Supercritical impregnation of polymers - ZyXEL NSA210
Supercritical impregnation of polymers - ZyXEL NSA210
Supercritical impregnation of polymers - ZyXEL NSA210
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
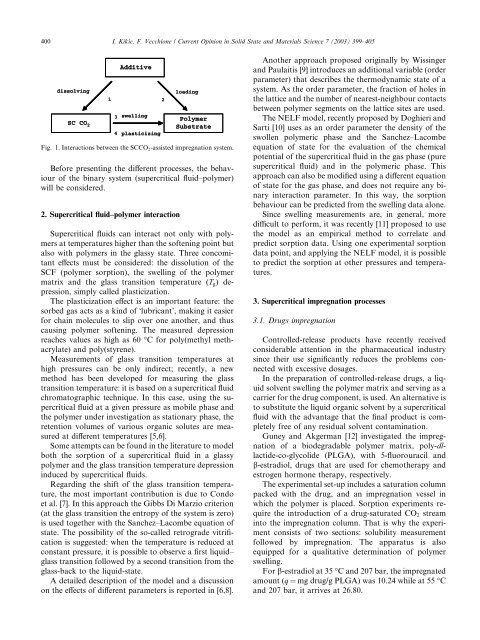
400 I. Kikic, F. Vecchione / Current Opinion in Solid State and Materials Science 7 (2003) 399–405<br />
dissolving<br />
SC CO 2<br />
1<br />
Additive<br />
3<br />
swelling<br />
4<br />
plasticizing<br />
Before presenting the different processes, the behaviour<br />
<strong>of</strong> the binary system (supercritical fluid–polymer)<br />
will be considered.<br />
2. <strong>Supercritical</strong> fluid–polymer interaction<br />
loading<br />
<strong>Supercritical</strong> fluids can interact not only with <strong>polymers</strong><br />
at temperatures higher than the s<strong>of</strong>tening point but<br />
also with <strong>polymers</strong> in the glassy state. Three concomitant<br />
effects must be considered: the dissolution <strong>of</strong> the<br />
SCF (polymer sorption), the swelling <strong>of</strong> the polymer<br />
matrix and the glass transition temperature (T g ) depression,<br />
simply called plasticization.<br />
The plasticization effect is an important feature: the<br />
sorbed gas acts as a kind <strong>of</strong> Ôlubricant’, making it easier<br />
for chain molecules to slip over one another, and thus<br />
causing polymer s<strong>of</strong>tening. The measured depression<br />
reaches values as high as 60 °C for poly(methyl methacrylate)<br />
and poly(styrene).<br />
Measurements <strong>of</strong> glass transition temperatures at<br />
high pressures can be only indirect; recently, a new<br />
method has been developed for measuring the glass<br />
transition temperature: it is based on a supercritical fluid<br />
chromatographic technique. In this case, using the supercritical<br />
fluid at a given pressure as mobile phase and<br />
the polymer under investigation as stationary phase, the<br />
retention volumes <strong>of</strong> various organic solutes are measured<br />
at different temperatures [5,6].<br />
Some attempts can be found in the literature to model<br />
both the sorption <strong>of</strong> a supercritical fluid in a glassy<br />
polymer and the glass transition temperature depression<br />
induced by supercritical fluids.<br />
Regarding the shift <strong>of</strong> the glass transition temperature,<br />
the most important contribution is due to Condo<br />
et al. [7]. In this approach the Gibbs Di Marzio criterion<br />
(at the glass transition the entropy <strong>of</strong> the system is zero)<br />
is used together with the Sanchez–Lacombe equation <strong>of</strong><br />
state. The possibility <strong>of</strong> the so-called retrograde vitrification<br />
is suggested: when the temperature is reduced at<br />
constant pressure, it is possible to observe a first liquid–<br />
glass transition followed by a second transition from the<br />
glass-back to the liquid-state.<br />
A detailed description <strong>of</strong> the model and a discussion<br />
on the effects <strong>of</strong> different parameters is reported in [6,8].<br />
2<br />
Polymer<br />
Substrate<br />
Fig. 1. Interactions between the SCCO 2 -assisted <strong>impregnation</strong> system.<br />
Another approach proposed originally by Wissinger<br />
and Paulaitis [9] introduces an additional variable (order<br />
parameter) that describes the thermodynamic state <strong>of</strong> a<br />
system. As the order parameter, the fraction <strong>of</strong> holes in<br />
the lattice and the number <strong>of</strong> nearest-neighbour contacts<br />
between polymer segments on the lattice sites are used.<br />
The NELF model, recently proposed by Doghieri and<br />
Sarti [10] uses as an order parameter the density <strong>of</strong> the<br />
swollen polymeric phase and the Sanchez–Lacombe<br />
equation <strong>of</strong> state for the evaluation <strong>of</strong> the chemical<br />
potential <strong>of</strong> the supercritical fluid in the gas phase (pure<br />
supercritical fluid) and in the polymeric phase. This<br />
approach can also be modified using a different equation<br />
<strong>of</strong> state for the gas phase, and does not require any binary<br />
interaction parameter. In this way, the sorption<br />
behaviour can be predicted from the swelling data alone.<br />
Since swelling measurements are, in general, more<br />
difficult to perform, it was recently [11] proposed to use<br />
the model as an empirical method to correlate and<br />
predict sorption data. Using one experimental sorption<br />
data point, and applying the NELF model, it is possible<br />
to predict the sorption at other pressures and temperatures.<br />
3. <strong>Supercritical</strong> <strong>impregnation</strong> processes<br />
3.1. Drugs <strong>impregnation</strong><br />
Controlled-release products have recently received<br />
considerable attention in the pharmaceutical industry<br />
since their use significantly reduces the problems connected<br />
with excessive dosages.<br />
In the preparation <strong>of</strong> controlled-release drugs, a liquid<br />
solvent swelling the polymer matrix and serving as a<br />
carrier for the drug component, is used. An alternative is<br />
to substitute the liquid organic solvent by a supercritical<br />
fluid with the advantage that the final product is completely<br />
free <strong>of</strong> any residual solvent contamination.<br />
Guney and Akgerman [12] investigated the <strong>impregnation</strong><br />
<strong>of</strong> a biodegradable polymer matrix, poly-dllactide-co-glycolide<br />
(PLGA), with 5-fluorouracil and<br />
b-estradiol, drugs that are used for chemotherapy and<br />
estrogen hormone therapy, respectively.<br />
The experimental set-up includes a saturation column<br />
packed with the drug, and an <strong>impregnation</strong> vessel in<br />
which the polymer is placed. Sorption experiments require<br />
the introduction <strong>of</strong> a drug-saturated CO 2 stream<br />
into the <strong>impregnation</strong> column. That is why the experiment<br />
consists <strong>of</strong> two sections: solubility measurement<br />
followed by <strong>impregnation</strong>. The apparatus is also<br />
equipped for a qualitative determination <strong>of</strong> polymer<br />
swelling.<br />
For b-estradiol at 35 °C and 207 bar, the impregnated<br />
amount (q ¼ mg drug/g PLGA) was 10.24 while at 55 °C<br />
and 207 bar, it arrives at 26.80.