN MEDICAL<br />
INDONESIA<br />
Estradial ll Elecsys and Cobas e<br />
Analyzers ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
MERCK Bismuth Sulfite Agar Acc.<br />
To Wilson-Blair MERCK KGaA., GERMANY PT. MERCK Tbk<br />
MERCK SS Agar for the Isolation of<br />
salmonella and Shigellae MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302807822 26/02/2013 MERCK Simmons Citrate Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302807823 25/02/2013<br />
MERCK Kligler Agar (Double Sugar<br />
Ion Agar acc. to KLIGLER) MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302807824 07/02/2013 Merck LEVINA EMB Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302807826 21/03/2013 MERCK Columbia Agar Base MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
20303807829 14/01/2013<br />
AKL<br />
20303807830 14/01/2013<br />
MICROPATH Febrile Antigen Kit Inc<br />
Control OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
MICROPATH Salmonella O Antigen<br />
D OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
AKL<br />
20101807850 30/01/2013<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
ADVIA Centaur Systems Calibrator 6 AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
AKL<br />
20101807851 30/01/2013<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
ADVIA Centaur Systems Calibrator 9 AG., GERMANY<br />
AKL<br />
20303807886 30/01/2013 ADVIA Centaur aHAVT ReadyPack<br />
AKL<br />
20303807887 30/01/2013<br />
AKL<br />
20306807889 30/01/2013<br />
AKL<br />
20305310147 29/01/2013<br />
SIEMENS ADVIA Centaur Hav IgM<br />
QC<br />
ADVIA Centaur Systems CEA<br />
Diluent<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
ABBOTT AXSYM SYSTEM HIV 1/2<br />
gO Controls ABBOTT GMBH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20303807892 29/01/2013<br />
AKL<br />
20303807894 29/01/2013<br />
AKL<br />
20101807896 01/02/2013<br />
ABBOTT Architect HAVAb-IgM<br />
Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT Architect HAVAb-IgM<br />
calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
CHEK - STIX Positive Control Strips<br />
for Urinalysis<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20301807870 31/01/2013 OXOID Cefadroxil CFR 30 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807872 31/01/2013 OXOID Cefoperazone CFP 75 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20301807876 31/01/2013 OXOID Ofloxacin OX 5 OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20305806965 29/01/2013 HUMAN SLE LATEX Test HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20207806964 10/01/2013 HUMAN Hemostat Fibrinogen HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101806962 10/01/2013<br />
HUMAN Hexagon hCG 1 - step<br />
Serum/Urine HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305806957 10/01/2013 HUMAN HBsAg ELISA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20306310014 10/01/2013 HUMAN Hexagon PSA HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303806959 08/01/2013 HUMAN Anti Streptolysine O (ASO) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20103806963 10/01/2013<br />
HUMAN Humadrug Cannabinoids<br />
(THC) HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101806966 08/01/2013 HUMAN Urea Liquicolor HUMAN GMBH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20205807383 19/02/2013 TC - Hemaxa + Hematology Analyzer TECO DIAGNOSTICS., USA PT. AKURAT INTAN MADYA<br />
AKL<br />
10208807613 31/01/2013 ABX EOSINOFIX HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20205807617 01/02/2013<br />
ABX PENTRA DF 120 and<br />
Accessories HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208807618 31/01/2013 ABX BASOLYSE HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208807619 31/01/2013 ABX DILUENT HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208807620 31/01/2013 ABX ALPHALYSE HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10208807633 31/01/2013 ABX FLUOCYTE HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20101807781 07/02/2013 DIALAB Creatinine Enzymatic DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20101807611 30/01/2013<br />
AKL<br />
10204310280 01/02/2013<br />
RAPIDLAB 1200 series Reagent<br />
Cartridge<br />
RAPIDLAB 1200 System Wash<br />
Cartridge<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
MANUFACTURING LTD., UK melalui<br />
SIEMENS AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10101310171 30/01/2013 ABBOTT AXSYM Cortisol Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20301807587 30/01/2013 OXOID Cefixime (CFM5) OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20303807484 14/01/2013<br />
MICROPATH Salmonella H Antigen<br />
a OMEGA DIAGNOSTICS LTD., UK PT. MULTIREDJEKI KITA<br />
AKL<br />
20305808199 29/01/2013 HUMAN Anti - HBs Elisa HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20102808086 31/01/2013<br />
RAPIDLAB 248 Critical Care<br />
Analyzer and Accessories<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10102702003 30/01/2013<br />
CLINITEK ATLAS Automated Urine<br />
Chemistry Analyzer and Accessories<br />
AKL<br />
10101808087 07/01/2013 SYSMEX TG Reagent L<br />
AKL<br />
20101310018 14/01/2013 SYSMEX T-Bill Reagent A<br />
SPARTON MEDICAL SYSTEMS USA untuk<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
INC., USA melalui SIEMENS AG., GERMANY<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
SYSMEX CORPORATION., JAPAN melalui<br />
SYSMEX ASIA PASIFIC PTE., LTD.,<br />
SINGAPORE<br />
PT. SIEMENS INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PT. SYSMEX INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20103808122 19/02/2013<br />
AKL<br />
20103808131 19/02/2013<br />
DIALAB Benzodiazepines (BZO)<br />
Control / Calibrator Set DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
DIALAB Amphetamines (AMP)<br />
Control / Calibrator Set DIALAB GmbH., AUSTRIA PT. MULTI SARANA MEDIKA<br />
AKL<br />
20207808091 08/01/2013 HUMAN HEMOSTAT aPTT-EL HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10303808092 08/01/2013 HUMAN Humatex ASO HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305808094 08/01/2013 HUMAN Humatex RF HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101808095 08/01/2013 HUMAN Humatrol N HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20103808096 29/01/2013 HUMAN Humadrug Cocaine (COC) HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305808097 29/01/2013 HUMAN Anti-HBc HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
30305808098 26/02/2013 HUMAN ANTI HIV 1/2 ELISA HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20305808099 29/01/2013 HUMAN HEXAGON HBsAg 1-Step HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
10101808100 08/01/2013 HUMAN Cholesterol Liquicolor HUMAN GmbH., GERMANY PT. SALI POLAPA BERSAMA<br />
AKL<br />
20101808108 18/03/2013<br />
AKL<br />
20101807959 29/01/2013<br />
AKL<br />
20101807963 29/01/2013<br />
ADVIA Chemistry ECRE-2<br />
Creatinine-2 Reagents (Enzymatic)<br />
SEKISUI DIAGNOSTICS P.E.I., CANADA<br />
untuk SIEMENS HEALTHCARE<br />
DIAGNOSTICS INC., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
ABBOTT AXSYM Cortisol Reagent<br />
Pack ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM Cortisol Standard<br />
Calibrator ABBOTT LABORATORIES., USA PT. ABBOTT INDONESIA<br />
AKL<br />
20101807964 10/01/2013 ABBOTT Hemocysteine Controls<br />
AKL<br />
20101807476 03/01/2013 ARCHITECT Intact PTH Calibrators<br />
AKL<br />
10204807950 31/01/2013<br />
ADVIA Centaur Total lgE Diluent<br />
Ready Pack<br />
AXIS - SHIELD DIAGNOSTICS LTD., UK<br />
untuk ABBOTT GMBH & CO. KG., GERMANY<br />
BIOKIT SA, SPAIN untuk ABBOTT GmbH &<br />
CO. KG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
10204807951 21/03/2013 ADVIA Centaur a TPO Diluent<br />
AKL<br />
20305807954 30/01/2013<br />
ADVIA Centaur Total IgE Ready<br />
Pack<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
Inc., USA melalui SIEMENS AG., GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20101808217 29/01/2013 ABBOTT CMV lgM Index Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20303310148 29/01/2013 ABBOTT CMV IgM Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
10302808226 21/03/2013<br />
AKL<br />
20103808241 31/01/2013<br />
AKL<br />
20103808244 01/02/2013<br />
AKL<br />
20101808247 18/03/2013<br />
MERCK Salmonella Enrichment<br />
Broth acc. To. RAPPAPORT MERCK KGaA., GERMANY PT. MERCK Tbk<br />
IMMULITE IMMULITE 1000 System<br />
Valproic Acid<br />
IMMULITE IMMULITE 1000<br />
Phenobarbital<br />
IMMULITE 2000 System CMB CK-<br />
MB<br />
AKL<br />
10101808248 31/01/2013 IMMULITE 1000 Systems IGFBP-3<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., UK melalui SIEMENS AG.,<br />
GERMANY<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
PT. SIEMENS INDONESIA<br />
AKL<br />
20305808209 19/02/2013 ERYCLONE Anti Human Globulin TULIP DIAGNOSTICS (P) LTD., INDIA PT. SUPRAMEDIKA PRIMA<br />
AKL<br />
20209808210 19/02/2013 ERYBANK Bovine Serum Albumin TULIP DIAGNOSTICS (P) LTD., INDIA PT. SUPRAMEDIKA PRIMA<br />
AKL<br />
20209808212 19/02/2013 ERYCLONE Anti AB Monoclonal TULIP DIAGNOSTICS (P) LTD., INDIA PT. SUPRAMEDIKA PRIMA<br />
AKL<br />
10302808256 21/03/2013 MERCK Trytose Broth MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302808257 21/03/2013 MERCK Urea Broth MERCK KGaA., GERMANY PT. MERCK Tbk<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
10302808258 29/01/2013<br />
MERCK Rambach Agar for the<br />
Identification of Salmonella MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302808259 31/01/2013 MERCK TCBS Agar (Vibrio Cholera) MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302808260 30/01/2013<br />
MERCK EMB Agar for the detection<br />
and isolation of photogenic<br />
Enterobacteriaceae MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302808261 31/01/2013 MERCK MacConkey Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10302808262 31/01/2013 MERCK Hektoen Enteric Agar MERCK KGaA., GERMANY PT. MERCK Tbk<br />
AKL<br />
10303808168 01/02/2013 MICROBACT Staph 12S OXOID LIMITED., UK PT. DIPA PUSPA LABSAINS<br />
AKL<br />
20103808321 08/01/2013<br />
AKL<br />
20103808318 08/01/2013<br />
AKL<br />
20103808317 08/01/2013<br />
AKL<br />
20103808316 08/01/2013<br />
AKL<br />
20205808178 31/01/2013<br />
AKL<br />
10101900013 01/02/2013<br />
AKL<br />
20101900016 01/02/2013<br />
AKL<br />
10101900024 01/02/2013<br />
AKL<br />
20305900025 31/01/2013<br />
AKL<br />
20305900026 31/01/2013<br />
AKL<br />
20303900027 01/02/2013<br />
AKL<br />
20303900028 01/02/2013<br />
AKL<br />
20303900030 01/02/2013<br />
AKL<br />
20101900034 01/02/2013<br />
dBEST One Step Rapid Test THC<br />
Strip Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
dBEST One Step Rapid Test OPI<br />
Card Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
dBEST One Step Rapid Test AMP<br />
Card Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
dBEST One Step Rapid Test THC<br />
Card Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
ADVIA 2120i Hematology System<br />
and Accessories<br />
AKL<br />
20101900019 31/01/2013 BATATEX DIRECT PLUS<br />
AKL<br />
20103808433 08/01/2013<br />
AKL<br />
20103808434 19/02/2013<br />
SIEMENS HEALTHCARE DIAGNOSTICS<br />
PRODUCTS LTD., USA melalui SIEMENS<br />
AG., GERMANY<br />
PT. SIEMENS INDONESIA<br />
ABBOTT AXSYM System Total T4<br />
Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Total T4<br />
Standard Calibrators ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Estradiol<br />
Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System HIV Ag /<br />
Ab Combo Controls (NEG, PC1,<br />
VLPC) ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System HIV Ag /<br />
Ab Combo Controls (NEG, PC1,<br />
PC2, VLPC) ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HAVAB -<br />
IgG Reagent Kit ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HAVAb - IgG<br />
Controls ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT ARCHITECT HAVAb - IgG<br />
Calibrator ABBOTT GmbH & CO. KG., GERMANY PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Estradiol<br />
Standard Calibrators ABBOTT LABORATORIES,USA PT. ABBOTT INDONESIA<br />
OMEGA DIAGNOSTICS LTD., UNITED<br />
KINGDOM<br />
PT. MULTIREDJEKI KITA<br />
dBEST One Step Rapid Test AMP<br />
Strip Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
dBEST One Step Rapid Test OPI<br />
Strip Test AMERITEK INC., USA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101900368 19/02/2013 ABBOTT Homocysteine Calibrators<br />
AKL<br />
10101900369 25/02/2013<br />
AKL<br />
20101900372 01/02/2013<br />
AKL<br />
10101900374 14/03/2013<br />
AKL<br />
20101900278 25/02/2013<br />
AXIS-SHIELD DIAGNOSTICS LTD., UK untuk<br />
ABBOTT GMBH CO & KG., GERMANY<br />
PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM System Prolactin<br />
Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Clinical Chemistry Acid<br />
Phosphatase ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT AXSYM SYSTEM FSH<br />
Reagent Pack ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ROCHE/HITACHI FE Standard<br />
Calibrator ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
10102900279 14/03/2013 ACCU CHEK Safe-T Pro Plus ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
AKL<br />
20305900280 25/02/2013<br />
AKL<br />
20305900282 25/02/2013<br />
AKL<br />
20101900211 10/01/2013 ACCU-CHECK Active<br />
AKL<br />
20101900212 10/01/2013<br />
PDF Creator - PDF4Free v3.0<br />
ROCHE/HITACHI IgG-2 Tina-quant<br />
lgG Gen.2 ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
ROCHE/HITACHI IgA-2 Tina-quant<br />
lgA Gen.2 ROCHE DIAGNOSTICS GmbH., GERMANY PT. ROCHE INDONESIA<br />
COBAS INTEGRA cobas c system<br />
TP2 Total Protein Gen.2<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
http://www.pdf4free.com<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA
AKL<br />
20305900213 10/01/2013<br />
ROCHE/HITACHI IgM-2 Tina-Quant<br />
IgM Gen.2<br />
AKL<br />
10102900214 04/01/2013 COBAS c 311 and Accessories<br />
AKL<br />
10102900215 31/01/2013<br />
ROCHE DIAGNOSTICS GMBH., GERMANY<br />
melalui ROCHE DIAGNOSTICS<br />
INTERNATIONAL LTD., SWITZERLAND<br />
HITACHI HIGH - TECHNOLOGIES CORP,<br />
JAPAN untuk ROCHE DIAGNOSTICS GmbH.,<br />
GERMANY melalui ROCHE DIAGNOSTICS<br />
INTL. LTD., SWITZERLAND<br />
PT. ROCHE INDONESIA<br />
PT. ROCHE INDONESIA<br />
PICCOLO Xpress Blood Chemistry<br />
Analyzer ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
10101900216 14/03/2013 PICCOLO Lipid Panel Plus ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
10101900218 31/01/2013 PICCOLO Lipid Panel ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900219 31/01/2013<br />
PICCOLO Comprehensive Metabolic<br />
Panel ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900220 31/01/2013 PICCOLO General Chemistry 13 ABAXIS INC., USA PT. MEGA UTAMA MEDICA<br />
AKL<br />
20101900292 19/02/2013<br />
AKL<br />
20101900299 01/02/2013<br />
AKL<br />
10101900300 14/03/2013<br />
AKL<br />
20101900289 25/02/2013<br />
ARCHITECT Homocysteine<br />
Calibrators<br />
ABBOTT ARCHITECT<br />
Homocysteine Reagent Kit<br />
ABBOTT ARCHITECT<br />
Homocysteine Controls<br />
ONETOUCH SureStep Hospital Test<br />
Strip<br />
AXIS-SHIELD DIAGNOSTICS LTD., UNITED<br />
KINGDOM untuk ABBOTT GMBH & CO. KG.,<br />
GERMANY<br />
AXIS-SHIELD DIAGNOSTICS LTD., UNITED<br />
KINGDOM untuk ABBOTT GMBH & CO. KG.,<br />
GERMANY<br />
AXIS-SHIELD DIAGNOSTICS LTD., UNITED<br />
KINGDOM untuk ABBOTT GMBH & CO. KG.,<br />
GERMANY<br />
LIFESCAN PRODUCTS LLC., USA untuk<br />
LIFESCAN INC., USA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. ABBOTT INDONESIA<br />
PT. JOHNSON & JOHNSON<br />
INDONESIA<br />
AKL<br />
20303900286 25/02/2013 PARACHECK PF Device ORCHID BIOMEDICAL SYSTEMS., INDIA PT. SUPRAMEDIKA PRIMA<br />
AKL<br />
21106700939 30/01/2013 VITROLIFE G - Thawkit Blast VITROLIFE AB., SWEDEN<br />
AKL<br />
21106200490 30/01/2013 VITROLIFE SpermGrad VITROLIFE AB., SWEDEN<br />
AKL<br />
21106310186 30/01/2013 VITROLIFE G - MM VITROLIFE AB., SWEDEN<br />
AKL<br />
20205901313 01/02/2013<br />
AKL<br />
10208901307 31/01/2013<br />
AKL<br />
20101901729 25/02/2013<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PT. DIPA PHARMALAB<br />
INTERSAINS<br />
PointCare NOW Reagent -<br />
CD4NOW Liquipack (Lyse) POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
PointCare NOW Reagent -<br />
CD4NOW Liquipack (Diluent) POINTCARE TECHNOLOGIES INC., USA PT. MEGA UTAMA MEDICA<br />
GONGDONG Vacuum Tube Sterile<br />
EDTA K-3 Purple Cap<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101901731 25/02/2013<br />
GONGDONG Vacuum Tube Sterile<br />
Sodium Heparin Green Cap<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101901732 25/02/2013<br />
GONGDONG Vacuum Tube Sterile<br />
Sodium Citrate Blue Cap<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101901733 25/02/2013<br />
GONGDONG Vacuum Tube Sterile<br />
Serum with Clot Activator<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101901734 25/02/2013<br />
AKL<br />
20101901929 25/02/2013<br />
AKL<br />
20303902425 26/02/2013<br />
AKL<br />
20303902422 26/02/2013<br />
AKL<br />
20101901041 21/03/2013<br />
AKL<br />
20101901042 21/03/2013<br />
AKL<br />
20101901037 21/03/2013<br />
GONGDONG Vacuum Tube Sterile<br />
Lithium Heparin Green Cap<br />
GONGDONG Vacuum Tube Sterile<br />
Glucose Grey Cap<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
ZHEJIANG GONGDONG MEDICAL<br />
TECHNOLOGY CO. LTD., CHINA<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. SETIA ANUGERAH MEDIKA<br />
ROCHE CMV IgG Elecsys and<br />
Cobas e Analyzer ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
ROCHE CMV IgM Elecsys and<br />
Cobas e Analyzer ROCHE DIAGNOSTICS GMBH., GERMANY PT. ROCHE INDONESIA<br />
IRMA TRUPOINT Blood Analysis<br />
System CC Cartridge<br />
IRMA TRUPOINT Blood Analysis<br />
System BG Cartridge<br />
IRMA TRUPOINT Blood analysis<br />
System CR Cartridge<br />
AKL<br />
20305901035 18/03/2013 INTEC HBsAg ELISA Test Kit<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
INTERNATIONAL TECHNIDYNE CORP.,<br />
USA<br />
INTEC PRODUCTS INC (XIAMEN)., P.R<br />
CHINA<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. DEMKA SAKTI<br />
PT. MITRASAMAYA SEJATI<br />
AKL<br />
10208901468 14/03/2013 CELL-DYN Emerald Diluent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20208901471 21/03/2013 CELL-DYN Reticulocyte Reagent ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20208901472 21/03/2013<br />
ABBOTT WBC Lyse (Cell-Dyn<br />
Ruby, Cell-Dyn 3200 System) ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com
AKL<br />
20208901473 21/03/2013<br />
AKL<br />
20208901475 21/03/2013<br />
ABBOTT CN-Free HGB / NOC Lyse<br />
(Cell-Dyn Ruby, Cell-Dyn 3200<br />
System) ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
ABBOTT Diluent / Sheath (Cell-Dyn<br />
Sapphire and Cell-Dyn Ruby<br />
systems) ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
AKL<br />
20209807116 31/01/2013 SPANCLONE Anti AB Monoclonal SPAN DIAGNOSTICS LTD., INDIA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
20101807916 03/01/2013 Cell Dyn 18 Plus Calibrator<br />
STRECK, USA for ABBOTT<br />
LABORATORIES, USA<br />
BECTON DICKINSON AND COMPANY (BD).,<br />
AKL<br />
20101010357 03/01/2013<br />
USA melalui BECTON DICKINSON<br />
BD Vacutainer Trace Element Serum HOLDINGS PTE., LTD., SINGAPORE<br />
AKL<br />
20101010360 03/01/2013<br />
AKL<br />
20101010361 03/01/2013<br />
AKL<br />
20305013133 01/02/2013<br />
BD Vacutainer No Additive (Z) Plus<br />
Tube<br />
BD Vacutainer Sodium Heparin (NH)<br />
75 USP Units plus Blood Collection<br />
Tubes<br />
ABBOTT m2000sp Instrument and<br />
Accessories (E-Series)<br />
AKL<br />
20303111109 01/02/2013 ASSURE Dengue IgA Rapid Test<br />
AKL<br />
10201111563 08/01/2013<br />
AKL<br />
10201111564 03/01/2013<br />
AKL<br />
10201111565 03/01/2013<br />
AKL<br />
10201111566 08/01/2013<br />
AKL<br />
10201111567 08/01/2013<br />
AKL<br />
10203111034 29/01/2013<br />
LEICA NOVOCASTRA NOVOLINK<br />
Polymer Detection System<br />
LEICA NOVOCASTRA Estrogen &<br />
Progestterone Receptor Antibodies<br />
(duo packs)<br />
LEICA NOVOCASTRA Epitope<br />
Retrieval Solution<br />
LEICA NOVOCASTRA c-erbB-2<br />
Oncoprotein (HER2) Antibodies<br />
LEICA NOVOCASTRA IHC Diluent<br />
Antibodies<br />
LEICA RM 2235 - Manual Rotary<br />
Microtome and Accessories<br />
BECTON DICKINSON AND COMPANY (BD).,<br />
USA melalui BECTON DICKINSON<br />
HOLDINGS PTE., LTD., SINGAPORE<br />
BECTON DICKINSON AND COMPANY (BD).,<br />
USA melalui BECTON DICKINSON<br />
HOLDINGS PTE., LTD., SINGAPORE<br />
ABBOTT MOLECULAR INC., USA<br />
MP BIOMEDICALS ASIA PACIFIC PTE. LTD.,<br />
SINGAPORE<br />
LEICA BIOSYSTEM NEWCASTLE LTD., UK<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM NEWCASTLE LTD., UK<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM NEWCASTLE LTD., UK<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM NEWCASTLE LTD., UK<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEM NEWCASTLE LTD., UK<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MICROSYSTEM<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. ABBOTT INDONESIA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. ANUGRAH ARGON MEDICA<br />
PT. SINERGI UTAMA<br />
SEJAHTERA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10203111303 03/01/2013<br />
LEICA ST4020 - Linier Stainer and<br />
Accessories<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH.,<br />
GERMANY melalui LEICA MYCROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
20305111637 06/03/2013 ABX Pentra SP Cal HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
20305111635 06/03/2013 ABX Pentra SP Control High HORIBA ABX SAS, FRANCE PT. DOS NI ROHA<br />
AKL<br />
10203111570 23/01/2013 LEICA DM 500 and Accessories<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH,<br />
GERMANY melalui LEICA MICROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10201111498 08/01/2013 LEICA SURGIPATH Orange G-6<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH,<br />
GERMANY melalui LEICA MICROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10201111494 08/01/2013<br />
LEICA SURGIPATH Harris<br />
Hematoxicil<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH,<br />
GERMANY melalui LEICA MICROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10201111495 08/01/2013 LEICA SURGIPATH EA-50<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH,<br />
GERMANY melalui LEICA MICROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
AKL<br />
10201111496 08/01/2013 LEICA SURGIPATH Eosin<br />
AKL<br />
10201111497 08/01/2013 LEICA SURGIPATH Xylene<br />
PDF Creator - PDF4Free v3.0<br />
LEICA BIOSYSTEMS NUSSLOCH GMBH,<br />
GERMANY melalui LEICA MICROSYSTEMS<br />
(SEA) PTE. LTD., SINGAPORE<br />
LEICA BIOSYSTEM RICHMOND INC., USA<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
http://www.pdf4free.com<br />
PT. BIOGEN SCIENTIFIC<br />
PT. BIOGEN SCIENTIFIC
AKL<br />
10203112476 23/01/2013 LEICA M80 and Accessories<br />
AKL<br />
20306112124 01/02/2013<br />
AKL<br />
20305112737 31/01/2013<br />
LEICA BIOSYSTEMS NUSSLOCH GmbH,<br />
Germany melalui LEICA MICROSYSTEMS (<br />
SEA) PTE, LTD, Singapore<br />
PT. BIOGEN SCIENTIFIC<br />
INNOGENETICS INNO-LiPA HLA-A<br />
Update INNOGENETICS N.V., BELGIUM PT. BEHRINDO NUSA PERKASA<br />
BIO-TECH Hepatitis B Surface<br />
Antibody (Anti-HBs) ELISA Kit ZHONGHAN BIO-TECH CO. LTD., CHINA PT. SETIA ANUGERAH MEDIKA<br />
AKL<br />
10204112986 08/01/2013 LEICA SURGIPATH Decalcifier I<br />
AKL<br />
10205112740 10/01/2013<br />
AKL<br />
30305113470 30/01/2013<br />
CARETIUM XC-A30 Automet<br />
Erythrocyte Sedimentation Rate<br />
Analyzer and Accessories<br />
MP Diagnostics HIV BLOT 2.2<br />
Western Blot Assay<br />
AKL<br />
10302702291 14/03/2013 BD PHOENIX PID<br />
AKL<br />
10101210326 01/02/2013<br />
LEICA BIOSYSTEMS RICHMOND INC., USA<br />
melalui LEICA MICROSYSTEMS (SEA) PTE.<br />
LTD., SINGAPORE<br />
CARETIUM MEDICAL INSTRUMENYS CO.<br />
LTD., CHINA<br />
MP BIOMEDICAL ASIA PACIFIC PTE., LTD.,<br />
SINGAPORE<br />
BECTON DICKINSON AND COMPANY (BD),<br />
USA melalui BECTON DICKINSON<br />
HOLDINGS PTE. LTD., SINGAPORE<br />
PT. BIOGEN SCIENTIFIC<br />
PT. SETIA ANUGERAH MEDIKA<br />
PT. DIASTIKA BIOTEKINDO<br />
PT. ANUGRAH ARGON MEDICA<br />
ABBOTT AXSYM System Estradiol<br />
Controls ABBOTT LABORATORIES, USA PT. ABBOTT INDONESIA<br />
PDF Creator - PDF4Free v3.0<br />
http://www.pdf4free.com