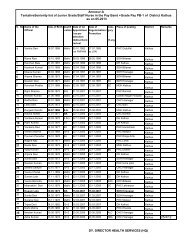
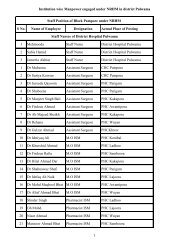
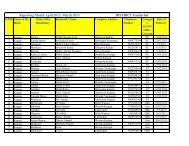
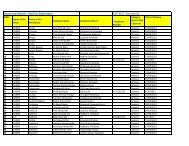
Guidelines for Setting Up Blood Storage Centres - NRHM Tripura
Guidelines for Setting Up Blood Storage Centres - NRHM Tripura
Guidelines for Setting Up Blood Storage Centres - NRHM Tripura
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
GUIDELINES<br />
APPROVAL OF THE BLOOD STORAGE FACILITY<br />
First referral Units, Community Health <strong>Centres</strong>, Primary Health <strong>Centres</strong> or any other hospitals are required to<br />
obtain approval from the State/Union Territory licensing authority. For this, an application has to be made as<br />
per the guidelines enclosed at Annexure I. The State Licensing Authority shall approve the blood storage unit<br />
after satisfying the conditions and facilities through inspection. The approval shall be valid up to a period of<br />
two years from the date of issue unless sooner suspended or cancelled. An application <strong>for</strong> renewal will have to<br />
be made three months prior to the date of expiry of the approval.<br />
Be<strong>for</strong>e applying <strong>for</strong> the approval, the storage centre will have to identify and obtain consent from the blood<br />
bank from where they will get the supply of blood/blood components. These could be licensed blood banks<br />
run by Government Hospitals/Indian Red Cross / Regional <strong>Blood</strong> Transfusion <strong>Centres</strong> only. In case the license<br />
of the parent blood bank/centre is cancelled, the license of the storage centre will also be automatically<br />
cancelled. The storage centres, can however, get affiliated to more than one blood bank/centre to ensure<br />
un-interrupted supplies, but a separate approval will be required in each case.<br />
1. REQUIREMENTS<br />
(i) Space :<br />
The area required <strong>for</strong> setting up the facility is only 10 square metres, well lighted, clean and<br />
preferably air-conditioned.<br />
(ii) Manpower :<br />
In the present phase no additional staff is required. One of the existing doctors and technicians<br />
should be designated <strong>for</strong> this purpose. They should be trained in the operation of blood storage<br />
centres and other basic procedures like storage, grouping, cross- matching and release of blood.<br />
The medical officer designated <strong>for</strong> this purpose will be responsible <strong>for</strong> overall working of the<br />
storage centre.<br />
(iii) Electricity :<br />
Regular 24 hours supply is essential. Provision of backup Generator is required.<br />
(iv) Equipment :<br />
Each FRU should have the following :<br />
1. <strong>Blood</strong> Bag Refrigerators having a storage capacity of 50 units of <strong>Blood</strong>.<br />
2. Deep Freezers <strong>for</strong> freezing ice packs required <strong>for</strong> transportation. The deep freezers available<br />
in the FRUs under the Immunisation Programme can be utilised <strong>for</strong> this purpose.<br />
3. Insulated Carrier boxes with ice packs <strong>for</strong> maintaining the cold chain during transportation of<br />
blood bags.<br />
4. Microscope and centrifuge: Since these are an integral part of any existing laboratory, these<br />
would already be available at the FRUs. These should be supplied only if they are not already<br />
available.<br />
4