VDP Specialty Drug List - 2013 05.xlsx - Texas Medicaid/CHIP ...
VDP Specialty Drug List - 2013 05.xlsx - Texas Medicaid/CHIP ...
VDP Specialty Drug List - 2013 05.xlsx - Texas Medicaid/CHIP ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
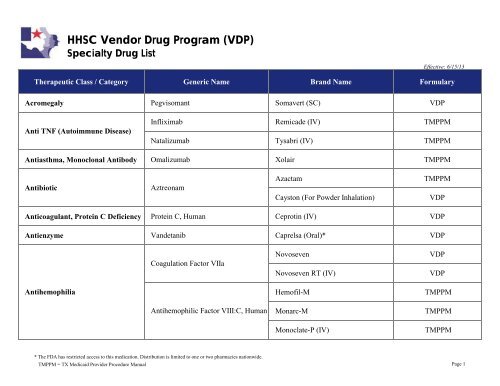
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Acromegaly Pegvisomant Somavert (SC) <strong>VDP</strong><br />
Anti TNF (Autoimmune Disease)<br />
Infliximab Remicade (IV) TMPPM<br />
Natalizumab Tysabri (IV) TMPPM<br />
Antiasthma, Monoclonal Antibody Omalizumab Xolair TMPPM<br />
Antibiotic<br />
Aztreonam<br />
Azactam<br />
Cayston (For Powder Inhalation)<br />
TMPPM<br />
<strong>VDP</strong><br />
Anticoagulant, Protein C Deficiency Protein C, Human Ceprotin (IV) <strong>VDP</strong><br />
Antienzyme Vandetanib Caprelsa (Oral)* <strong>VDP</strong><br />
Coagulation Factor VIIa<br />
Novoseven<br />
Novoseven RT (IV)<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Antihemophilia<br />
Hemofil-M TMPPM<br />
Antihemophilic Factor VIII:C, Human<br />
Monarc-M TMPPM<br />
Monoclate-P (IV) TMPPM<br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 1
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Antihemophilic Factor VIII:C, Human Koate DVI <strong>VDP</strong><br />
Antihemophilic Factor VIII,<br />
Recombinant<br />
Kogenate FS<br />
Recombinate<br />
Helixate FS<br />
Kogenate FS with Bio-Set<br />
Advate<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Antihemophilia<br />
Xyntha<br />
Refacto<br />
Autoplex T<br />
<strong>VDP</strong><br />
TMPPM<br />
<strong>VDP</strong><br />
Anti-Inhibitor Coagulant Complex<br />
Feiba-VH<br />
Feiba NF<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Factor XIII Human Corifact (IV) <strong>VDP</strong><br />
Coagulation Factor IX, Recombinant Benefix (IV) <strong>VDP</strong><br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 2
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Bebulin<br />
<strong>VDP</strong><br />
Factor IX Complex, Human<br />
Profilnine SD<br />
Bebulin VH<br />
<strong>VDP</strong><br />
TMPPM<br />
Proplex T (IV)<br />
TMPPM<br />
Antihemophilia<br />
Factor IX Human, Purified<br />
Mononine<br />
Alphanine SD (IV)<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Humate-P<br />
<strong>VDP</strong><br />
Antihemophilic Factor VIII / Von<br />
Willebrand Factor Complex, Human<br />
Wilate (IV)<br />
<strong>VDP</strong><br />
Alphanate<br />
<strong>VDP</strong><br />
Antipsychotic Olanzapine Zyprexa Relprevv TMPPM<br />
Antiviral Ganciclovir Cytovene (IV) <strong>VDP</strong><br />
Antiviral / For HIV Infection Zidovudine Retrovir (IV) <strong>VDP</strong><br />
Bisphosphonate, Calcium Regulator Pamidronate Disodium Aredia (IV) <strong>VDP</strong><br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 3
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Eculizumab Soliris TMPPM<br />
Aralast<br />
<strong>VDP</strong><br />
Prolastin<br />
<strong>VDP</strong><br />
Blood Modifier Agent For Alpha-1-<br />
Antitrypsin Deficiency<br />
Alpha-1 Proteinase Inhibitor Human<br />
Zemaira<br />
Aralast NP<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Prolastin-C<br />
<strong>VDP</strong><br />
Glassia (IV)<br />
<strong>VDP</strong><br />
Abiraterone Acetate Zytiga (Oral) <strong>VDP</strong><br />
Dactinomycin Cosmegen (IV) <strong>VDP</strong><br />
Cancer<br />
Vemurafenib Zelboraf (Oral) <strong>VDP</strong><br />
Oncovin<br />
TMPPM<br />
Vincristine Sulfate<br />
Vincasar PFS (IV)<br />
TMPPM<br />
Vinblastine Sulfate Velban (IV) TMPPM<br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 4
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Pegaspargase Oncaspar (IM/IV) <strong>VDP</strong><br />
Mitoxantrone HCI Novantrone <strong>VDP</strong><br />
Mitoxantrone HCI Otn Mitoxantrone (IV) <strong>VDP</strong><br />
Vismodegib Erivedge* <strong>VDP</strong><br />
Axitinib Inlyta <strong>VDP</strong><br />
Doxorubicin HCL Doxil TMPPM<br />
Cancer<br />
Cytarabine<br />
Cytosar-U (IV)<br />
Tarabine PFS (Intrathecal)<br />
TMPPM<br />
TMPPM<br />
Bevacizumab Avastin (IV) TMPPM<br />
Decitabine Dacogen (IV) TMPPM<br />
Zoledronic Acid Zometa (IV) TMPPM<br />
Azacitidine Vidaza (SQ) (IV) TMPPM<br />
Docetaxel Taxotere (IV) TMPPM<br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 5
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Fulvestrant Faslodex (IV) TMPPM<br />
Cancer<br />
Ipilimumab Yervoy TMPPM<br />
Ado-Trastuzumab Emtansine Kadcyla TMPPM<br />
Cataplexy, Narcolepsy Sodium Oxybate Xyrem (Oral Solution)* <strong>VDP</strong><br />
Endocine-Metabolic Agent Mecasermin Increlex (SQ) <strong>VDP</strong><br />
Endocine-Metabolic Agent, Enzyme<br />
Endocrine-Metabolic Agent,<br />
Hormone<br />
Galsulfase Naglazyme (IV) <strong>VDP</strong><br />
Agalsidase Beta Fabrazyme (IV) <strong>VDP</strong><br />
Corticotropin H.P. Acthar <strong>VDP</strong><br />
Alglucosidase Alfa<br />
Myozyme<br />
Lumizyme (IV)<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Enzyme<br />
Idursulfase Elaprase <strong>VDP</strong><br />
Imiglucerase Cerezyme (IV) <strong>VDP</strong><br />
Alglucerase Ceredase (IV) TMPPM<br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 6
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Enzyme Laronidase Aldurazyme (IV) <strong>VDP</strong><br />
Somatropin Genotropin <strong>VDP</strong><br />
Humatrope<br />
<strong>VDP</strong><br />
Norditropin<br />
<strong>VDP</strong><br />
Nutropin<br />
<strong>VDP</strong><br />
Tev-Tropin<br />
<strong>VDP</strong><br />
Growth Hormones<br />
E-Coli / Mammalian Derived<br />
Omnitrope<br />
Genotropine Miniquick<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Nutropin Aq Pen.<br />
<strong>VDP</strong><br />
Saizen<br />
<strong>VDP</strong><br />
Serostim<br />
<strong>VDP</strong><br />
Zorbtive (SC)<br />
<strong>VDP</strong><br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 7
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Hereditory Angioedema (HAE)<br />
C1 Estrase Inhibitor, Human<br />
Cinryze (IV)<br />
Berinert (IV)<br />
<strong>VDP</strong><br />
TMPPM<br />
Immune Modulator Lenalidomide Revlimid <strong>VDP</strong><br />
Baygam<br />
TMPPM<br />
Biogam<br />
TMPPM<br />
Carimune<br />
TMPPM<br />
Gammagard<br />
TMPPM<br />
Immune Serum<br />
Immune Globulin<br />
Sandoglobulin Gamimune N<br />
Gammar-P<br />
TMPPM<br />
<strong>VDP</strong><br />
Hizentra<br />
<strong>VDP</strong><br />
Gamunex<br />
<strong>VDP</strong><br />
Gamunex-C<br />
<strong>VDP</strong><br />
Polygam<br />
<strong>VDP</strong><br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 8
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Privigen<br />
<strong>VDP</strong><br />
Immune Serum<br />
Immune Globulin<br />
Flebogamma<br />
Gammaked<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Octagam (IV)<br />
<strong>VDP</strong><br />
Cytomegalovirus IG, Human Cytogam (IV) <strong>VDP</strong><br />
Multiple Sclerosis Dalfampridine Ampyra <strong>VDP</strong><br />
Myleofibrosis Ruxolitinib Jakafi* <strong>VDP</strong><br />
Oral Antineoplastic Sorafenib Nexavar <strong>VDP</strong><br />
Opioid Antagonist Naltrexone Extended Release Vivitrol (IM) TMPPM<br />
Ambrisentan Letairis <strong>VDP</strong><br />
Pulmonary Arterial Hypertension<br />
Agents<br />
Epoprostenol Sodium<br />
Flolan*<br />
Veletri (IV)*<br />
<strong>VDP</strong><br />
<strong>VDP</strong><br />
Treprostinil Tyvaso <strong>VDP</strong><br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 9
HHSC Vendor <strong>Drug</strong> Program (<strong>VDP</strong>)<br />
<strong>Specialty</strong> <strong>Drug</strong> <strong>List</strong><br />
Effective: 6/15/13<br />
Therapeutic Class / Category Generic Name Brand Name Formulary<br />
Pulmonary Arterial Hypertension<br />
Agents<br />
Treprostinil Remodulin (IV) <strong>VDP</strong><br />
RSV Infection Palivizumab Synagis (IM) <strong>VDP</strong><br />
Treatment Of Hyperammonemia<br />
NAGS Deficiency<br />
Carglumic Acid Carbaglu (Oral) <strong>VDP</strong><br />
* The FDA has restricted access to this medication. Distribution is limited to one or two pharmacies nationwide.<br />
TMPPM = TX <strong>Medicaid</strong> Provider Procedure Manual<br />
Page 10