Universal Set of Dyes for Digital Inkjet Textile Printing
Universal Set of Dyes for Digital Inkjet Textile Printing
Universal Set of Dyes for Digital Inkjet Textile Printing
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
NTC Project C03-PH01<br />
6<br />
mono-functional aldehyde, 9-anthraldehyde, in combination with methylamine, were reacted<br />
under similar conditions. Although the one-pot process was attractive <strong>for</strong> use in combinatorial<br />
syntheses, excessive reaction time [72-96 hours], and production <strong>of</strong> toxic hydrogen cyanide gas<br />
as a by-product, made this synthetic route undesirable.<br />
H 3C<br />
N<br />
O<br />
H<br />
H<br />
HN<br />
CH 3<br />
H<br />
NaBH 3 CN<br />
CH 2<br />
CH 3<br />
CH 2<br />
boroxin<br />
CH3CN<br />
K 2 CO 3<br />
Reflux<br />
B(OH) 2<br />
C<br />
H 2<br />
N<br />
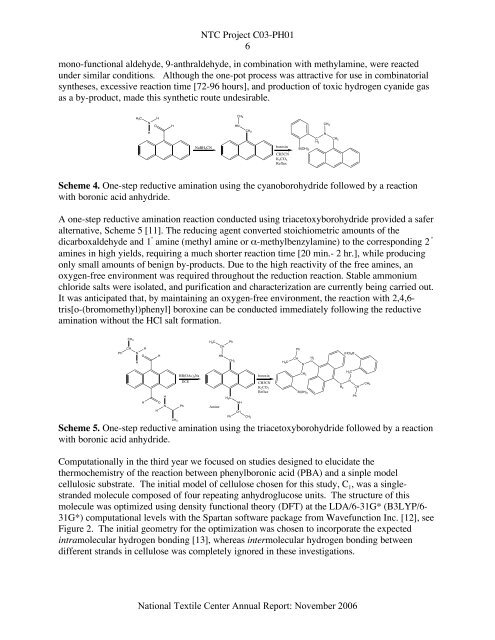
Scheme 4. One-step reductive amination using the cyanoborohydride followed by a reaction<br />
with boronic acid anhydride.<br />
A one-step reductive amination reaction conducted using triacetoxyborohydride provided a safer<br />
alternative, Scheme 5 [11]. The reducing agent converted stoichiometric amounts <strong>of</strong> the<br />
dicarboxaldehyde and 1 º amine (methyl amine or α-methylbenzylamine) to the corresponding 2 º<br />
amines in high yields, requiring a much shorter reaction time [20 min.- 2 hr.], while producing<br />
only small amounts <strong>of</strong> benign by-products. Due to the high reactivity <strong>of</strong> the free amines, an<br />
oxygen-free environment was required throughout the reduction reaction. Stable ammonium<br />
chloride salts were isolated, and purification and characterization are currently being carried out.<br />
It was anticipated that, by maintaining an oxygen-free environment, the reaction with 2,4,6-<br />
tris[o-(bromomethyl)phenyl] boroxine can be conducted immediately following the reductive<br />
amination without the HCl salt <strong>for</strong>mation.<br />
CH 3<br />
H 3C<br />
Ph<br />
Ph<br />
CH<br />
N<br />
H<br />
O<br />
H<br />
H<br />
CH<br />
HN<br />
CH 2<br />
H 3C<br />
Ph<br />
CH<br />
N<br />
H 2<br />
C<br />
(HO) 2B<br />
H<br />
HB(OAc) 3 Na<br />
DCE<br />
H 2C<br />
boroxin<br />
CH3CN<br />
K 2 CO 3<br />
Reflux<br />
CH 2<br />
B(OH) 2<br />
C<br />
H 2<br />
H 2C<br />
N<br />
Ph<br />
CH<br />
CH 3<br />
H<br />
H<br />
O<br />
N<br />
Ph<br />
Amine<br />
NH<br />
CH<br />
CH 3<br />
Ph CH 3<br />
Scheme 5. One-step reductive amination using the triacetoxyborohydride followed by a reaction<br />
with boronic acid anhydride.<br />
Computationally in the third year we focused on studies designed to elucidate the<br />
thermochemistry <strong>of</strong> the reaction between phenylboronic acid (PBA) and a sinple model<br />
cellulosic substrate. The initial model <strong>of</strong> cellulose chosen <strong>for</strong> this study, C 1 , was a singlestranded<br />
molecule composed <strong>of</strong> four repeating anhydroglucose units. The structure <strong>of</strong> this<br />
molecule was optimized using density functional theory (DFT) at the LDA/6-31G* (B3LYP/6-<br />
31G*) computational levels with the Spartan s<strong>of</strong>tware package from Wavefunction Inc. [12], see<br />
Figure 2. The initial geometry <strong>for</strong> the optimization was chosen to incorporate the expected<br />
intramolecular hydrogen bonding [13], whereas intermolecular hydrogen bonding between<br />
different strands in cellulose was completely ignored in these investigations.<br />
National <strong>Textile</strong> Center Annual Report: November 2006