Do not prepack list - American Society of Health System Pharmacists
Do not prepack list - American Society of Health System Pharmacists
Do not prepack list - American Society of Health System Pharmacists
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
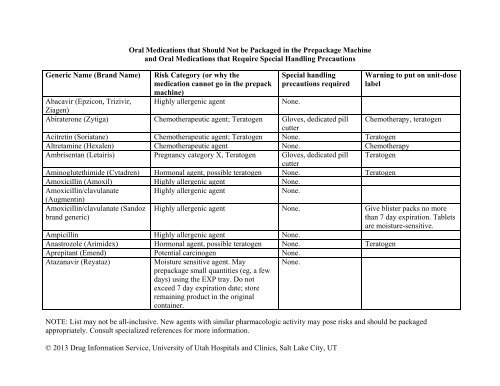
Generic Name (Brand Name)<br />
Oral Medications that Should Not be Packaged in the Prepackage Machine<br />
and Oral Medications that Require Special Handling Precautions<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Special handling<br />
precautions required<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT<br />
Warning to put on unit-dose<br />
label<br />
Abacavir (Epzicon, Trizivir, Highly allergenic agent None.<br />
Ziagen)<br />
Abiraterone (Zytiga) Chemotherapeutic agent; Teratogen Gloves, dedicated pill Chemotherapy, teratogen<br />
cutter<br />
Acitretin (Soriatane) Chemotherapeutic agent; Teratogen None. Teratogen<br />
Altretamine (Hexalen) Chemotherapeutic agent None. Chemotherapy<br />
Ambrisentan (Letairis) Pregnancy category X, Teratogen Gloves, dedicated pill Teratogen<br />
cutter<br />
Aminoglutethimide (Cytadren) Hormonal agent, possible teratogen None. Teratogen<br />
Amoxicillin (Amoxil) Highly allergenic agent None.<br />
Amoxicillin/clavulanate Highly allergenic agent None.<br />
(Augmentin)<br />
Amoxicillin/clavulanate (Sandoz<br />
brand generic)<br />
Highly allergenic agent None. Give b<strong>list</strong>er packs no more<br />
than 7 day expiration. Tablets<br />
are moisture-sensitive.<br />
Ampicillin Highly allergenic agent None.<br />
Anastrozole (Arimidex) Hormonal agent, possible teratogen None. Teratogen<br />
Aprepitant (Emend) Potential carcinogen None.<br />
Atazanavir (Reyataz)<br />
Moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities (eg, a few<br />
days) using the EXP tray. <strong>Do</strong> <strong>not</strong><br />
exceed 7 day expiration date; store<br />
remaining product in the original<br />
container.<br />
None.
Generic Name (Brand Name)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Special handling<br />
precautions required<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT<br />
Warning to put on unit-dose<br />
label<br />
Axitinib (Inlyta) Chemotherapeutic agent None. Chemotherapy<br />
Azathioprine (Imuran) Teratogen; Immunosuppressant agent Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
Teratogen,<br />
Bexarotene (Targretin) Chemotherapeutic agent; Teratogen None. Chemotherapy, teratogen<br />
Bicalutamide (Casodex) Hormonal agent, possible teratogen None.<br />
Bosentan (Tracleer) Pregnancy category X, Teratogen Gloves, dedicated pill<br />
cutter<br />
Teratogen<br />
Bosutinib (Bosulif) Chemotherapeutic agent None. Chemotherapy<br />
Busulfan (Myleran) Chemotherapeutic agent None. Chemotherapy<br />
Cabozantinib (Cometriq) Chemotherapeutic agent None. Chemotherapy<br />
Capecitabine (Xeloda) Chemotherapeutic agent None. Chemotherapy<br />
Cabergoline (<strong>Do</strong>stinex) Hormonal agent, possible teratogen None.<br />
Carbamazepine (eg, Epitol,<br />
Equetro, Tegretol, Tegretol<br />
XR)<br />
Carbidopa/levodopa orallydisintegrating<br />
tablets (Parcopa)<br />
Possible teratogen None. Teratogen<br />
Highly-moisture sensitive agent<br />
Cefaclor (Ceclor) Highly allergenic agent None.<br />
Cefadroxil (Duricef) Highly allergenic agent None.<br />
Cefdinir (Omnicef) Highly allergenic agent None.<br />
Cefditorin (Spectracef) Highly allergenic agent None.<br />
Cefprozil (Cefzil) Highly allergenic agent None.<br />
Cefuroxime (Ceftin) Highly allergenic agent None.<br />
Cephalexin (Keflex) Highly allergenic agent None.<br />
Dry hands before<br />
handling medication.<br />
Open product package<br />
just before use.
Generic Name (Brand Name)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Cephradine (Velosef) Highly allergenic agent None.<br />
Cefpodoxime (Vantin) Highly allergenic agent None.<br />
Ceftibuten (Cedax) Highly allergenic agent None.<br />
Special handling<br />
precautions required<br />
Chloral Hydrate (Som<strong>not</strong>e) Requires black box disposal per<br />
RCRA.<br />
None.<br />
Chlorambucil (Leukeran) Chemotherapeutic agent, Requires None.<br />
black box disposal per RCRA.<br />
Clonazepam (eg, Klonopin) Possible teratogen None. Teratogen<br />
Cloxacillin (Cloxapen) Highly allergenic agent None.<br />
Colchicine Carcinogen, or potential carcinogen None. Carcinogen<br />
Cotrimoxazole (Bactrim,<br />
Bactrim DS, Cotrim, Cotrim DS,<br />
Septra, Septra DS)<br />
Highly allergenic agent None.<br />
Warning to put on unit-dose<br />
label<br />
Black box disposal / RCRA<br />
Chemotherapy<br />
Black box disposal / RCRA<br />
Crizotinib (Xalkori) Chemotherapeutic agent None. Chemotherapy<br />
Cyclophosphamide (Cytoxan) Chemotherapeutic agent, Requires<br />
black box disposal per RCRA.<br />
None.<br />
Chemotherapy<br />
Black box disposal / RCRA<br />
Cyclosporine (Sandimmune,<br />
Neoral, Gengraf)<br />
Teratogen; Immunosuppressant agent Place unit dose oral<br />
suspension in a separate<br />
Teratogen<br />
plastic bag for delivery<br />
Danazol Possible teratogen None. Teratogen<br />
Dasatinib (Sprycel) Chemotherapeutic agent None. Chemotherapy<br />
Dicloxacillin (Dynapen) Highly allergenic agent None.<br />
Divalproex sodium (eg, Teratogen None. Teratogen<br />
Depakote, Depakote ER)<br />
Dronedarone (Multaq) Teratogen, or potential teratogen None. Teratogen<br />
Dutasteride (Avodart) Teratogen None. Teratogen<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT
Generic Name (Brand Name)<br />
Efavirenz / Emtricitabine /<br />
Ten<strong>of</strong>ovir (Atripla)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Highly-moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities (up to 7<br />
tablets) in EXP tray. <strong>Do</strong> <strong>not</strong> exceed 1<br />
month expiration date; store remaining<br />
product in the original container.<br />
Special handling<br />
precautions required<br />
Dry hands before<br />
handling medication<br />
Efavirenz Possible teratogen None Teratogen<br />
Emtricitabine/Ten<strong>of</strong>ovir<br />
Dry hands before<br />
(Truvada)<br />
handling medication.<br />
Highly-moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities (up to 7<br />
tablets) in EXP tray. <strong>Do</strong> <strong>not</strong> exceed 1<br />
month expiration date; store remaining<br />
product in the original container.<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT<br />
Warning to put on unit-dose<br />
label<br />
Entecavir (Baraclude) Possible teratogen None Teratogen<br />
Enzalutamide (Xtandi) Hormonal agent, possible teratogen None.<br />
Erlotinib (Tarceva) Chemotherapeutic agent None. Chemotherapy<br />
Estramustine (Emcyt) Chemotherapeutic agent None. Chemotherapy<br />
Etoposide (VePesid) Chemotherapeutic agent None. Chemotherapy<br />
Everolimus (Afinitor, Zortress) Chemotherapeutic agent;<br />
Immunosuppressant agent<br />
None.<br />
Exemestane (Aromasin) Hormonal agent, possible teratogen None. Teratogen<br />
Finasteride (Propecia, Proscar) Teratogen None. Teratogen<br />
Fish oil capsules Capsules explode in <strong>prepack</strong> machine None.<br />
Flucytosine (Ancobon) Teratogen or potential teratogen Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery.<br />
Chemotherapy<br />
Teratogen<br />
Fludarabine (Oforta) Chemotherapeutic agent None. Chemotherapy<br />
Fluoxymesterone (Halotestin) Hormonal agent, possible teratogen None.<br />
Flutamide (Eulexin) Hormonal agent, possible teratogen None.
Generic Name (Brand Name)<br />
Ganciclovir (Cytovene)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Teratogen; Mutagen or potential<br />
mutagen; Carcinogen or potential<br />
carcinogen; Immunosuppressant agent<br />
Special handling<br />
precautions required<br />
Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
Warning to put on unit-dose<br />
label<br />
Teratogen<br />
Gefitinib (Iressa) Chemotherapeutic agent None. Chemotherapy<br />
Hydroxyurea (eg, Hydrea, Chemotherapeutic agent None. Chemotherapy<br />
Droxia)<br />
Imatinib (Gleevec) Chemotherapeutic agent None. Chemotherapy<br />
Isotretinoin (Accutane) Teratogen None. <strong>Do</strong> <strong>not</strong> repackage, Teratogen<br />
keep in original package.<br />
Lamivudine / Zidovudine Mutagen or potential mutagen; None.<br />
Mutagen, Carcinogen<br />
(Combivir)<br />
Carcinogen or potential carcinogen<br />
Lapatinib (Tykerb) Chemotherapeutic agent None. Chemotherapy<br />
Leflunomide (Arava) Teratogen; Immunosuppressant agent None. Teratogen<br />
Lenalidomide (Revlimid) Chemotherapeutic agent; Teratogen None. <strong>Do</strong> <strong>not</strong> repackage, Chemotherapy, teratogen<br />
keep in original package.<br />
Letrozole (Femara) Hormonal agent, possible teratogen None. Teratogen<br />
Levodopa/carbidopa orallydisintegrating<br />
tablets (Parcopa)<br />
Highly-moisture sensitive agent<br />
Dry hands before<br />
handling medication.<br />
Open product package<br />
just before use.<br />
Lomustine (CeeNu) Chemotherapeutic agent None. Chemotherapy<br />
Lopinavir/ritonavir (Kaletra)<br />
Highly-moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities in EXP<br />
tray. <strong>Do</strong> <strong>not</strong> exceed 2 week expiration<br />
date; store remaining product in the<br />
original container.<br />
None.<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT
Generic Name (Brand Name)<br />
Magnesium chloride entericcoated<br />
tablets (Slow-Mag)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Highly-moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities (eg, a few<br />
days) using the EXP tray. <strong>Do</strong> <strong>not</strong><br />
exceed 7 day expiration date; store<br />
remaining product in the original<br />
container.<br />
Special handling<br />
precautions required<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
None.<br />
Medroxyprogesterone (Provera) Hormonal agent, possible teratogen None. Teratogen<br />
Megestrol (Megace) Hormonal agent, possible teratogen Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
Teratogen<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT<br />
Warning to put on unit-dose<br />
label<br />
Melphalan (Alkeran)<br />
Chemotherapeutic agent, Requires<br />
black box disposal per RCRA.<br />
None.<br />
Mercaptopurine (Purinethol) Chemotherapeutic agent None. Chemotherapy<br />
Methotrexate (Folex) Chemotherapeutic agent None. Chemotherapy<br />
Methoxsalen (8-MOP,<br />
Teratogen None. Teratogen<br />
Oxsoralen, Oxsoralen Ultra)<br />
Methyltestosterone (Android) Hormonal agent, possible teratogen None. Teratogen<br />
Mifepristone (Korlyn, Mifeprex) Abortifacient None. Abortifacient<br />
Misoprostol (Cytotec) Teratogen None. Teratogen<br />
Mitotane (Lysodren) Chemotherapeutic agent None. Chemotherapy<br />
Mycophenolate m<strong>of</strong>etil<br />
(Cellcept)<br />
Mycophenolate sodium<br />
(Myfortic)<br />
Mutagen or potential mutagen;<br />
Teratogen or potential teratogen;<br />
Immunosuppressant agent<br />
Mutagen or potential mutagen;<br />
Teratogen or potential teratogen;<br />
Immunosuppressant agent<br />
Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
None.<br />
Chemotherapy<br />
Black box disposal / RCRA<br />
Mutagen<br />
Teratogen<br />
Mutagen<br />
Teratogen<br />
Nilotinib (Tasigna) Chemotherapeutic agent None. Chemotherapy
Generic Name (Brand Name)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Special handling<br />
precautions required<br />
Nilutamide (Nilandron) Hormonal agent, possible teratogen None. Teratogen<br />
Omega-3 Fatty Acids Capsules explode in <strong>prepack</strong> machine None.<br />
Oxacillin (Bactocill) Highly allergenic agent None.<br />
Oxandrolone (Oxandrin) Teratogen; Hormonal agent, possible None.<br />
Teratogen<br />
teratogen<br />
Oxcarbazepine (eg, Trileptal) Possible teratogen None. Teratogen<br />
Pancreatic enzyme replacement<br />
products (Creon, Pancreaze,<br />
Pertzye, Ultresa, Viokace,<br />
Zenpep)<br />
Highly-moisture sensitive agent. Send<br />
as 100-count bottle. Store in original<br />
bottle with dessicant (if included).<br />
None.<br />
Warning to put on unit-dose<br />
label<br />
Paroxetine (Paxil) Teratogen or potential teratogen None. Teratogen<br />
Pazopanib (Votrient) Chemotherapeutic agent None. Chemotherapy<br />
Penicillin (Beepen VK, Betapen<br />
VK, Bopen VK, Pen-Vee K,<br />
Pfizerpen VK, Suspen, V-Cillin<br />
K, Veetids)<br />
Highly allergenic agent None.<br />
Phenoxybenzamine (eg,<br />
Dibenzyline)<br />
Mutagen or potential mutagen;<br />
Carcinogen or potential carcinogen<br />
None.<br />
Phentermine (Adipex-P, Requires black box disposal per None.<br />
Phentercot, Suprenza)<br />
RCRA.<br />
Pomalidomide (Pomalyst) Chemotherapeutic agent None. Chemotherapy<br />
Ponatinib (Iclusig) Chemotherapeutic agent None. Chemotherapy<br />
Prasugrel (Effient) Highly moisture sensitive <strong>Do</strong> <strong>not</strong> repackage. Keep<br />
in original unit-dose<br />
packaging.<br />
Procarbazine (Matulane) Chemotherapeutic agent None. Chemotherapy<br />
Mutagen, Carcinogen<br />
Black box disposal / RCRA<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT
Generic Name (Brand Name)<br />
Pyridostigmine (eg, Mestinon,<br />
Mestinon Timespan)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Highly-moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities (eg, a few<br />
days) using the EXP tray. <strong>Do</strong> <strong>not</strong><br />
exceed 7 day expiration date; store<br />
remaining product in the original<br />
container.<br />
Special handling<br />
precautions required<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
None.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT<br />
Warning to put on unit-dose<br />
label<br />
Raloxifene (Evista) Hormonal agent, possible teratogen None. Teratogen<br />
Rasagiline (Azilect)<br />
Mutagen or potential mutagen; None.<br />
Mutagen, Carcinogen<br />
Carcinogen or potential carcinogen<br />
Regorafenib (Stivarga) Chemotherapeutic agent None. Chemotherapy<br />
Ribavirin (Rebetol) Teratogen None. Teratogen<br />
Risperidone (Risperdal) Carcinogen or potential carcinogen;<br />
Teratogen or potential teratogen<br />
Place unit dose oral<br />
suspension in a separate<br />
Carcinogen<br />
Teratogen<br />
plastic bag for delivery.<br />
Ritonavir (Norvir)<br />
Capsules are sensitive to heat. Capsules Store in refrigerator.<br />
may explode in <strong>prepack</strong> machine<br />
Ruxolitinib (Jakafi) Chemotherapeutic agent None. Chemotherapy<br />
Selenium<br />
Requires black box disposal per None.<br />
Black box disposal / RCRA<br />
RCRA.<br />
Sirolimus (Rapamune) Immunosuppressant agent Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
Sorafenib (Nexavar) Chemotherapeutic agent None. Chemotherapy<br />
Sulfadiazine Highly allergenic agent None.<br />
Sulfapyridine Highly allergenic agent None.<br />
Sulfasalazine Highly allergenic agent None<br />
Sulfisoxazole (Gantrisin) Highly allergenic agent None.
Generic Name (Brand Name)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Special handling<br />
precautions required<br />
Warning to put on unit-dose<br />
label<br />
Sunitinib (Sutent) Chemotherapeutic agent None. Chemotherapy<br />
Tacrolimus (eg, Hecoria, Carcinogen or potential carcinogen; Place unit dose oral Carcinogen<br />
Prograf)<br />
Immunosuppressant agent<br />
suspension in a separate<br />
Tamoxifen (Nolvadex,<br />
Soltamox)<br />
Hormonal agent, possible teratogen<br />
plastic bag for delivery.<br />
Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
Teratogen<br />
Temozolomide (Temodar) Chemotherapeutic agent None. Chemotherapy<br />
Ten<strong>of</strong>ovir (Viread)<br />
Highly-moisture sensitive agent. May<br />
<strong>prepack</strong>age small quantities (up to 7<br />
tablets) in EXP tray. <strong>Do</strong> <strong>not</strong> exceed 1<br />
month expiration date; store remaining<br />
product in the original container.<br />
Dry hands before<br />
handling medication.<br />
Testolactone (Teslac) Hormonal agent, possible teratogen None. Teratogen<br />
Tetracycline (eg, Sumycin) Mutagen or potential mutagen; Possible None.<br />
Mutagen<br />
teratogen<br />
Thalidomide (Thalomid) Chemotherapeutic agent; Teratogen None. <strong>Do</strong> <strong>not</strong> repackage, Chemotherapy, teratogen<br />
keep in original package.<br />
Thioguanine (Thioguanine Chemotherapeutic agent None. Chemotherapy<br />
Tabloid)<br />
Topotecan (Hycamtin) Chemotherapeutic agent None. Chemotherapy<br />
Toremifene (Fareston) Hormonal agent, possible teratogen None. Teratogen<br />
Tretinoin (Vesanoid) Chemotherapeutic agent; Teratogen None. Chemotherapy, teratogen<br />
Trientine (Syprine) Potential teratogen, irritant None Possible teratogen, do <strong>not</strong><br />
open capsule<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT
Generic Name (Brand Name)<br />
Valganciclovir (Valcyte)<br />
Risk Category (or why the<br />
medication can<strong>not</strong> go in the <strong>prepack</strong><br />
machine)<br />
Teratogen; Mutagen or potential<br />
mutagen ; Immunosuppressant agent<br />
Special handling<br />
precautions required<br />
Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT<br />
Warning to put on unit-dose<br />
label<br />
Teratogen<br />
Valproic acid (eg, Depacon, Teratogen None. Teratogen<br />
Depakene, Stavzor)<br />
Vandetanib (Caprelsa) Chemotherapeutic agent None. Chemotherapy<br />
Vemurafenib (Zelboraf) Chemotherapeutic agent None. Chemotherapy<br />
Vigabatrin (Sabril) Teratogen None. Teratogen<br />
Vismodegib (Erivedge) Chemotherapeutic agent None. Chemotherapy<br />
Vorinostat (Zolinza)<br />
Warfarin sodium (Coumadin,<br />
Jantoven)<br />
Zidovudine (Retrovir)<br />
Chemotherapeutic agent; Mutagen or<br />
potential mutagen<br />
Requires black box disposal per<br />
RCRA.<br />
Mutagen or potential mutagen,<br />
Carcinogen or potential carcinogen<br />
None.<br />
None.<br />
Ziprasidone (Geodon) Possible teratogen None.<br />
Zonisamide (Zonegran) Mutagen or potential mutagen; None.<br />
Teratogen or potential teratogen<br />
Agents Used to Clean the Prepackage Machine<br />
Refer to the user manual for general cleaning instructions.<br />
Place unit dose oral<br />
suspension in a separate<br />
plastic bag for delivery<br />
Chemotherapy, mutagen<br />
Black box disposal / RCRA<br />
Mutagen, Carcinogen<br />
Mutagen<br />
Teratogen<br />
In the event that one <strong>of</strong> the drugs <strong>list</strong>ed above is accidentally placed in the <strong>prepack</strong>age machine, either <strong>of</strong> the following agents may be<br />
used to clean the machine depending on the type <strong>of</strong> drug that was accidently placed in the machine.<br />
1. Clorox – any chemotherapeutic drug
2. Alcohol – any other drug<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT
References<br />
University <strong>Health</strong> Care Policy Manual: Safe Handling <strong>of</strong> Hazardous Drugs.<br />
http://intranet.uuhsc.utah.edu/standards/org_wide/med_mgmt/Safe-Handling-<strong>of</strong>-Hazardous-Drugs.pdf. Updated November 2012.<br />
Accessed December 10, 2012.<br />
ASHP Technical assistance bulletin on handling cytoxic and hazardous drugs. Am J Hosp Pharm. 1990;47(5):1033-1049.<br />
Department <strong>of</strong> <strong>Health</strong> and Human Services. Centers for Disease Control and Prevention. National Institute for Occupational Safety<br />
and <strong>Health</strong>. Preventing occupational exposure to antineoplastic and other hazardous drugs in health care settings. NIOSH Publication<br />
No. 2004-165. http://www.cdc.gov/niosh/docs/2004-165/2004-165d.html. Published 2004. Accessed May 4, 2006.<br />
Department <strong>of</strong> <strong>Health</strong> and Human Services. Centers for Disease Control and Prevention. National Institute for Occupational Safety<br />
and <strong>Health</strong>. NIOSH <strong>list</strong> <strong>of</strong> antineoplastic and other hazardous drugs in healthcare settings 2010. NIOSH Publication No. 2010-167.<br />
http://www.cdc.gov/niosh/docs/2010-167/pdfs/2010-167.pdf. Published September 2010. Accessed September 15, 2010.<br />
Department <strong>of</strong> <strong>Health</strong> and Human Services. Centers for Disease Control and Prevention. National Institute for Occupational Safety<br />
and <strong>Health</strong>. NIOSH <strong>list</strong> <strong>of</strong> antineoplastic and other hazardous drugs in healthcare settings 2012. NIOSH Publication No. 2012-150.<br />
http://www.cdc.gov/niosh/docs/2012-150/pdfs/2012-150.pdf. Published June 2012. Accessed July 20, 2012.<br />
McEvoy GK, Litvak K, Welsh OH, et al, eds. AHFS 2013 Drug Information. Bethesda, MD: <strong>American</strong> <strong>Society</strong> <strong>of</strong> <strong>Health</strong>-<strong>System</strong><br />
<strong>Pharmacists</strong>; 2013.<br />
National Institute for Occupational Safety and <strong>Health</strong>. Process for updating the <strong>list</strong> <strong>of</strong> hazardous drugs (Appendix A) for the NIOSH<br />
Alert on hazardous drugs. NIOSH <strong>Do</strong>cket #105. http://www.cdc.gov/niosh/review/public/105/default.html. Published 2004. Accessed<br />
July 9, 2007.<br />
United States Department <strong>of</strong> Labor. Occupational Safety & <strong>Health</strong> Administration. Occupational Safety & <strong>Health</strong> Administration.<br />
OSHA Technical Manual. Section VI. Chapter 2. Controlling occupational exposure to hazardous drugs. Available at:<br />
http://www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2_html. Published 1995. Accessed October 9, 2007.<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT
Wickersham RM, Novak KK, eds. Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer <strong>Health</strong>, Inc.; 2013.<br />
NOTE: List may <strong>not</strong> be all-inclusive. New agents with similar pharmacologic activity may pose risks and should be packaged<br />
appropriately. Consult specialized references for more information.<br />
© 2013 Drug Information Service, University <strong>of</strong> Utah Hospitals and Clinics, Salt Lake City, UT