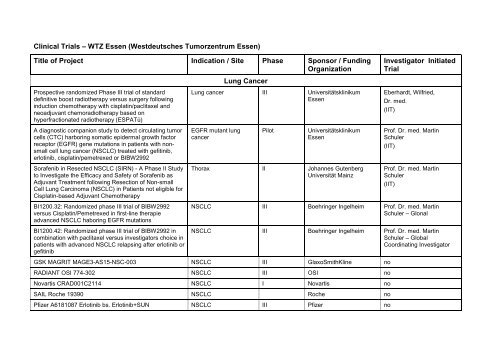
Clinical Trials â WTZ Essen (Westdeutsches Tumorzentrum Essen ...
Clinical Trials â WTZ Essen (Westdeutsches Tumorzentrum Essen ...
Clinical Trials â WTZ Essen (Westdeutsches Tumorzentrum Essen ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Clinical</strong> <strong>Trials</strong> – <strong>WTZ</strong> <strong>Essen</strong> (<strong>Westdeutsches</strong> <strong>Tumorzentrum</strong> <strong>Essen</strong>)<br />
Title of Project Indication / Site Phase Sponsor / Funding<br />
Organization<br />
Investigator Initiated<br />
Trial<br />
Prospective randomized Phase III trial of standard<br />
definitive boost radiotherapy versus surgery following<br />
induction chemotherapy with cisplatin/paclitaxel and<br />
neoadjuvant chemoradiotherapy based on<br />
hyperfractionated radiotherapy (ESPATü)<br />
Lung Cancer<br />
Lung cancer III Universitätsklinikum<br />
<strong>Essen</strong><br />
Eberhardt, Wilfried,<br />
Dr. med.<br />
(IIT)<br />
A diagnostic companion study to detect circulating tumor<br />
cells (CTC) harboring somatic epidermal growth factor<br />
receptor (EGFR) gene mutations in patients with nonsmall<br />
cell lung cancer (NSCLC) treated with gefitinib,<br />
erlotinib, cisplatin/pemetrexed or BIBW2992<br />
EGFR mutant lung<br />
cancer<br />
Pilot<br />
Universitätsklinikum<br />
<strong>Essen</strong><br />
Prof. Dr. med. Martin<br />
Schuler<br />
(IIT)<br />
Sorafenib in Resected NSCLC (SIRN) - A Phase II Study<br />
to Investigate the Efficacy and Safety of Sorafenib as<br />
Adjuvant Treatment following Resection of Non-small<br />
Cell Lung Carcinoma (NSCLC) in Patients not eligible for<br />
Cisplatin-based Adjuvant Chemotherapy<br />
Thorax II Johannes Gutenberg<br />
Universität Mainz<br />
Prof. Dr. med. Martin<br />
Schuler<br />
(IIT)<br />
BI1200.32: Randomized phase III trial of BIBW2992<br />
versus Cisplatin/Pemetrexed in first-line therapie<br />
advanced NSCLC haboring EGFR mutations<br />
BI1200.42: Randomized phase III trial of BIBW2992 in<br />
combination with paclitaxel versus investigators choice in<br />
patients with advanced NSCLC relapsing after erlotinib or<br />
gefitinib<br />
NSCLC III Boehringer Ingelheim Prof. Dr. med. Martin<br />
Schuler – Glonal<br />
NSCLC III Boehringer Ingelheim Prof. Dr. med. Martin<br />
Schuler – Global<br />
Coordinating Investigator<br />
GSK MAGRIT MAGE3-AS15-NSC-003 NSCLC III GlaxoSmithKline no<br />
RADIANT OSI 774-302 NSCLC III OSI no<br />
Novartis CRAD001C2114 NSCLC I Novartis no<br />
SAIL Roche 19390 NSCLC Roche no<br />
Pfizer A6181087 Erlotinib bs. Erlotinib+SUN NSCLC III Pfizer no
AURORA Nerviano AURA-6202-006<br />
BERLINStudie Carboplatin + Irinotecan vs Carboplatin +<br />
Etoposid<br />
Breast, small cell lung<br />
and non small cell lung<br />
cancers. (closed for<br />
pancreatic, ovarial and<br />
colorectal cancers)<br />
II Nerviano no<br />
SCLC III AIO no<br />
Bayer 310101 Epothilon-Studie SCLC I / II Schering no<br />
START EMR 63325-001 NSCLC III Merck no<br />
TIE ML19747 NSCLC II AIO/Roche no<br />
TREAT / AIO NSCLC II AIO/Lilly no<br />
Sanofi Aventis VEGF-Trap VITAL NSCLC II Sanofi Aventis no<br />
Astra Zeneca REASON NSCLC n/a Astra Zeneca no<br />
Novartis ATTRACT2 NSCLC III Novartis no<br />
Pfizer A8081005 NSCLC II Pfizer no<br />
Pfizer A8081007 NSCLC III Pfizer no<br />
Roche Humab NO21160 NSCLC Roche no<br />
Bayer ESCAPE 11961 NSCLC III Bayer no<br />
Merck EMD72000-031 NSCLC II Merck no<br />
Pfizer A8501001 NSCLC III Pfizer no<br />
Bayer PTK NSCLC II Bayer no<br />
Astra Zeneca D4200C00032 NSCLC III Astra Zeneca no<br />
Novartis Zometa CZOL446G2419 NSCLC III Novartis no<br />
Bayer 12006 NEXUS NSCLC III Bayer no<br />
Astra Zeneca EPICLIN NSCLC n/a Astra Zeneca no<br />
Celgene AMR PH GL 2007 CL 001 SCLC III Celgene no
Novartis ATTRACT1 NSCLC III Novartis no<br />
BI1200.23 NSCLC IIb/III Boehringer Ingelheim no<br />
Stem Cell Transplantation<br />
Autologous or allogeneic transplantation following<br />
conventional chemotherapy in younger Patients (18-60<br />
yrs) with mature ALK negative peripheral T-cell<br />
lymphoma (DSHNHL 2006-1A)<br />
Aggressive<br />
T-cell lymhoma<br />
Phase III <strong>Clinical</strong> Trial Center HH Prof.Dr. N. Schmitz<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
AML- SCT<br />
Allogenic stem cell<br />
transplantation acute<br />
myeloic leucemia<br />
N / A<br />
Hannover clinical Trial<br />
Center<br />
PD Dr. med. Martin Sauer<br />
<strong>Clinical</strong> Phase III trial to compare Treosulfan-based<br />
conditioning therapy with Busulfan-based reducedintensity<br />
conditioning (RIC) prior to allogeneic stem cell<br />
transplantation in patients with AML or MDS considered<br />
ineligible to standard conditioning regimens (MC-<br />
FludT.14/L)]<br />
Acute myeloid leukemia<br />
Myelodysplastic<br />
syndromes<br />
Phase III MEDAC GmbH Prof. Dr. D. W. Beelen<br />
(coordinating investigator)<br />
Phase II study Allogeneic Stem Cell Transplantation with<br />
Treosulfan, VP-16 and Cyclophosphamide for patients<br />
with Acute Lymphoblastic Leukemia (ALL) not eligible for<br />
TBI-containing regimens (Allo-SCT -- Treo-VP16-Cyclo /<br />
ALL)<br />
Acute lymphoblastic<br />
leukemia<br />
Phase II<br />
CTS<br />
(<strong>Clinical</strong> Trial Solutions)<br />
MEDAC GmbH<br />
Prof. Dr. D. W. Beelen,<br />
<strong>Essen</strong><br />
Prof. Dr. N. Kröger ,<br />
Hamburg<br />
(coordinating investigators)<br />
AML: Phase II study of haploidentical hematopoietic cell<br />
transplantation with CD3/CD19 depleted grafts after a<br />
reduced intensity conditioning regimen (E 410/2007)<br />
Acute myeloid leukemia Phase II University Hospital<br />
Tübingen<br />
PD Dr. W. Bethge,Tübingen<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Lenalidomide maintenance therapy in patients with MDS<br />
or AML with cytogenetic abnormalities involving<br />
monosomy 5 or del5q after allogeneic hematopoietic<br />
stem cell transplantation - LENAMAINT<br />
Acute myeloid leukemia<br />
Myelodysplastic<br />
syndromes<br />
Phase I/II ClinAssess Leverkusen PD Dr. U. Platzbecker,<br />
Dresden<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen
Multicenter randomized study comparing oral<br />
valganciclovir versus intravenous ganciclovir in patients<br />
following allogeneic stem cell transplantation (ML 22371)<br />
Cytomegalovirus<br />
infection<br />
Phase III IFE Europe GmbH Prof. Dr. H. Einsele,<br />
Würzburg<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Evaluation of allogeneic cell transplantation in acute<br />
myeloid leukemia I (ETAL I)<br />
Acute myeloid leukemia Phase III DFG Prof. Dr. M. Bornhäuser,<br />
Dresden<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Prospektive offene multizentrische Phase II-Studie zur<br />
Effektivität von Palifermin (Kepivance) in der Prophylaxe<br />
der Mukositis nach allogener Stamzelltransplantation mit<br />
myeloablativer Ganzkörperbestrahlung (OSHO 76)<br />
Phase-III study on the value of allogeneic stem cell transplantation<br />
in poor-risk chronic lymphocytic leukemia<br />
(GCLLSG/GCTSG CLL-X2 trial)<br />
Akute und chronische<br />
Leukämien<br />
Chronic lymphocytic<br />
leukemia<br />
Phase III WISP GmbH PD. Dr. H. Sayer, Jena<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Phase III DFG Prof. Dr. P. Dreger,<br />
Heidelberg<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Autologous-allogeneic tandem stem cell transplantation<br />
and maintenance therapy with Thalidomide/DLI for<br />
patients with multiple myeloma and age ≤ 60 years: a<br />
phase II-study<br />
Muliple myeloma Phase II University Medical Center<br />
Hamburg-Eppendorf<br />
Prof. Dr. N. Kröger,<br />
Hamburg<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Double-blind, placebo-controlled, randomized multicenter<br />
phase III trial to access the efficacy of Sorafenibmaintenance<br />
therapy in Flt3-ITD positive AML in<br />
complete remission<br />
Acute myeloid leukemia Phase II KKS Marburg Prof Dr. A. Neubauer<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen
A randomized, multicenter phase III-study to compare the<br />
efficacy and safety of early pre-emptive versus minimal<br />
residual disease (MRD)-triggered administration of<br />
Imatinib Mesylate (STI571, Glivec) after stem cell<br />
transplantation for Ph+/BCR-ABL+ acute lymphoblastic<br />
leukemia (Ph+ALL).<br />
Acute lymphoblastic<br />
leukemia<br />
Phase III Univ. Frankfurt Prof. Dr. O.G. Ottmann,<br />
Frankfurt<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
A randomized, risk and age adapted comparison of the<br />
dose-dense regimen S-HAM (sequential high-dose<br />
cytosine arabinoside and mitoxantrone) versus standard<br />
double induction for initial chemotherapy of adult patients<br />
with acute myeloid leukemia<br />
Acute myeloid leukemia Phase III DKH Prof. Dr. W. Hiddemann,<br />
München<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Multizentrische Therapieoptimierungsstudie der akuten<br />
lymphatischen Leukaemie bei Erwachsenen und<br />
Adoleszenten ab 18 Jahren (GMALL 07/2003) -<br />
Therapieoptimierung durch Evaluation der minimalen<br />
Resterkrankung -<br />
Randomisierter kontrollierter Vergleich von Imatinib vs.<br />
Imatinib und Interferon-alpha vs. Imatinib 800 mg mit<br />
Prüfung des Stellenwertes der allogenen<br />
Stammzelltransplantation bei neu diagnostizierter CML in<br />
chronischer Phase<br />
Acute lymphoblastic<br />
leukemia<br />
Chronische myeloische<br />
Leukämie<br />
Phase III DKH Prof. Dr. D. Hoelzer,<br />
Frankfurt<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Phase IV DKH Prof. Dr. R. Hehlmann,<br />
Mannheim<br />
(coordinating investigator)<br />
PI <strong>Essen</strong>:<br />
Prof. Dr. D. W. Beelen<br />
Prospective multicentre validation of National Insitutes of<br />
Health criteria of chronic graft-versus-host –disease in<br />
children and adolescents following allogenic stem cell<br />
transplation<br />
cGvHD-Project N / A St. Anna-Kinderspital,<br />
Wien<br />
Melanoma<br />
Dr. med. Lawitschka<br />
Klinische Phase-III Studie der ADO "Individualisierte<br />
Therapie nach Chemosensitivitätsprofil versus DTIC<br />
beim metastasierten Melanom"<br />
Metastasiertes<br />
Melanom Stadium IIIC<br />
nicht resezierbar und<br />
Stadium IV<br />
III ADO Prof. Dr. Selma Ugurel,<br />
Uniklinikum Würzburg<br />
Arbeitsgemeinschaft<br />
Dermatologische Onkologie<br />
(ADO)
Adjuvant immunotherapie with anti-CTLA-4 monoclonal<br />
antibody (ipilimumab) versus placebo after complete<br />
resection of high-risk Stage III melanoma: A<br />
Randomized, double-blind Phase 3 trial of the EORTC<br />
Melanoma Group (BMS CA184 029)<br />
A double blind, randomized, placebo controlled Phase III<br />
Study to assess the efficacy of recMAGE-A3+AS15 ASCI<br />
as adjuvant therapy in Patients with MAGE-A3 positive<br />
resected stage III melanoma<br />
An open, single-arm trial to assess the clinical activity of<br />
recMAGE-A3 + AS15 in Patients with unresectable<br />
MAGE-A3-positive, metastasic cutaneous melanoma<br />
ADO-CTCL-3 (TARADO): Multizentrisches Therapie-<br />
Protokoll zur Bexaroten (Targretin®)-Monotherapie bei<br />
vorbehandeltem CTCL Stadium ≥Ib mit klarer Zuordnung<br />
einer EORTC-Diagnose<br />
PRAME-AS15-MEL-001 (met) An open , dose-escalation<br />
Phase I/II study to assess the safety, immunogeneticity<br />
and clinical activity of recPRAME + AS15 Antigen-<br />
Specific Cancer Immunotherapeutic as first-line<br />
treatment of patients with PRAME-positive metastati<br />
Protocol H8K-MC-JZAO A Randomized Phase 3 Study of<br />
Tasisulam Administered as an Intravenous Infusion on<br />
Day 1 of a 28-Day Cycle vs. Paclitaxel as Second-Line<br />
Treatment in Patients with Metastatic Melanoma<br />
Malignes Melanom<br />
Stadium III komplett<br />
resezierbar<br />
MAGE-A3-positives<br />
Melanom Stadium III<br />
MAGE-A3-positives<br />
nicht resezierbares<br />
Melanom Stadium III<br />
oder IV M1a<br />
Mycosis fungoides,<br />
Sezary-Syndrome, 10-<br />
30 CD30-positive CTCL,<br />
lymphomatoide<br />
Papulosen, NK-<br />
Lymphome<br />
Phase I: Stadium IV,<br />
M1b und M1c und<br />
Patienten mit vollständig<br />
reseziertem Tumor im<br />
Stadium IV Phase II:<br />
Stadium III, messbar<br />
und nicht resezierbar,<br />
einschließlich in-transit<br />
Metastasen und<br />
Patienten im Stadium<br />
M1a<br />
Malignes Melanonom im<br />
Stadium IV<br />
III<br />
Bristol-Myers-Squibb/<br />
EORTC<br />
no<br />
III Glaxo Smith Kline no<br />
III Glaxo Smith Kline no<br />
/ Cephalon Prof. Dr. Reinhard Dummer,<br />
Universitätsspital Zürich,<br />
Arbeitsgemeinschaft<br />
Dermatologische<br />
Onkologie(ADO)<br />
I / II Glaxo Smith Kline no<br />
III Eli - Lilly no
The TEAM Trial (Tasigna Efficacy in advanced<br />
Melanoma): A randimized, phase III, open-label, multicenter,<br />
two-arm study to compare the efficacy of Tasigna<br />
® versus dacarbazine (DTIC) in treatment of Patients<br />
with inoperable metastatic acral, mucosal or c<br />
A Randomized, open label, controlled, multicenter,<br />
Phase III Study in previously untreated Patient with<br />
unresectable Stage IIIc or Stage IV Melanoma with<br />
V600-Positive BRAF mutation Receiving RO5185426 or<br />
Dacarbazine<br />
SIMPS: An international, prospective, blinded clinical<br />
study designated to determine the safety and<br />
effetiveness of the SciBase III device designed to<br />
distinguish between malignant melanoma and benign<br />
lesions using electrical impedance spectroscopy<br />
A randomized, double-blind, vehicle-controlled,<br />
multicenter<br />
trial of topically administered LDE225 cream (0.75% bid)<br />
to<br />
evaluate clearance of Basal Cell Carcinoma in adult<br />
patients with Nevoid Basal Cell Carcinoma Syndrome<br />
Randomisierte Phase III Studie zum Vergleich von<br />
intravenous versus intraarteriell verabreichtem<br />
Fotemustin bei Patienten mit Lebermetastasen eines<br />
Aderhautmelanoms<br />
NOA-05 phase II trial of procarbazine and lomustine<br />
chemotherapy (PC) in newly diagnosed gliomatosis<br />
cerebri<br />
Therapie für c-Kit-<br />
Mutation positive<br />
Patienten des Exon 11<br />
oder 13, sowie c-Kit<br />
Mutation Y822D /<br />
Y823D des Exon 17 bei<br />
einem metastasierten<br />
Schleimhautmelanom,<br />
akralen- oder kutanen<br />
Melanom<br />
Für BRAF-positive<br />
Patienten mit Malignem<br />
Melanom im nicht<br />
resezierbaren Stadium<br />
IIIC oder Stadium IV<br />
Patienten mit Verdacht<br />
auf maligne Läsion<br />
BCC Gorlin Golz<br />
Syndrom<br />
III Novartis no<br />
III Roche no<br />
MPG SciBase AB no<br />
III Novartis no<br />
Uvealmelanoma III EORTC no<br />
Neuroectodermal Tumors<br />
Gliomatosis cerebri /<br />
Brain<br />
Multicentric<br />
phase II study<br />
N/A<br />
Prof. Dr. U. Herrlinger,<br />
Bonn
HIT- HGG<br />
HIT-REZ<br />
HIT-HGG - CilMetro<br />
High Grade<br />
Glioblastoma<br />
High Grade Gliomas<br />
and Refracotry /<br />
Relapse PNET and<br />
Medulloblastoma<br />
refractory High Grade<br />
Gliomas<br />
Phase II UK Halle / S. PD Dr. med. Kramm<br />
Phase II UK Bonn Prof. Dr. med. Fleischhack<br />
Phase II UK Halle / S PD Dr. med. Kramm<br />
HIT-REZ.<br />
intrathecal therapy with<br />
liposomal cytarabine<br />
( DepoCyte)<br />
Phase II<br />
Mundipharma Research<br />
UK Bonn<br />
Prof. Dr. med. Fleischhack<br />
DIRECTOR – Dose intensified rechallenge with<br />
temozolomide, one week on one week off versus three<br />
weeks on one week off in patients with progressive or<br />
recurrent glioblastoma<br />
Glioblastoma / Brain<br />
Multicentric<br />
phase II study<br />
Schering-Plough<br />
Prof. Dr. M. Weller, Zurich<br />
NOA-08 randomized phase III trial of 1 week on/1 week<br />
off temozolomide versus involved-field radiotherapy in<br />
elderly (older than age 65) patients with newly diagnosed<br />
anaplastic astrocytoma or glioblastoma (Methusalem)<br />
Glioblastoma / Brain<br />
Multicentric<br />
phase III study<br />
Essex Pharma / Schering-<br />
Plough<br />
Prof. Dr. W. Wick,<br />
Heidelberg<br />
AWB P04739 study on concomitant and adjuvant<br />
temozolomide and radiotherapy for the treatment of<br />
glioblastoma<br />
Glioblastoma / Brain<br />
Multicentric<br />
post marketing<br />
survaillance<br />
study<br />
N/A<br />
Prof. Dr. W. Stummer,<br />
Heidelberg<br />
Whole Brain radiotherapy (WBRT) vs.WBRT and<br />
integrated boost using helical tomotherapy for patients<br />
with multiple brain metastases – a multicentre<br />
randomized phase II trial<br />
Brain metastases<br />
Randomized<br />
Phase II<br />
Stuschke<br />
Other<br />
Ariad AP23573-07-302 STS II ARIAD no<br />
EORTC 62931 STS III EORTC no
EORTC 62961 STS III EORTC no<br />
EORTC 62072 STS III EORTC no<br />
Sabine STS Surveillance Merck & Co no<br />
AP23573-07-302 A pivotal trial to determine the efficacy<br />
and safety of AP23573 when administered as<br />
maintenance therapiy to patients with metastastic softtissue<br />
or bone sarcomas<br />
EORTC 62061: Randomized phase II study of brostallicin<br />
(PNU-166196A) versus doxorubicin as first line<br />
chemotherapy in patients with advanced or metastatic<br />
soft tissue sarcoma<br />
EORTC 62072: A randomized double blind phase IIIof<br />
Pazopanib versus placebo in patients with soft tissue<br />
sarcoma whose disease has progressed during or<br />
following prior therapy<br />
STS II ARIAD no<br />
STS III EORTC no<br />
STS III EORTC no<br />
CARIN<br />
WX/60-006<br />
CRAD001W2301 (Bolero-3)<br />
L00070 IN 308 B0 (Vinflunin vs Alkylanz)<br />
Treatment reality and survival (tumor registry)<br />
Metastatic breast<br />
cancer<br />
Metastatic breast<br />
cancer<br />
Metastatic breast<br />
cancer<br />
Metastatic breast<br />
cancer<br />
Metastatic breast<br />
cancer<br />
II, randomis. Iomedico no<br />
III Wilex AG no<br />
III Novartis no<br />
III Pierre Fabre no<br />
N/A IOmedico no<br />
Phase I study of imatinib and LBH589 in imatinib- and<br />
sunitinib-refractory gastrointestinal stromal tumors<br />
GIST I Universitätsklinikum<br />
<strong>Essen</strong><br />
PD Dr. Sebastian Bauer<br />
(IIT)<br />
An open-label, multi-center study to evaluate the efficacy<br />
of nilotinib in adult patients with gastrointestinal stromal<br />
tumors resistant to imatinib and Sunitinib<br />
(CAMN107DDE05)<br />
GIST II Novartis no
CAMN107G2301 GIST III Novartis no<br />
S.A.K.K.57-07 GIST II S.A.K.K. no<br />
CAMN107G2301 A randomized, open-label, multi-center<br />
phase III study to evaluate the efficacy and safety of<br />
nilotinib versus imatinib in adult patients with<br />
unresectable or metastatic gastrointestinal stromal tumor<br />
GIST)<br />
GIST III Novartis no<br />
CAMN107DDE05 GIST III Novartis no<br />
AMN107A2201: A randomized, open-label, multi-center<br />
study to evaluate the efficacy of nilotinib versus best<br />
supportive care with or without a tyrosine kinase inhibitor<br />
(investigator’s choice) in adult patients with<br />
gastrointestinal stromal tumors resistant to both imatinib<br />
and sunitinib<br />
CRAD001C2454 Everolimus Multicenter, single-arm,<br />
two-stage phase II trial of RAD001 (everolismus) with<br />
Glivec in Glivec-resistant patients with progressive GIST<br />
CAM107A2201 Nilotinib A randomized, open-label,<br />
multi-center study to evaluate the efficacy of nilotinib<br />
versus best supportive care with or without a tyrosine<br />
kinase inhibitor in adult patients with gastrointestinal<br />
stromal tumors resistant to both imatinib and sunitinib.<br />
A Phase 3, Randomized, Double-Blind, Placebo-<br />
Controlled, Multi-Center Study Evaluating the Efficacy<br />
and Safety of IPI-504 in Patients with Metastatic and/or<br />
Unresectable Gastrointestinal Stromal Tumors Following<br />
Failure of at Least Imatinib and Sunitinib<br />
Merck Expand EMR 20048-052<br />
Ganymed GM-IMAB-01<br />
GIST III Novartis no<br />
GIST II Novartis no<br />
GIST III Novartis no<br />
GIST III Infinity no<br />
Advanced Esophago-<br />
Gastric Cancer<br />
Advanced<br />
gastroesophageal<br />
cancer<br />
III Merck no<br />
I Ganymed no<br />
GC-CIF-2005 met. gastric cancer II AIO no
Sunitinib Sutent SU11248 Gastric cancer II AIO no<br />
GC-DOR-2004<br />
Adenocarcinoma,<br />
gastroesophageal<br />
transitional<br />
I / II AIO no<br />
EORTC 62061 Brostallicin WTS III EORTC no<br />
Multizentrische Phase II-Studie mit<br />
Pemetrexed bei Patienten mit vorbehandelten<br />
metastasierten Weichteilsarkomen<br />
Randomisierte Phase II-Studie von Trofosfamid versus<br />
Adriamycin bei älteren Patienten mit unvorbehandeltem<br />
metastasiertem Weichteilsarkom<br />
WTS II AIO Prof. Jörg Hartmann, Kiel<br />
WTS II AIO Prof. Jörg Hartmann, Kiel<br />
GEN205<br />
Merck ADVANTAGE<br />
Lilly H3E-MC-S123 Phase 2 study of pemetrexed in<br />
combination with cisplatin and cetuximab in recurrent of<br />
metastatic squamous cell carcinoma of the head and<br />
neck<br />
Head and Neck<br />
carcinoma<br />
Head and Neck<br />
carcinoma<br />
Head and Neck<br />
carcinoma<br />
II Genmab no<br />
II Merck no<br />
II Lilly no<br />
Lilly H3E-MC-JMHR<br />
Head and Neck<br />
Carcinoma<br />
III Lilly no<br />
CeFCiD<br />
Head and Neck<br />
carcinoma<br />
II<br />
Charite Universitätsklinik<br />
Berlin<br />
Prof. Keilholz Charite Berlin<br />
(IIT)<br />
DCC<br />
Head and Neck<br />
carcinoma<br />
II<br />
Charité Universitätsklinik<br />
Berlin<br />
Dr. Dr. Raguse Charite<br />
Berlin (IIT)<br />
CETAX<br />
Head and Neck<br />
carcinoma<br />
II<br />
Charité Universitätsklinik<br />
Berlin<br />
Prof. Keilholz Charite Berlin<br />
(IIT)<br />
Novartis RECORD2 Renal Cell Carcinoma II Novartis no<br />
GSK COMPARZ Renal Cell Carcinoma III GlaxoSmithKline no
CESAR C-II-006 Renal Cell Carcinoma II CESAR no<br />
Pfizer AXIS A4061032 RCC III Pfizer no<br />
Novartis CRAD001L2401 RCC IIIb Novartis no<br />
Pfizer A6181037 Metastatic RCC II Pfizer no<br />
Prospektive, randomisierte, kontrollierte, unizentrische,<br />
offene, Studie zur Thromboembolieprophylaxe mit<br />
Enoxaparin bei nicht-chirurgischen onkologischen<br />
Patienten unter systemischer antineoplastischer<br />
Therapie<br />
Solide Tumore II Universitätsklinikum<br />
<strong>Essen</strong><br />
Prof. Dr. med. M. E.<br />
Scheulen<br />
(IIT)<br />
Astra Zeneca 14-er Solid tumors I Astra Zeneca no<br />
A phase I, open-label, study of the safety, tolerability and<br />
pharmacokinetics of pazopanib in combination with<br />
gemcitabine and gemcitabine plus cisplatin for advanced<br />
solid tumors<br />
solid tumors I GlaxoSmithKline no<br />
A randomized, double-blind, placebo-controlled study<br />
comparing aflibercept versus placebo on the QTc interval<br />
in Cancer patients treated with docetaxel.<br />
solid tumors I Sanofi-Aventis<br />
Deutschland GmbH<br />
no<br />
A Phase I, Open-Label study of the safety, tolerability,<br />
and pharmacokinetics of Angiocal (PRS-050-PEG40) in<br />
patients with solid tumor<br />
Open label, dose escalation trial of oral 4SC-205 in<br />
patients with advanced malignancies: First-in-man study<br />
of a newly developed, oral inhibitor of kinesin-spindle<br />
protein, EG 5 (AEGIS)<br />
An open phase I single dose escalation study of two<br />
dosing schedules of BI811283 administered<br />
intravenously over 24 h continuous infusion in patients<br />
with advanced solid tumor with repente administration in<br />
patients with clinical benefit<br />
A Phase I, open label study evaluating the<br />
Pharmacokinetics of components of S-1 in patients with<br />
varying degrees of renal function (S-1111)<br />
solid tumors I Pieris AG no<br />
solid tumors I 4SC AG no<br />
Solid tumors I/IIa Boehringer no<br />
Solid tumors I Taiho no
A Phase I Dose Escalation study on the tolerability and<br />
activity of TriN 2755 in patients with advanced solid<br />
tumors and sarcomas using two different dosage regimes<br />
Phase II double-blind placebo-controlled trial of CY-503<br />
in patients with chemotherapy-refractory metastatic<br />
colorectal cancer<br />
Solid tumors I TriN no<br />
Colorectal cancer II CYTAVIS no<br />
Bayer Correct BAY73-4506 / 14387 Met. CRC III Bayer Health Care no<br />
Maurice EMR 200020-34 CRC II Merck no<br />
Merck OPUS EMR 62202-047 CRC II Merck no<br />
Schering PTK I CRC III Schering No<br />
Schering PTK II CRC III Schering No<br />
Petacc8 Colon-Ca III Pierre Fabre no<br />
Astra Zeneca Horizon-II Met. CRC II Astra Zeneca no<br />
Celim Met. Hep. CRC II AIO no<br />
Amgen 20050181 Panitumumab Met. CRC II Amgen no<br />
Roche Beat Met. CRC n.a. Roche no<br />
Merck MK-0646 Met. CRC I Merck no<br />
Velour Met. CRC III Sanofi Aventis no<br />
Biweekly Cetuximab in combination with FOLFOX-6 as<br />
first-line treatment in metastatic colorectal cancer<br />
patients (CEBIFOX)<br />
Tolerability and efficacy of bevacizumab, capecitabine<br />
and oxaliplatin as first-line therapy in patients with<br />
metastatic colorectal cancer (NERO)<br />
Phase-II-study, radio-chemo-therapy (neoadjuvant) with<br />
Capecitabine and Oxaliplatin and Bevacizumab for<br />
patients with advanced rectum-ca (translational research<br />
project) BEV-XELOX-RT)<br />
Colorectal cancer II Universitätsklinikum<br />
<strong>Essen</strong><br />
Tanja Trarbach,<br />
Dr. med., MSc<br />
(IIT)<br />
Colorectal cancer II Roche Tanja Trarbach,<br />
Dr. med., MSc<br />
rectum cancer II Universitätsklinikum<br />
Schleswig Holstein<br />
Prof. Dr. med. Dunst.<br />
Lübeck<br />
(IIT)
A prospective angiogenic imaging study with DCE-MRI<br />
and DCE-USI in patients with colorectal cancer and liver<br />
metastases receiving sunitinib in addition to 5-FU, folic<br />
acid and irinotecan (FOLFIRI) as 1 st line therapy(C-II-<br />
005)<br />
Colorectal Cancer II CESAR Prof. Dr. med. M. E.<br />
Scheulen<br />
(IIT)<br />
AIO KRK 0204 CRC I7II AIO no<br />
CEBIFOX CRC II Merck, Sanofi Aventis no<br />
AIO KRK 0504
Two-arm, randomized, open-label, phase IIIb study<br />
investigating the safety of 2 hour i.p. infusion of<br />
catumaxomab with and without prednisolone<br />
premedication in patients with malignant ascites due to<br />
epithelial cancer<br />
AMGEN 20050244<br />
malignant ascites due to<br />
epithelial cancer<br />
Bone metastasis,<br />
advanced Cancer<br />
(excluding Breast and<br />
Prostate) or Multiple<br />
Myeloma<br />
IIIb Fresenius Biotech GmbH no<br />
III AMGEN no<br />
Phase II study to evaluate Glivec (Imatinib Mesylate) to<br />
induce protression arrest in aggressive fibromatosis /<br />
desmoids tumors not amenable to surgical resection with<br />
R0 intent or accompanied by unacceptable function loss<br />
Desmoid Tumors II IIT Prof. Peter Hohenberger,<br />
Mannheim<br />
Saladax<br />
Bayer REASON<br />
Valuation of Eligibility of<br />
5-FU-concentration in<br />
clinical routine<br />
Ovarian Cancer/<br />
Prostate Cancer<br />
N/A Saldax Biomedical no<br />
II Bayer no<br />
Bingo Advanced biliary cancer II Institut Gustave Roussy,<br />
France<br />
no<br />
SPOT Oesophageal Cancer I/II Sanofi-Aventis no<br />
Pilot-Study of dose-intensified radiotherapy according to<br />
PET-CT in combination with induction- and simultaneous<br />
chemotherapy (SPOT)<br />
Oesophageal Cancer I/II Universitätsklinikum<br />
<strong>Essen</strong><br />
Prof. Dr. med. Martin<br />
Stuschke (IIT)<br />
HD-PEI +/- NESF<br />
met. „poor prognosis“<br />
germ cell tumor<br />
II Amgen no<br />
Phase I/II study with temsirolimus versus no add-on in<br />
patients with castration resistant prostate cancer (CRPC)<br />
receiving first-line Docetaxel chemotherapy (C-II-007)<br />
Positron-Emissions-Tomographie-gesteuerte Therapie<br />
aggressiver Lymphome (PETAL)<br />
Prostate Cancer I/II CESAR Prof. Dr. med. M. E.<br />
Scheulen<br />
(IIT)<br />
Aggressive Lymphome III German Cancer Aid Prof. Dr. U. Dührsen
Valuation of Eligibility of 5-FU-concentration in clinical<br />
routine (SALADAX)<br />
Observational Study to explore dermatological side<br />
effects of patients receiving an Anti-EGFR therapy<br />
multiple N/A Universitätsklinikum<br />
<strong>Essen</strong><br />
Skin N/A Universitätsklinikum<br />
<strong>Essen</strong><br />
Tanja Trarbach,<br />
Dr. med., MSc<br />
(IIT)<br />
Tanja Trarbach,<br />
Dr. med., MSc (IIT)<br />
Pacet-Cup Unknown Primary II AIO no