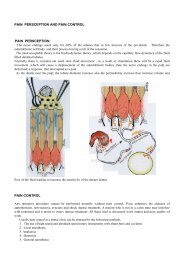
Assistant Lecture Aayad Amaar Concentration Most chemical ...
Assistant Lecture Aayad Amaar Concentration Most chemical ...
Assistant Lecture Aayad Amaar Concentration Most chemical ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
College of Dentistry<br />
Inorganic Chemistry<br />
<strong>Assistant</strong> <strong>Lecture</strong><br />
<strong>Aayad</strong> <strong>Amaar</strong><br />
<strong>Concentration</strong><br />
<strong>Most</strong> <strong>chemical</strong> reactions occur in a liquid solvent/solute (i.e.,<br />
solution) environment. Typically the solute(s) in a solution will be<br />
the reactants. Since stoichiometric calculations require amounts of<br />
reactants, we need a way to express amounts of reactants when<br />
they are in solution. The way chemists do this is through the<br />
concept of concentration. <strong>Concentration</strong> is a way to express the<br />
amount of solute per unit amount of solution/solvent. There are<br />
several ways that concentration can be expressed. A few of these<br />
are:<br />
ppm (parts per million)<br />
ppb (parts per billion)<br />
Weight/weight percent (w/w %)<br />
Weight/volume percent (w/v %)<br />
Volume/volume percent (v/v %)<br />
molarity<br />
ppm and ppb<br />
Parts per million (ppm) and parts per billion (ppb) are examples<br />
of expressing concentrations by mass. These units turn out to be<br />
convenient when the solute concentrations are very small (almost<br />
trace amounts). For example, if a solution has 1 ppm solute this<br />
would mean that 1 g of solution would have one "millionth" gram<br />
of solute. Equivalently, 1 kg of this solution will have 1 mg of<br />
solute etc... By definition we have:<br />
For example, suppose a 155.3 g sample of pond water is found to<br />
have 1.7x10 -4 g of phosphates. What is the concentration of<br />
phosphates in ppm?
College of Dentistry<br />
Inorganic Chemistry<br />
A similar procedure would be followed to calculate ppb. In the<br />
above example the pond water would be 1100 ppb.<br />
Now suppose we have 400 g sample of pond water and it has a<br />
concentration of 3.5 ppm dissolved nitrates. What is the mass of<br />
dissolved nitrates in this sample?<br />
Weight/Weight %<br />
This concentration unit is similar to ppm or ppb except it<br />
focuses on the solute as a percent (by mass) of the total solution.<br />
It is appropriate for relatively large solute concentrations, by<br />
definition we have<br />
As an example considers 5 g sugar dissolved in 20 g of water.<br />
What is the w/w% concentration of sugar in this solution?<br />
Now suppose we have 450 g of NaCl solution that is 35 NaCl<br />
w/w %. What is the mass of NaCl?<br />
Weight/Volume Percent<br />
The concentration of a solution is defined as the amount of solute<br />
dissolved in a specified amount of solution,<br />
If we define the amount of solute as the mass of solute (in grams)<br />
and the amount of solution in volume units (milliliters),<br />
concentration is expressed as the ratio
College of Dentistry<br />
Inorganic Chemistry<br />
This concentration can then be expressed as a percentage by<br />
multiplying the ratio by the factor 100%. This result in<br />
The percent concentration expressed in this way is called<br />
weight/volume percent, or % (W/V). Thus<br />
Example<br />
Volume/Volume %<br />
When the solute is a liquid sometimes it is convenient to<br />
express its concentration in volume/volume percent (v/v %). The<br />
definition of v/v % is<br />
Wine has about 12 mL of alcohol (ethanol) per 100 mL of<br />
solution. Wine would have the following v/v % alcohol content:<br />
Molarity<br />
Molarity is the most useful concentration for <strong>chemical</strong> reaction<br />
in solution because it directly relates moles of solute to volume of<br />
solution. The definition of molarity is
College of Dentistry<br />
Inorganic Chemistry<br />
As an example, suppose we dissolve 23 g of ammonium chloride<br />
(NH 4 Cl) in enough water to make 145 mL of solution. What is the<br />
molarity of ammonium chloride in this solution?<br />
Now, suppose we have a beaker with 175 mL of a 0.55 M HCl<br />
solution. How many moles of HCl is in this beaker?<br />
As a final example suppose we have a solution of 0.135 M<br />
NaCl and we need 1.2 moles of NaCl. What volume of the NaCl<br />
solution is required?<br />
Dilution<br />
Often it is necessary to take a concentrated solution and dilute<br />
it. However, we want to dilute it in a controlled way so that we<br />
know the concentration after dilution. The way this is done can be<br />
extracted from the following figure of dilution:<br />
The solute is concentrated in the beaker on the left. Adding<br />
water dilutes the solution as shown with the beaker on the right.<br />
However, note that although the concentration changes upon<br />
dilution, the number of solute molecules does not. In other words
College of Dentistry<br />
Inorganic Chemistry<br />
the number of moles of solute is the same before and after<br />
dilution. Since Moles = Molarity x Volume (i.e., moles= M x V)<br />
we end up with the following equation relating molarity and<br />
volume before and after dilution:<br />
Mi x Vi = Mf x Vf<br />
Where i and f stand for initial and final. Suppose we need 150 mL<br />
of 0.25 M NaCl. On the shelf we find a bottle of 2M NaCl. What<br />
do we do? the concentrated molarity is Mi and the volume needed<br />
is Vi. We need to determine Vi and can do so by rearranging the<br />
above equation and doing the resulting calculation:<br />
Thus, we need 18.8 mL of the 2M NaCl solution, put it in a<br />
beaker and add enough water to make 150 mL of solution. The<br />
resulting solution will have a molarity of 0.25 M NaCl.
This document was created with Win2PDF available at http://www.daneprairie.com.<br />
The unregistered version of Win2PDF is for evaluation or non-commercial use only.