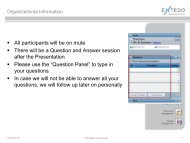
OPENeCTD formerly - Extedo
OPENeCTD formerly - Extedo
OPENeCTD formerly - Extedo
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>formerly</strong><br />
<strong>OPENeCTD</strong><br />
Agenda: Wednesday, 13 April 2011 – Day 1<br />
08:00 – 09:30<br />
09:30 – 09:45<br />
09:45 – 10:15<br />
10:15 – 10:45<br />
• Registration<br />
• Opening eRA2011 – Tore Bergsteiner & Martin Schmid<br />
• Electronic Submissions in Europe – EMAs Roadmap and Vision – Hans Georg Wagner (European Medicines Agency)<br />
• eSubmission: The Good, The Bad, The Future – Michael Schaub (Regulatory Pharma Net)<br />
10:45 – 11:15 Coffee Break<br />
11:15 – 12:00<br />
12:00 – 12:30<br />
• Your eRegulatory World – Winds of Change – Steve Scribner (International Life Science Solutions)<br />
• Current Status of Electronic Regulatory Affairs Standards in Europe – Vito Strasberger (Nanokinetik)<br />
12:30 – 14:00 Lunch – sponsored by ProductLife<br />
14:00 – 15:30 A1 Submission Planning & Tracking – How to manage<br />
registrations<br />
• B.Braun´s View on an Integrated Regulatory<br />
Application Landscape and How to Get There –<br />
Harald Weis (B.Braun)<br />
• Swissmedic‘s Submission Tracking and Reviewing<br />
Now and in the Future – What is in it for the<br />
Applicants? – Susanne Kienberger (Swiss Authority,<br />
Swissmedic)<br />
• Submission Planning & Tracking of Agency<br />
Communication – Ruedi Blattmann (Life Science<br />
Consulting Partners, LSCP)<br />
B1<br />
EU-Regulation & Validation<br />
• Validation of eCTD and NeeS – An Industry<br />
Perspective – Alastair Nixon (GSK)<br />
• The Improved Validation Criteria – Karin Gröhndal<br />
(Swedish Authority, MPA)<br />
• e-Submission Validation in Europe – Balancing National<br />
Agencies‘ Requirements with an Applicants‘<br />
Desire for a Harmonized Dossier – Karl-Heinz<br />
Loebel (Pharmalex)<br />
C1<br />
Sponsoring Partner Track<br />
• Managing Electronic Submission Documents and<br />
Correspondence – Cyril Walsh (QUMAS)<br />
• From Paper-Dossier to eCTD – Andreas Treptow<br />
(Optimal Systems)<br />
15:30 – 16:00 Coffee Break<br />
16:00 – 17:30 A2 Customer Success Stories<br />
B2<br />
Worldwide Standards and Interoperability<br />
C2<br />
Sponsoring Partner Track<br />
• How to Replace Three Legacy eSubmission<br />
Systems with a New One – Adam Aparicio (Merck)<br />
• Global eSubmission management – act local and<br />
and think global – the Angelini Group experience –<br />
Maria Angela Gatto (Angelini)<br />
• CanReg’s eCTD Success Story and Lessons<br />
Learned – Ted Hanebach (CanReg)<br />
• Introduction to the IRISS Forum (Implementation<br />
of Regulatory Information Submission Standards) –<br />
Deanna Murden (ePharmaCMC, LLC)<br />
• An Update on the IRISS ETICS III Project: The EU<br />
Arm of the Study – John-Paul Smith (Celgene)<br />
• Electronic Submissions in the Far East –<br />
Harv Martens (EXTEDO)<br />
• Sharepoint Validation Services – Klavs Esbjerg<br />
(Epista IT)<br />
eRegulatory Affairs 2011 / CONFERENCE ANNOUNCEMENT<br />
3