Total Synthesis Highlights
Total Synthesis Highlights
Total Synthesis Highlights
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
近 年 来 完 成 的 天 然 产 物 的 全 合 成 简 介<br />
<strong>Total</strong> <strong>Synthesis</strong> <strong>Highlights</strong><br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/totalsynthesis.shtm<br />
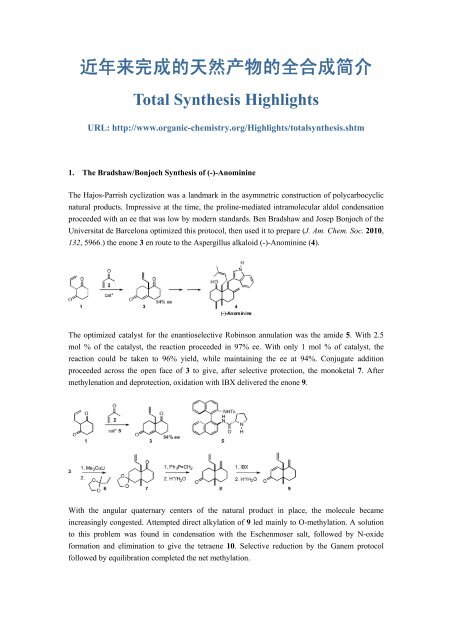
1. The Bradshaw/Bonjoch <strong>Synthesis</strong> of (-)-Anominine<br />
The Hajos-Parrish cyclization was a landmark in the asymmetric construction of polycarbocyclic<br />
natural products. Impressive at the time, the proline-mediated intramolecular aldol condensation<br />
proceeded with an ee that was low by modern standards. Ben Bradshaw and Josep Bonjoch of the<br />
Universitat de Barcelona optimized this protocol, then used it to prepare (J. Am. Chem. Soc. 2010,<br />
132, 5966.) the enone 3 en route to the Aspergillus alkaloid (-)-Anominine (4).<br />
The optimized catalyst for the enantioselective Robinson annulation was the amide 5. With 2.5<br />
mol % of the catalyst, the reaction proceeded in 97% ee. With only 1 mol % of catalyst, the<br />
reaction could be taken to 96% yield, while maintaining the ee at 94%. Conjugate addition<br />
proceeded across the open face of 3 to give, after selective protection, the monoketal 7. After<br />
methylenation and deprotection, oxidation with IBX delivered the enone 9.<br />
With the angular quaternary centers of the natural product in place, the molecule became<br />
increasingly congested. Attempted direct alkylation of 9 led mainly to O-methylation. A solution<br />
to this problem was found in condensation with the Eschenmoser salt, followed by N-oxide<br />
formation and elimination to give the tetraene 10. Selective reduction by the Ganem protocol<br />
followed by equilibration completed the net methylation.
Under anhydrous conditions, the oxide derived from the allylic selenide 12 did not rearrange. On<br />
the addition of water, the rearrangement proceeded smoothly. Protection and hydroboration<br />
converted 13 into 14. The bulk of the folded molecule protected the exo methylene of 14, so<br />
hydrogenation followed by protection and oxidation delivered 15.<br />
Conjugate addition of indole to 15 set the stage for oxidation and bis-methylenation to give 17.<br />
Selective Ru-mediated cross coupling with 18 followed by deprotection then completed the<br />
synthesis of (-)-Anominine (4), which proved to be the enantiomer of the natural product.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2011, March 7.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2011/07March.shtm<br />
2. The Boger <strong>Synthesis</strong> of (-)-Vindoline<br />
The periwinkle-derived alkaloids vinblastine (2a) and vincristine (2b) are still mainstays of cancer<br />
chemotherapy. The more complex half of these dimeric alkaloids, vindoline (1), presents a<br />
formidable challenge for total synthesis. Building on his previous work (Org. Lett. 2005, 7, 4539.),<br />
Dale L. Boger of Scripps, La Jolla devised (J. Am. Chem. Soc. 2010, 132, 3685.) a strikingly<br />
simple solution to this problem, based on sequential cycloaddition.
The starting point for the synthesis was the ester 3, derived from D-asparagine. This was extended<br />
to 4, condensation of which with 5 gave the enol ether 6. On heating, 7 cyclized to 8, which lost<br />
N 2 to give the zwitterion 9. Addition of the intermediate 9 to the indole then gave 10. In one<br />
reaction, the entire ring system of vindoline, appropriately oxygenated, was assembled, with the<br />
original stereogenic center from D-asparagine directing the relative and absolute configuration of<br />
the final product.<br />
To complete the synthesis, the pendant carbon on 11 had to be incorporated into the pentacyclic<br />
skeleton. After adjusting the relative configuration of the secondary alcohol, the N was rendered<br />
nucleophilic by reduction of the amide to the amine. Oxidation delivered 14, that on activation as<br />
the tosylate smoothly rearranged to the ketone 15. Reduction and regioselective dehydration then<br />
completed the synthesis of vindoline (1).
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2011, February 7.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2011/07February.shtm<br />
3. The Tanino <strong>Synthesis</strong> of (-)-Glycinoeclepin A<br />
(-)-Glycinoeclepin A (3) is effective at picogram/mL concentrations as a hatch-stimulating agent<br />
for the soybean cyst nematode. Approaching the synthesis of 3, Keiji Tanino of Hokkaido<br />
University envisioned (Chem. Lett. 2010, 39, 835. DOI: 10.1246/cl.2010.835) the convergent<br />
coupling of the allylic tosylate 2 with the bridgehead anion 1. The assembly of the fragment 2 was<br />
particularly challenging, as the synthesis would require the establishment not just of the two<br />
adjacent cyclic quaternary centers, but also control of the relative configuration on the side chain.<br />
The preparation of 1 began with the prochiral diketone 3. Enantioselective reduction of the mono<br />
enol ether 4 set the absolute configuration of 5. Iodination followed by cyclization then completed<br />
the assembly of 1.<br />
The construction of the bicyclic tosylate 2 began with m-methyl anisole (7). Following the<br />
Rubottom procedure, Birch reduction followed by mild hydrolysis gave the ketone 8. Epoxidation<br />
followed by β-elimination delivered the racemic 9, which was exposed to lipase to give, after<br />
seven days, the residual alcohol in 40% yield and high ee.
The side chain nitrile was prepared from the diol 12. Homologation gave the nitrile 14, that was<br />
equilibrated to the more stable enol ether 15. The two cyclic quaternary centers of 3 were set in a<br />
single step, by the conjugate addition of the anion of 16 to the crystalline enone 11. Mild<br />
hydrolysis of 17 gave the keto aldehyde, that underwent aldol condensation to give the enone 18.<br />
The hydroboration of 19 followed by coupling of the intermediate organoborane with 20 delivered<br />
21 with 94:6 relative diastereocontrol. Formylation of the enone 22 followed by triflation and<br />
reduction then led to 2.<br />
Although the ketone 1 could be deprotonated with LDA, the only product observed, even at -78°C,<br />
was the derived aldol dimer. The metalated dimethylhydrazone 25, in contrast, coupled smoothly<br />
with 2 to give, after hydrolyis, the desired adduct 26. Pd-mediated carboxylation of the enol<br />
triflate followed by selective oxidative cleavage and hydrolysis then completed the synthesis of<br />
(-)-Glycinoecleptin A (3).<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2011, January 3.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2011/03January.shtm<br />
4. The Chen <strong>Synthesis</strong> of (-)-Nakiterpiosin<br />
(-)-Nakiterpiosin (3), isolated from the thin encrusting sponge Terpios hoshinota, has an IC 50<br />
against murine P388 leukemia cells of 10 ng/mL. Chuo Chen of UT Southwestern Medical Center
developed (J. Am. Chem. Soc. 2010, 132, 371.) a practical synthetic route to 3 based on the<br />
convergent coupling of 1 and 2.<br />
The preparation of 1 was based on the intramolecular [4+2] cyclization of the furan 9, prepared by<br />
Friedel-Crafts acylation of furan (4) with maleic anhydride (5). The absolute configuration of the<br />
secondary alcohol was set by Noyori reduction, using sodium formate as the hydride source.<br />
The cyclization of 9 to 10 proceeded with high diastereocontrol, presumably by way of a chelated<br />
transition state. As expected, cyclization of the silyl ether of 9 delivered the complementary<br />
diastereomer. As the cyclization of 9 was readily reversible, it was taken quickly to the bromide<br />
11. Oxidative cleavage of the diol followed by selective reduction and protection then completed<br />
the synthesis of 1.<br />
The preparation of 2 began with the commercial bromo acid 12. The enantiomerically-enriched<br />
epoxide 13 was constructed in the usual way, by homologation of the aldehyde to the allylic<br />
alcohol followed by Sharpless epoxidation. On exposure to the Yamamoto catalyst, 13 smoothly<br />
rearranged to the aldehyde 14. Condensation of 14 with 15 then gave 16, with only minimal<br />
erosion of enantiomeric excess over the two steps.<br />
Unfortunately, 16 was the incorrect diastereomer, so it had to be inverted. With the aldehyde 17 in<br />
hand, conversion to the dichloride followed by functional group interchange completed the<br />
construction of 2.
Carbonylative coupling of 1 and 2 led to the enone 18. The photochemical Nazarov cyclization of<br />
18 proceeded with the expected high diastereocontrol, to give, after epimerization, the desired<br />
trans-anti-trans product. Deprotection then completed the synthesis of (-)-Nakiterpiosin (3). It is<br />
noteworthy that the full A-ring functionality of 3 was compatible with the conditions of the<br />
photochemical cyclization.<br />
The work of Chen toward the total synthesis of (-)-nakiterpoisin (3) led to a correction of the<br />
relative configurations both of the dichloromethyl substituent and of the secondary bromide. The<br />
availability of 3 by total synthesis is particularly exciting, because it has been shown to interfere<br />
with the Hedgehog signaling pathway. There is the potential, based on this activity, that<br />
derivatives of 3 may prove useful as adjuncts in cancer chemotherapy.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, December 6.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/06December.shtm<br />
5. The Shair <strong>Synthesis</strong> of Cephalostatin 1<br />
The cephalostatins and ritterazines, represented by Cephalostatin 1 (3), have the remarkable<br />
property of inducing apoptosis in apoptosis-resistant malignant cell lines. The total synthesis (J.<br />
Am. Chem. Soc. 2010, 132, 275. DOI: 10.1021/ja906996c) of 3 by Matthew D. Shair of Harvard<br />
University required the practical preparation of the complex hexacyclic ketones 1 and 2.<br />
The preparation of 1 started with irradiation of commercial hecogenin acetate 4 to give the known<br />
aldehyde 5. Reaction of 5 with N-phenyltriazolenedione (6) led to the ketal 7. Oxidative cleavage<br />
generated an aldehyde, that on reduction and allylation was converted to 8. Acid-mediated<br />
cyclization led to 9.
The sidechain of 9 was removed, giving 10, that was selectively reduced, leading to 11.<br />
Intramolecular aldol condensation gave 12. The relative configuration of the spiroketal 1 was<br />
established by kinetic bromoetherification of the alcohol 13, followed by free radical reduction of<br />
the resulting tertiary bromide, and acid-catalyzed equilibration.<br />
The synthesis of 2 began with the inexpensive steroid 14. Following the Schönecker protocol, C-H<br />
functionalization led to the ketone 15. Pd-mediated coupling of the derived enol triflate with the<br />
alkyne gave 16, that was oxidized and cyclized to 17. Simmons-Smith conditions converted the<br />
dihydrofuran of 17 into the cyclopropane, that was again opened kinetically with bromine (NBS)<br />
to set the relative configuration of the spiroketal. Free radical reduction followed by protection<br />
and oxidation then completed the preparation of 2.<br />
The coupling of 1 and 2 (not illustrated) to form the central pyrazine of 3 followed the precedent<br />
of Fuchs, combining the 2-azido ketone derived from 1 with the 2-amino methoxime derived from<br />
2. Remarkably, tens of milligrams of 1 and of 2 were prepared, assuring a reasonable supply of 3<br />
for further studies.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, November 1.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/01November.shtm
6. The Overman <strong>Synthesis</strong> of Briarellin F<br />
Briarellin F (4) is an elegant representative of the complex polycyclic ethers produced by soft<br />
corals such as Briareum abestinum. Larry E. Overman of the University of California, Irvine<br />
developed (J. Org. Chem. 2009, 74, 5458.) a triply-convergent approach to 4, the central feature of<br />
which was the Prins-pinacol combination of 1 with 2 to give 3.<br />
The aldehyde 2 was assembled by Wittig homologation of the aldehyde 5 with the phosphorane 6,<br />
followed by metalation and formylation. The aldehyde 10 was prepared by opening the<br />
enantiomerically-pure epoxide 8 with the acetylide 9.<br />
Hydroboration of carvone 11 could not be effected with sufficient diastereocontrol. As an<br />
alternative, the mixture of diols was oxidized to the lactone 12. Kinetic quench of the derived silyl<br />
ketene acetal followed by reduction led to the diastereomerically-pure crystalline diol 13. This key<br />
intermediate will have many other applications in target-directed synthesis.<br />
The ketone 14 was converted to the alkenyl iodide 15 by stannylation of the enol triflate, followed<br />
by exposure of the stannane to N-iodosuccinimide. Addition of the alkenyl iodide 15 to the<br />
aldehyde 10 gave the diol 1 as an inconsequential 3:1 mixture of diastereomers. This mixture was<br />
combined with the aldehyde 2 to give, via Lewis acid-mediated rearrangement of the<br />
initially-prepared acetal, the aldehyde 3.
The aldehyde 3 was readily decarbonylated by irradiation in dioxane. Face-selective Al-mediated<br />
epoxidation of the derived homoallylic alcohol proceeded with 10:1 selectivity, and subsequent<br />
MCPBA epoxidation of the cyclohexene was also secured with 10:1 facial control. This set the<br />
stage for the triflic anhydride-mediated closure of the six-membered ring ether. The<br />
Nozaki-Hiyama-Kishi cyclization of 18 proceeded with remarkable selectivity, delivering<br />
Briarellin E (19) as a single diastereomer. Dess-Martin oxidation converted 19 into Briarellin F<br />
(4).<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, October 4.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/04October.shtm<br />
8. The Baran <strong>Synthesis</strong> of Vinigrol<br />
The diterpene vinigrol (3), isolated from Virgaria nigra F-5408, has eluded total synthesis for<br />
more than twenty years. Attempts to construct the four-carbon bridge on a preformed cis-decalin<br />
have been unavailing. Phil S. Baran of Scripps/LaJolla solved (Angew. Chem. Int. Ed. 2008, 47,<br />
3054, ; J. Am. Chem. Soc. 2009, 131, 17066, ) this problem by adding the extra C-C bond of 1,<br />
that could then be cleaved in course of a Grob fragmentation, leading to 2.<br />
The preparation of 1 started with the dihydroresorcinol derivative 4. Diels-Alder addition of the<br />
ester 5 gave 6, with a modest 2:1 dr. Addition of allyl magnesium chloride to the derived aldehyde<br />
7 proceeded with 6:1 dr. The resulting triene was conformationally sufficiently constrained that<br />
cyclization to 8 proceeded at room temperature over two weeks, or more conveniently at 105°C<br />
for 90 minutes. With 8 in hand, oxidation to the ketone allowed installation of the additional<br />
methyl group of 9. Desilylation followed by OH-directed reduction set the relative configuration<br />
of 1 correctly for the Grob fragmentation to the Z-alkene 2.
There were two remaining problems in the synthesis. The alkene of 2 had to be converted to the<br />
methylated tertiary alcohol, and the ketone had to be elaborated to the ene diol. While seemingly<br />
straightforward, the congested tricyclic skeleton of 2 made many common transformations<br />
difficult. The solution to the former problem was found in the selective dipolar addition of<br />
bromonitrile oxide. Reduction of the ketone then enabled HO-directed hydrogenation of the<br />
alkene, that otherwise was resistant. Dehydration followed by reduction with LiAlH 4 gave the<br />
desired methyl group bearing a primary amine, that was removed by free radical reduction of the<br />
corresponding isonitrile, to give 12.<br />
With 12 in hand, the end of the synthesis appeared to be in sight. In fact, the reduction of a variety<br />
of oxidized intermediates proved difficult. In the end, a sequence that did not require reduction<br />
proved effective. Dihydroxylation of 12 gave a diol, selective oxidation of which delivered the<br />
α-hydroxy ketone 13. Formation of the trisylhydrazone followed by Shapiro reaction gave the<br />
intermediate alkenyl anion, that was trapped with formaldehyde to give the long-sought Vinigrol<br />
(3).<br />
Vinigrol (3) produced by this sequence was racemic. The absolute configuration of the ring<br />
system was set in the course of the Diels-Alder addition of 5 to 4. Use of a chiral catalyst in this<br />
step could set the stage for an enantioselective synthesis of 3.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, September 6.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/06September.shtm
9. The Nicolaou <strong>Synthesis</strong> of (+)-Vannusal<br />
The correct assignment of relative configuration for portions of a complex structure that are<br />
remote one from another can present substantial difficulties. This was brought home in the course<br />
of the synthesis of (+)-Vannusal (3) described (Angew. Chem. Int. Ed. 2009, 48, 5642,; 5648, ) by<br />
K. C. Nicolau of Scripps/La Jolla. In fact, they prepared several alternative diastereomers,<br />
including the originally assigned structure, before finally coming to 3, the spectra of which<br />
matched those of the natural product.<br />
Their synthetic strategy was based on the late-stage convergent coupling of the aldehyde 13 with<br />
the iodide 19, leading to 1. The preparation of 13 began with conjugate addition of the 1-propenyl<br />
Grignard reagent 5 to the cyclohexenone 4. Deprotection, oxidation and acetal formation led to 6,<br />
that cyclized with high diastereocontrol to 7. Carbomethoxylation of the ketone followed by<br />
Mn(OAc) 3 cyclization delivered the highly strained norbornane 8 as a single diastereomer.<br />
Condensation of the derived ketone 9 with acetone (10) followed by reduction set the three<br />
remaining ternary stereogenic centers of 13. O-Alkylation of the aldehyde 11 followed by Claisen<br />
rearrangement established the alkylated quaternary center. Functional group manipulation then<br />
converted 12 into 13.<br />
The preparation of the iodide 19 began with the diene 14. Hydroboration followed by acetylation<br />
provided the meso diol. Enzymatic hydrolysis proceeded with high enantioselectivity, to give 15.<br />
Opening of the epoxide 16 with 2-propenyl lithium gave the trans alcohol, that was converted to<br />
the requisite cis alcohol 17 by Mitsunobu esterification followed by hydrolysis. Shapiro iodination<br />
of 18 then delivered 19.
The iodide 19 was enantiomerically pure, but the aldehyde 13 was racemic, so coupling of the two<br />
led to 1 and its diastereomer. The cyclization of 1 with SmI 2 proceeded with remarkable<br />
diastereocontrol, to give the desired 2 directly. Deprotection and oxidation then completed the<br />
synthesis of (+)-Vannusal B (3).<br />
It is noteworthy that throughout this synthesis, the radicals AZADO (20) and 1-Me-AZADO (21),<br />
developed by Professor Iwabuchi ( 2010, March 8), more efficient than the traditional<br />
TEMPO, were used to effect selective catalytic oxidation.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, August 2.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/02August.shtm<br />
10. The Nicolaou <strong>Synthesis</strong> of (+)-Hirsutellone B<br />
(+)-Hirsutellone B (3), isolated from the insect pathogenic fungus Hirsutella nivea BCC 2594,<br />
shows good activity (MIC = 0.78 μg/mL) against Mycobacterium tuberculosis. Approaching the<br />
synthesis of 3, K. C. Nicolaou of Scripps/La Jolla envisioned and reduced to practice (Angew.<br />
Chem. Int. Ed. 2009, 49, 6870. ) a spectacular tandem intramolecular epoxide opening - internal<br />
Diels-Alder cyclization (1 → 2) that established all three of the carbocyclic rings of 3 with the<br />
proper relative and absolute configuration.
The construction of 1 began with commercial (R)-(+)-citronellal (4). Wittig homologation<br />
established the (Z)-iodide 5. Selective ozonolysis followed by condensation with the phosphorane<br />
7 set the stage for Jørgensen-Córdova epoxidation (Tetrahedron Lett. 2006, 47, 99.) with H 2 O 2<br />
and a catalytic amount of the Hayashi catalyst 9. Condensation of 10 with the phosphorane 11<br />
followed by Cu-catalyzed coupling of 12 with the organostannane 13 completed the assembly of<br />
1.<br />
This approach underscores the strategic advantages the Jørgensen-Córdova epoxidation has over<br />
the Sharpless protocol. It is not necessary to reduce the aldehyde to the allyic alcohol, then<br />
reoxidize. Further, the Jørgensen-Córdova epoxidation, using catalytic 9, is operationally easier<br />
than the Sharpless procedure, that often uses stoichiometric amounts of tartrate ester.<br />
The cyclization of 1 proceeded by way of 14, with the newly formed stereogenic center having the<br />
diene equatorial on the cyclohexane. Endo cycloaddition catalyzed by the Lewis acid in the<br />
solution then gave 2. The facility with which the cyclization of 14 set both the substituents and the<br />
stereogenic centers of 2 raises the possibility that the biosynthesis might also follow such an<br />
internal [4 + 2] cycloaddition.
To complete the synthesis of 3, it was necessary to construct the strained paracyclophane. The<br />
authors took advantage of the facile cyclization of the thiolate liberated from 19, then installed the<br />
ring-contracted alkene with a Ramburg-Bäcklund rearrangement. They completed the synthesis of<br />
(+)-Hirsutellone B (3) by exposing the ketone 22 to NH 3 in CH 3 OH/H 2 O.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, July 5.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/05July.shtm<br />
11. The Magnus <strong>Synthesis</strong> of (±)-Codeine<br />
Although there have been many synthetic approaches to morphine and its methyl ether codeine (3),<br />
the pentacyclic structure of these Papaver alkaloids continues to intrigue organic chemists. Philip<br />
Magnus of the University of Texas devised (J. Am. Chem. Soc. 2009, 131, 16045.) an elegant<br />
route to 3 based on the conversion of 1 to 2 by way of an intramolecular Michael addition.<br />
The starting point for the synthesis was the commercial bromoaldehyde 4. Coupling with 5<br />
delivered the substituted biphenyl 6, that was carried on to the mixed bromo acetal 8. On exposure<br />
to fluoride ion, 8 was desilylated, and the intermediate phenoxide cyclized with impressive facility<br />
to give 1. Exposure of 1 to nitromethane delivered the tetracyclic 2. This reaction apparently was<br />
initiated by Henry addition of the nitromethane to the aldehyde. The intramolecular Michael<br />
addition of the intermediate Henry adduct then proceeded to give the desired cis diastereomer of<br />
the newly formed ring. Finally, loss of water gave 2.<br />
Conjugate reduction of the nitroalkene 2 led to 9 with remarkable diastereocontrol. Exposure of 9<br />
to LiAlH 4 converted the nitro group to the amine, and the enone to the allylic alcohol. On<br />
exposure to acid, the hemiacetal was hydrolyzed. The liberated aldehyde underwent reductive
amination with the free amine, while at the same time ionic cyclization closed the ether ring.<br />
N-Acylation completed the conversion to 10.<br />
The ether 10 had previously been converted to codeine, and then, in a single demethylation step,<br />
to morphine. In that synthesis, the alkene of 10 was directly epoxidized. The resulting “up”<br />
epoxide reacted only sluggishly with phenylselenide anion, and the relative configuration of the<br />
resulting allylic alcohol had to be inverted by oxidation followed by reduction. In the current<br />
synthesis, exposure of the alkene 10 to dibromohydantoin under aqueous conditions, to form the<br />
bromohydrin, effected concomitant arene bromination, to give, after base treatment, the “down”<br />
epoxide 12. Phenylselenide opening of the epoxide was then facile, and the product allylic alcohol<br />
had the correct relative configuration for codeine and morphine. The extra Br was of no<br />
consequence, as it was removed by the final LiAlH 4 reduction.<br />
Except for the stereogenic center of the acetal, the dienone 1 is prochiral. This raises the exciting<br />
possibility that it may be possible to set the absolute configuration of codeine and thus of<br />
morphine by asymmetric catalysis of the Henry reaction of 1.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, June 7.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/07June.shtm<br />
12. The Dixon <strong>Synthesis</strong> of (-)-Nakadomarin A<br />
(-)-Nakadomarin A (4), isolated from the sponge Amphimedon sp. off the coast of Okinama,<br />
shows interesting antifungal and antibacterial activity. The key step in the total synthesis of 4<br />
reported (J. Am. Chem. Soc. 2009, 131, 16632. ) by Darren J. Dixon of the University of Oxford<br />
was the diastereoselective addition of the enantiomerically-pure ester 1 to the prochiral nitroalkene<br />
2.
The assembly of 2 began with the linchpin ketophosphonate 5. Alkylation of the dianion of 5 with<br />
allyl bromide followed by direct condensation of the resulting monoanion with the diacetate 6<br />
gave 7. On exposure to aqueous acid, 7 cyclized to the furan. Oxidation of the liberated primary<br />
alcohol followed by condensation with nitromethane then completed the preparation of 2.<br />
The starting material for the synthesis of 1 was the enantiomerically-pure pyroglutamate<br />
derivative 8. Sulfide displacement followed by N-alkylation with the bromide 10 delivered 11.<br />
Oxidation followed by deprotection then set the stage for the intramolecular Julia-Kocienski<br />
cyclization, that gave 12 with the expected (eight-membered ring) high geometric control.<br />
Addition of the ester 1 to Michael acceptors proceeded across the open face of the lactam, but it<br />
was still necessary to control the face of the nitro alkene 2 to which the lactam anion added.<br />
Catalysis of the addition with the urea 13 delivered 3 with 10:1 diasterocontrol.<br />
Mannich condensation of the nitroalkane 3 with formaldehyde and the amine 14 gave the<br />
bis-lactam 15, conveniently as a single diastereomer. After free radical removal of the nitro group,<br />
it was necessary to achieve selective reduction of the δ-lactam in the presence of the γ-lactam.<br />
Low temperature LiAlH 4 was found to be effective. Direct reduction of the resulting hemiaminal<br />
with formic acid led to the monolactam 16. The hemiaminal from monoreduction of 16 was found<br />
to be unstable and sensitive to over-reduction. Nevertheless, exposure of 16 to Dibal at low<br />
temperature followed by acid-mediated cyclization delivered the diamine 17.
Cyclization of the free base of 17 with the first generation Grubbs catalyst gave (-)-Nakadomarin<br />
A (4) as the minor component of a 40:60 Z/E mixture. Carrying out the cyclization on the<br />
camphorsulfonate salt improved the ratio to 63:37 Z/E.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, May 3.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/03May.shtm<br />
13. The Corey <strong>Synthesis</strong> of (+)-Lupeol<br />
The total synthesis of lupeol was one of the crowning achievements of the Robinson<br />
annulation/reductive alkylation approach to stereocontrolled polycarbocyclic construction<br />
developed by Gilbert Stork (J. Am. Chem. Soc. 1971, 93, 4945. ). It is a measure of the progress of<br />
organic synthesis since that time that E. J. Corey of Harvard University could devise (J. Am. Chem.<br />
Soc. 2009, 131, 13928.) an enantioselective synthesis of (+)-lupeol (3) that could be carried out by<br />
a single co-worker. The key step in the synthesis was the Lewis acid-mediated cyclization of 1 to<br />
2.<br />
The preparation of 1 began with the enantioselective epoxidation of farnesol acetate (4). To this<br />
end, asymmetric dihydroxylation delivered the diol 5. Selective mesylation followed by exposure<br />
to dilute methoxide effected ring closure to the epoxide, but also removed the acetate, so this had<br />
to be reapplied.
The synthesis of the aromatic portion of 1 started with the phenol 7. Protection as the very bulky<br />
triisopropylsilyl ether was important for the success of the subsequent cyclization, perhaps<br />
because it discouraged complexation of the Lewis acid with the aryl ether. Metalation followed by<br />
formylation delivered the aldehyde 8, that was reduced and carried on to the bromide 9. The<br />
derived Grignard reagent coupled smoothly with 6 under Li 2 CuCl 4 catalysis.<br />
The cyclization of 1 to 2 proceeded with remarkable efficiency (43%!), for a reaction in which<br />
three new carbon-carbon bonds, four rings and five new stereogenic centers were established. It is<br />
particularly noteworthy that the cyclization cleanly set the trans, anti, trans, anti tetracyclic<br />
backbone of (+)-lupeol (3).<br />
To complete the synthesis of 3, the less substituted alkene of 2 was selectively hydrogenated, then<br />
CH 3 Li was added to give 10. Hydrolysis and dehydration yielded 11, that was reduced and<br />
equilibrated to 12. On brief exposure to MsCl/Et 3 N, 13 cyclized to (+)-lupeol (3).<br />
It is a measure of the remarkable efficiency of this synthesis of (+)-lupeol (3) that it provided<br />
sufficient material to enable studies of the rearrangement of 3 under acidic conditions to other
pentacyclic triterpenes, including, inter alia, germanicol, α-amyrin, β-amyrin and taraxasterol<br />
(14).<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, April 5.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/05April.shtm<br />
14. The Trost <strong>Synthesis</strong> of (-)-Pseudolaric Acid B<br />
(-)-Pseudolaric Acid B (3), isolated from the bark of the golden larch Pseudolarix kaempferi,<br />
shows potent antifungal activity. A key step in the total synthesis of 3 described (J. Am. Chem.<br />
Soc. 2008, 130, 16424. ) by Barry M. Trost of Stanford University was the free radical cyclization<br />
of 1 that established the angular ester and the trans ring fusion of 2 and thus of 3.<br />
To prepare the bicyclic skeleton of 1, the authors envisioned the Rh-mediated intramolecular<br />
addition of the alkyne of 11 to the alkenyl cyclopropane. The acyclic centers of 11 were<br />
established by Noyori hydrogenation of (equilibrating) racemic 4. One enantiomer reduced much<br />
more quickly than did the other, leading to 5. The absolute configuration of the cyclopropane was<br />
set by Charette cyclopropanation of the monosilyl ether of the inexpensive diol 8. The two<br />
components were then coupled using a Corey-Schlosser protocol. Alkylation of the ylide 10 with<br />
7 gave a new phosphonium salt, that in situ was deprotonated and condensed with the aldehyde 9.<br />
The resulting betaine was deprotonated and quenched, then exposed again to base to give the trans<br />
alkene 11. It is important in this procedure to use PhLi as the base, since the alkyl lithium can<br />
displace the alkyl group on phosphorus.<br />
The product from Ru-catalyzed cyclization was the expected 1,4-diene 12. Fortunately, it was<br />
found that TBAF desilylation led to concomitant alkene migration, to give the more stable<br />
conjugated diene 13. Selective epoxidation of the more electron-rich alkene followed by exposure<br />
to strong base then delivered 14, with the requisite angular oxygenation established.
Pseudolaric Acid B (3) would be derived from cyclization of the selenocarbonate of a tertiary<br />
alcohol. In fact, however, attempted cyclization of such selenocarbonates led only to<br />
decarboxyation and reduction. Even with the selenocarbonate 1 prepared from the secondary<br />
alcohol, the cyclization to 2 required careful optimization, including using not AIBN, but<br />
azobis(dicyclohexylcarbonitrile) as the radical initiator.<br />
Acetylide addition to the ketone 15 could be effected with high diastereocontrol, but lactone<br />
construction proved elusive. Alkaline conditions led quickly to addition of the angular hydroxyl to<br />
the activated alkene in the seven-membered ring. Eventually it was found that the ester exchange<br />
catalyst 16 developed by Otera delivered 17, that could be carried on to (-)-Pseudolaric Acid B<br />
(3).<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, March 1.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/01March.shtm<br />
15. The Nakada <strong>Synthesis</strong> of (-)-FR182877<br />
The Streptomyces metabolite (-)-FR182877 (3) binds to and stabilizes microtubules, showing the<br />
same potency of anticancer activity as Taxol. Masahisa Nakada of Waseda University assembled<br />
(Angew. Chem. Int. Ed. 2009, 48, 2580.) the hexacyclic ring system of 3 by the tandem<br />
intramolecular Diels-Alder – intramolecular hetero Diels-Alder cyclization of 1, generating seven<br />
new stereogenic centers in a single step.<br />
The construction of the pentaene substrate 1 started with the known aldehyde 4, prepared by<br />
homologation of commercial ethyl 3-methyl-4-oxocrotonate. Addition of the propionyl
oxazolidine anion 5 proceeded with high diastereocontrol, to give 6. The acyl oxazolidinone was<br />
not an efficient acylating agent, so it was converted to the Weinreb amide. Protection and<br />
deprotection then delivered the allylic acetate 7.<br />
The key step in the pentaene assembly was the carefully optimized Negishi-Wipf methylation of 8,<br />
followed by Pd-mediated coupling of the alkenyl organometallic so generated with the allylic<br />
acetate, to give 9. Condensation of the derived keto phosphonate 11 with the known aldehyde 12<br />
then delivered the enone 13.<br />
The Nakada group has worked extensively on the intramolecular Diels-Alder reaction of<br />
substrates such as 1. They have shown that protected anti diols such as 1 cyclize with substantial<br />
diastereocontrol and in the desired sense. In contrast, cyclizations of protected syn diols proceed<br />
with poor diastereocontrol. The enone 13 was therefore reduced to the anti diol and protected,<br />
leading to 14. Oxidation of 14 at room temperature led to a complex mixture, but slow oxidation<br />
at elevated temperature delivered 2. Although the yield of 2 was not much better than if the<br />
reactions were carried out sequentially, first the intramolecular Diels-Alder cyclization, then the<br />
intramolecular hetero Diels-Alder cyclization, with the cascade protocol pure 2 was more readily<br />
separated from the reaction matrix.<br />
With 2 in hand, there was still the challenge of assembling the seven-membered ring. Cyclization<br />
was effected with an intramolecular Heck protocol. The two diastereomers of the allylic alcohol<br />
15 cyclized with comparable efficiency. Ir-catalyzed alkene migration then converted the allylic<br />
alcohols to a mixture of ketones, that was equilibrated to give the more stable diasteromer.
Reduction of the ketone then set the last stereogenic center of 3. Deprotection and subsequent<br />
lactone formation completed the synthesis of (-)-FR182877 (3).<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, February 1.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/01February.shtm<br />
16. The Williams <strong>Synthesis</strong> of (-)-4-Hydroxydictyolactone<br />
(-)-4-Hydroxydictyolactone (3), representative of the cyclononene xenicanes isolated from the<br />
Dictyotacae algae, readily isomerizes thermally to the more stable (Z)-6,7-isomer. Attempts to<br />
directly form this strained ring system appeared to be fraught with difficulty. David R. Williams<br />
of Indiana University envisoned (J. Am. Chem. Soc. 2009, 131, 9038.) that use of Suzuki coupling<br />
might ameliorate some of the strain, since at the point of commitment to bond formation, the Pd<br />
center would be included in the forming ring. This analysis led specifically to the trans ether 1, as<br />
cyclization of the trans ether appeared likely to be more facile than would cyclization of the<br />
alternative cis diastereomer.<br />
The first challenge was the assembly of the array the four contiguous alkylated stereogenic centers<br />
of 1. To this end, the Z secondary ester 7 was prepared from the acetonide 4, available from<br />
mannitol, and (R)-(+)-citronellic acid, prepared by oxidation of the commercial aldehyde.<br />
Addition of 7 to LDA led to decomposition, but inverse addition of LDA to a mixture of the ester,<br />
TMSCl and Et 3 N smoothly delivered the ketene silyl acetal. On warming, Ireland-Claisen<br />
rearrangement of the ketene silyl acetal led to the acid 8 with remarkable diastereocontrol.<br />
The last alkylated stereogenic center of 1 was installed by reductive cyclization of the formate<br />
ester 9. Again, the cyclization proceeded with remarkable diastererocontrol. While the
intramolecular reaction of in situ prepared allyl metals is well precedented, the addition to a<br />
formate ester had not previously been reported.<br />
Although 11 appears to be ready for the long-awaited Suzuki coupling, in fact the TIPS protecting<br />
group substantially slowed hydroboration. The free alcohol/methyl acetal was the best substrate<br />
for hydroboration, but the free alcohol entered into other side reactions. After extensive<br />
experimentation, a happy medium was found with the methyl acetal/TBS ether 1.<br />
Selenylation of the lactone 12 followed by oxidative elimination of the selenide delivered the<br />
expected Z alkene. Removal of the silyl protecting group had to precede introduction of the second<br />
alkene, as the product 3 deteriorated rapidly on exposure to the alkaline conditions of TBAF<br />
cleavage.<br />
Strained medium rings such as the (E,Z)-cyclononadiene assembled in this study have been<br />
particularly challenging to prepare. It will be interesting to see how generally useful the Suzuki<br />
coupling turns out to be for the construction of such rings.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, January 4.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/04January.shtm<br />
2009 年<br />
17. The Davies/Williams <strong>Synthesis</strong> of (-)-5-epi-Vibsanin E<br />
There are currently 61 known vibsane-type diterpenes, as exemplified by (-)-5-epi-Vibsanin E (3).<br />
The first synthesis of 3, described (J. Am. Chem. Soc. 2009, 131, 8329. ) by Huw M. L. Davies of<br />
Emory University and Craig M. Williams of the University of Queensland, was based on the<br />
enantioselective seven-membered ring construction developed by the Davies group and the end<br />
game established by the Williams group. A key step in the synthesis was the intramolecular hetero<br />
Diels-Alder cyclization of 1 to 2.
The absolute configuration of 1 was set by the Rh-mediated cyclopropanation of 4 with the diazo<br />
ester 5. Though closely related to the α-diazo β-keto ester 6, the alkene of 5 donates electron<br />
density to the intermediate Rh carbene, making it more susceptible to the influence of the chiral<br />
ligands. The alkene of the enol ether then participated in the Cope rearrangement, delivering 8.<br />
Routine functional group transformation then converted 8 to 1, that cyclized smoothly to 2.<br />
The enol ether of 2 was reduced with high diastereocontrol to give 10. The ketone was installed by<br />
allylic oxidation, setting the stage for attachment of the two pendant sidechains of 3 by conjugate<br />
addition followed by enolate trapping. Cu-catalyzed addition of the α-oxygenated organolithium<br />
12 proceeded well in the presence of TMS-Cl, to establish the silyl enol ether 13. Allylation of the<br />
regenerated enolate proceeded at oxygen, but the enol ether 14 so prepared rearranged to the<br />
desired C-alkylated product 15 on microwave heating.<br />
The synthesis endgame was based on an unusual transformation, the addition to the keto aldehyde<br />
16 of the phosphonium salt 17, developed (Tetrahedron 2008, 64, 6482.) by the Williams group.<br />
This allowed the introduction of the complete vinyl ester array of (-)-5-epi-Vibsanin E (3).
This synthesis illustrates the power of the elegant enantioselective seven-membered ring<br />
construction developed by the Davies group. The Williams phosphonium salt will also have<br />
general applicability. In a simpler manifestation, conversion of an aldehyde to, e.g., the enol<br />
benzoate, followed by exposure to dilute methoxide, will allow the conversion of an aldehyde to<br />
the aldehyde one carbon longer, without the acidic hydrolysis usually required for such a<br />
transformation.<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2009, December 7.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2009/07December.shtm<br />
18.The Kobayashi <strong>Synthesis</strong> of (-)-Norzoanthamine<br />
The Zoanthus alkaloids, exemplified by (-)-norzoanthamine (3a) and zoanthamine (3b), show<br />
promising activity against osteoporosis. Susumu Kobayashi of the Tokyo University of Science<br />
assembled (Angew. Chem. Int. Ed. 2009, 48, 1400, ; Angew. Chem. Int. Ed. 2009, 48, 1404, ) the<br />
challenging tricyclic core of 3a employing the intramolecular Diels-Alder cyclization of 1 to 2.<br />
The cyclopentane of 1 served as useful scaffolding, even though it was cleaved en route to 3a.<br />
The cyclohexane ring of 1 has five of its six positions substituted, including three that are<br />
alkylated quaternary centers. The starting point for the preparation of 1 was the<br />
enantiomerically-pure Hajos-Parrish ketone 4, containing the first of the those quaternary centers.<br />
Conjugate addition of MeLi established the second quaternary center. The less stable endo alkyl<br />
branch of 1 was installed by conjugate addition to the more reactive α-methylene ketone of the<br />
cross-conjugated 5, followed by kinetic quench. Addition of vinyl cuprate across the open face of<br />
the enone 7 then established the final quaternary center, setting the stage for the intramolecular<br />
Diels-Alder reaction. The silyl enol ether from the cyclization of 1 was not stable, so it was<br />
directly oxidized to the enone 2.
The keto phosphonate 16 for the last two rings of 3a was prepared from the previously-reported<br />
crystalline glutamic acid-derived mesylate 12. Reduction and homologation delivered the ester 14,<br />
that was condensed with the phosphonate anion 15 to give 16.<br />
The congested cyclopentanone 17, derived from 2, was most efficiently deprotonated with n-BuLi.<br />
Exposure of the resulting silyl enol ether to ozone led to the α-hydroxylated product 18.<br />
Unexpectedly but happily, oxidative cleavage of 18 delivered, after deprotection and reprotection,<br />
the more congested aldehyde 19. This cleavage may be proceeding by tautomerization of 18 to the<br />
regioisomeric keto alcohol. The aldehyde 19 was condensed with the keto phosphonate 16, to give,<br />
after hydrogenation, the keto lactone 20. A series of oxidation state adjustments then completed<br />
the synthesis of (-)-norzoanthamine (3a).<br />
The preparation of 3a outlined here underlines the importance of developing new methods for<br />
concise stereocontrolled carbocyclic construction. The utility of an enantiomerically-pure bicyclic<br />
scaffold such as 4 for subsequent relative stereocontrol is particularly apparent<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2009, November 2.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2009/02November.shtm
19.The Castle <strong>Synthesis</strong> of (-)-Acutumine<br />
The complex tetracyclic alkaloid (-)-acutumine 3, isolated from the Asian vine Menispermum<br />
dauricum, shows selective T-cell toxicity. The two adjacent cyclic all-carbon quaternary centers of<br />
3 offered a particular challenge. Steven L. Castle of Brigham Young University solved (J. Am.<br />
Chem. Soc. 2009, 131, 6674.) this problem by effecting net enantioselective conjugate allylation<br />
of the enantiomerically pure substrate 1 to give 2 with high diastereocontrol.<br />
The starting coupling partners (Org. Lett. 2006, 8, 3757,; Org. Lett. 2007, 9, 4033,) for the<br />
synthesis were the Weinreb amide 4, prepared over several steps from 2,3-dimethoxyphenol, and<br />
the diastereomerically- and enantiomerically-pure cyclopentenyl iodide 5, prepared by singlet<br />
oxygenation of cyclopentadiene followed by enzymatic hydrolysis. Transmetalation of 5 by the<br />
Knochel protocol, addition of the resulting organometallic to 4 and enantioselective (and therefore<br />
diastereoselective) reduction of the resulting ketone delivered the alcohol 6. Methods for installing<br />
cyclic halogenated stereogenic centers are not well developed. Exposure of the allylic alcohol to<br />
mesyl chloride gave the chloride 7 with inversion of absolute configuration. Remarkably, this<br />
chlorinated center was carried through the rest of the synthesis without being disturbed.<br />
A central step in the synthesis of 3 was the spirocyclization of 7 to 8. Initially, iodine atom<br />
abstraction generated the aryl radical. The diastereoselectivity of the radical addition to the<br />
cyclopentene was set by the adjacent silyloxy group. The α-keto radical so generated reacted with<br />
the Et 3 Al to give a species that was oxidized by the oxaziridine to the α-keto alcohol, again with<br />
remarkable diastereocontrol.<br />
Conjugate addition to the cyclohexenone 1 failed, so an alternative strategy was developed,<br />
diastereoselective 1,2-allylation of the ketone followed by oxy-Cope rearrangement. The<br />
stereogenic centers of 1 are remote from the cyclohexenone carbonyl, so could not be used to<br />
control the facial selectivity of the addition. Fortunately, the stoichiometric enantiomerically-pure
Nakamura reagent delivered the allyl group preferentially to one face of the ketone 1, to give 9.<br />
The subsequent sigmatropic rearrangement to establish the very congested second quaternary<br />
center of 2 then proceeded with remarkable facility, at 0°C for one hour.<br />
Oxidative cleavage to the aldehyde followed by reductive amination gave 10, that looks as though<br />
it could be poised for intramolecular displacement of the secondary chloride. Nonetheless, Lewis<br />
acid mediated ionization followed by cyclization proceeded smoothly, to establish the fourth ring<br />
of the natural product. Oxidation state adjustment then completed the synthesis of (-)-Acutumine<br />
(3).<br />
The face selective enone allylation followed by oxy-Cope rearrangement (1 → 2), a highlight of<br />
the approach presented here, will have many applications in target-directed synthesis.<br />
20.The Trost <strong>Synthesis</strong> of (-)-Ushikulide A<br />
(-)-Ushikolide A (4), isolated from a culture broth of Streptomyces sp. IUK-102, showed powerful<br />
activity against murine splenic lymphocyte proliferation (IC 50 = 70 nM). The most important<br />
player in the synthesis of 4 described (J. Am. Chem. Soc. 2008, 130, 16190. ) by Barry M. Trost of<br />
Stanford University was the ProPhenol ligand 1.<br />
The precursor 2 was prepared by coupling the mesylate 7, the alkyne 12, and the aldehyde 13. The<br />
first role of catalyst 1 was in mediating the enantioselective coupling of commercial 5 with 6 to<br />
give, after saponification and CuCl decarboxylation, the mesylate 7. The preparation of 12 began<br />
with the Noyori hydrogenation of the ester 8 to the alcohol 9 in the expected high ee. Note that<br />
although this transformation was carried out at 1800 psi, such reductions proceed well and in
similar ee at 60°C and 60 psi. Brown crotylation of the derived aldehyde 10 delivered 11, that was<br />
homologated to the alkyne 12.<br />
The third fragment 13 was prepared by chiral auxiliary directed aldol condensation. Combination<br />
of 12 with 13 was followed by Au-mediated cyclization, converting the internal alkyne of 14 to<br />
the spiroketal of 15. Pd-catalyzed coupling of 15 with 7 then led to 2 with high diastereocontrol.<br />
The aldol addition of the enolate of 17 to 18 proved elusive under the usual conditions, but with<br />
30 mol % of the Zn catalyst 1 the reaction proceeded smoothly, to deliver 19 with high<br />
diastereocontrol.<br />
To complete the synthesis, hydroboration with 9-BBN was effected on the free carboxylic acid 3,<br />
and Pd-mediated coupling of the derived borane was carried out with the free iodo alcohol 2. As a<br />
result, the product hydroxy acid 20 could be taken directly to the subsequent macrolactonization.
21. The Overman Syntheses of Nankakurines A and B<br />
The tetracyclic alkaloids Nankakurine A and Nankakurine B were isolated from the club moss<br />
Lycopodium hamiltonii. A preliminary study of the biological activity of Nankakurine A<br />
suggested that it could induce secretion of neurotrophic factors and promote neuronal<br />
differentiation. The key step in the first syntheses of Nankakurine A and of Nankakurine B,<br />
reported (J. Am. Chem. Soc. 2008, 130, 11297.) by Larry E. Overman of the University of<br />
California, Irvine was the intriguing intramolecular aza-Prins cyclization of 1 to 2.<br />
The starting material for the synthesis was 5-methyl cyclohexenone 6, prepared from (R)-pulegone.<br />
The diene 5 was prepared from the alkyne 4, following the procedure developed by Diver. There<br />
were two issues in developing the Diels-Alder addition of the enone 4 to the diene 6. The first was<br />
the relative lack of reactivity of 4 as a dienophile. The other issue was the ready epimerization of<br />
the product ketone 9. Both of these problems were solved using the activation method devised by<br />
Gassman. Condensation of 4 with 7 in the presence of the bis-silyl ether 7 and the diene 6 at<br />
cryogenic temperatures led to the ketal 8. It is thought that the active dienophile was the cation 11.<br />
Gentle hydrolysis of the ketal 8 was effected with minimal epimerization. Reductive amination<br />
with the hydrazide 10 proceeded with high diastereocontrol, to give the precursor 1.<br />
The intramolecular aza-Prins cyclization of 1 to 2 proceeded well, though the desired tetracyclic 2<br />
was only observed when base was included in the reaction medium. In the absence of base,<br />
tricyclic alkenes dominated.
Reduction of the N-N bond of 2 proceeded smoothly with freshly prepared SmI 2 . After reductive<br />
methylation, hydrogenation removed the benzyl ether, and AlH 3 converted the benzamide to the<br />
benzyl amine. At low temperature, mesylation of the alcohol was apparently faster than<br />
mesylation of the secondary amine, enabling cyclization to 14. Removal of the benzyl protecting<br />
group gave Nankakurine A, which was successfully methylated to give Nankakurine B.<br />
The completion of a total synthesis is an anxious moment, as for the first time it is possible to<br />
compare spectra of the synthetic material with those reported for the natural product. There is<br />
always a concern as to whether or not the spectra are being acquired under precisely the same<br />
conditions employed by those who did the initial isolation. This is particularly true for very polar<br />
molecules such as these diamines. In fact, the spectra in CD 3 OD did not initially match, but on the<br />
addition of small amounts of CF 3 CO 2 H they were brought into congruence.<br />
Although in this synthesis the starting enantiomerically-pure cyclohexenone 4 was derived from<br />
natural sources, one could imagine that enantioselective conjugate methylation of cyclohexenone<br />
or a derivative could get one into the same manifold.<br />
22. The Keck <strong>Synthesis</strong> of Epothilone B<br />
The total synthesis of Epothilone B (4), the first natural product (with Epothilone A) to show the<br />
same microtubule-stabilizing activity as paclitaxel (Taxol®), has attracted a great deal of attention<br />
since that activity was first reported in 1995. The total synthesis of 4 devised (J. Org. Chem. 2008,<br />
73, 9675.) by Gary E. Keck of the University of Utah was based in large part on the<br />
stereoselective allyl stannane additions (e.g. 1 + 2 → 3) that his group originated.<br />
The allyl stannane 2 was prepared from the acid chloride 5. Exposure of 5 to Et 3 N generated the<br />
ketene, that was homologated with the phosphorane 6 to give the allene ester 7. Cu-mediated<br />
conjugate addition of the stannylmethyl anion 8 then delivered 2.
The benzyloxy aldehyde 1 was prepared from the ester 9 by reduction with Dibal.<br />
Felkin-controlled 1,2-addition of the allyl stannane 2 established the relative configuration of the<br />
secondary alcohol of 3, that was then used to control the relative configuration of the new alcohol<br />
in 10. Addition of the crotyl borane 12 to the derived aldehyde 11 also proceeded with high<br />
diastereocontrol.<br />
The other component of 4 was prepared from the aldehyde 14. Enantioselective allylation, by the<br />
method the authors developed, delivered the alcohol 16. The Z trisubstituted alkene was then<br />
assembled by condensing the aldehyde 17 with the phosphorane 18. Dibal reduction of the product<br />
lactone 19 gave a diol, the allylic alcohol of which was selectively converted to the chloride with<br />
the Corey-Kim reagent. Hydride reduction then delivered the desired homoallylic alcohol, that<br />
was converted to the phosphonium salt 21. Condensation of 21 with 13 gave the diene, that was<br />
carried on to Epothilone B (4).<br />
The synthesis of Epothilone B (4) as originally conceived by the authors depended on ring-closing<br />
metathesis of the triene 22. They prepared 22, but on exposure to the second-generation Grubbs<br />
catalyst it was converted only to 23. The authors concluded that the trans acetonide kept 22 in a<br />
conformation that did not allow the desired macrocyclization.
23. The Johnson <strong>Synthesis</strong> of Zaragozic Acid C<br />
The zaragozic acids, exemplified by Zaragozic Acid C (3), are picomolar inhibitors of cholesterol<br />
biosynthesis. Jeffrey S. Johnson of the University of North Carolina developed (J. Am. Chem. Soc.<br />
2008, 130, 17281.) an audacious silyl glyoxylate cascade approach to the oxygenated backbone<br />
fragment 1. Intramolecular aldol cyclization converted 1 to 2, setting the stage for the construction<br />
of 3.<br />
The lactone 2 includes five stereogenic centers, two of which are quaternary. The authors were<br />
pleased to observe that exposure of 4 to vinyl magnesium bromide (5) led, via condensation, silyl<br />
transfer, condensation, and again silyl transfer, to a species that was trapped with t-butyl<br />
glyoxylate (6) to give 7 as a single diastereomer. This one step assembled three of the stereogenic<br />
centers of 2, including both of the quaternary centers. The alcohol 7 so prepared was racemic, so<br />
the wrong enantiomer was separated by selective oxidation. Intramolecular aldol condensation of<br />
the derived α-benzyloxy acetate 1 then completed the construction of 2.<br />
Addition of the alkyl lithium 8, again as a single enantiomerically-pure diasteromer, to 2 gave the<br />
hemiketal 9. Exposure of 9 to acid initially gave a mixture of products, but this could be induced<br />
to converge to the tricyclic ester 10. To convert 10 to 11, the diastereomer that was needed for the<br />
synthesis, two of the stereogenic centers had to be inverted. This was accomplished by exposure to<br />
t-BuOK/t-amyl alcohol, followed by re-esterification. The inversion of the secondary hydroxyl<br />
group was thought to proceed by retro-aldol/re-aldol condensation.
Debenzylation of 11 followed by acetylation delivered 12, an intermediate in the Carreira<br />
synthesis of the zaragozic acids. Following that precedent, the ring acetates of 12 were selectively<br />
removed, leaving the acetate on the side chain. Boc protection was selective for the endo ring<br />
secondary hydroxyl, leaving the exo ring secondary hydroxyl available for condensation with the<br />
enantiomerically-pure acid 13. Global deprotection then completed the synthesis of Zaragozic<br />
Acid C (3).<br />
The key to the success of this synthesis of the complex spiroketal 3 was the assembly of 7 in one<br />
step as a single diastereomer from the readily-available building blocks 4, 5, and 6. This process,<br />
reminiscent of group transfer polymerization, will be a useful complement to the cascade<br />
organocatalyzed aldol condensations that have recently been developed.<br />
24. The Carter <strong>Synthesis</strong> of (-)-Lycopodine<br />
Rich G. Carter of Oregon State University described (J. Am. Chem. Soc. 2008, 130, 9238 ) the first<br />
enantioselective synthesis of the Lycopodium alkaloid (-)-lyopodine (3). A key step in the<br />
assembly of 3 was the diastereoselective intramolecular Michael addition of the keto sulfone of 1<br />
to the enone, leading to the cyclohexanone 2.<br />
The key cyclization substrate 1 bore a single secondary methyl group. While that could have been<br />
derived from a natural product, it was operationally easier to effect chiral auxiliary controlled<br />
conjugate addition to the crotonyl amide 4, leading, after methoxide exchange, to the ester 5. The<br />
authors reported that double deprotonation with LiTMP gave superior results, vs. LDA or BuLi, in<br />
the condensation of 6 with 5 to give 7. Metathesis with pentenone 8 gave the intramolecular<br />
Michael substrate 1.
The authors thought that they would need a chiral catalyst to drive the desired stereocontrol in the<br />
cyclization of 1 to 2. As a control, they tried an achiral base first, and were pleased to observe the<br />
desired diastereomer crystallize from the reaction mixture in 89% yield. The structure of 2 was<br />
confirmed by X-ray crystallography.<br />
To prepare for the intramolecular Mannich condensation, the azide was reduced to give the imine,<br />
and the methyl ketone was converted to the silyl enol ether. Under Lewis acid conditions, the<br />
sulfonyl group underwent an unanticipated 1,3-migration, to give 11. Cyclization of 12 then<br />
delivered the crystalline 14. Reduction converted 14 to the known (in racemic form) ketone 15.<br />
To complete the synthesis, the amine 15 was alkylated with 16 to give the alcohol 17. Oppenauer<br />
oxidation followed by aldol condensation delivered the cyclized enone, that was reduced with the<br />
Stryker reagent to give (-)-Lycopodine (3).<br />
Both the cyclization of 1 to 2 and the cyclization of 9 to 14 are striking. It may be that the steric<br />
demand of the phenylsulfonyl group destabilizes the competing transition state for the cyclization<br />
of 1.<br />
25.
The Hoveyda <strong>Synthesis</strong> of (-)-Clavirolide C<br />
Conjugate addition-enolate trapping, a strategy originally developed by Gilbert Stork, has become<br />
a powerful method for stereocontrolled ring construction. A key step in the synthesis of<br />
(-)-Clavirolide C (3) reported (J. Am. Chem. Soc. 2008, 130, 12904) by Amir H. Hoveyda of<br />
Boston College occurred early on, with the enantioselective conjugate addition of Me 3 Al to 1 to<br />
give the silyl enol ether 2. Enantioselective conjugate addition to establish a quaternary center β<br />
on a cyclohexanone had been established (2008, August 18), but not yet on cyclopentanones.<br />
Professor Hoveyda found that a modified form of the Ag catalyst that they had published earlier,<br />
in combination with the Lewis acidic AlMe 3 , effected conjugate addition to 1 in 84% ee.<br />
Quenching of the reaction mixture with triethylsilyl triflate led to the enol silyl ether 2.<br />
The assembly of the 11-membered ring of 3 also began with an enantioselective conjugate<br />
methylation, of the lactone 4 with Me 2 Zn, again using a catalyst developed by Professor Hoveyda.<br />
Opening of the lactone 5 followed by Swern oxidation gave the Weinreb amide 6, that was<br />
homologated and reduced to give 7.<br />
Addition of n-BuLi to 2 regenerated the enolate. There were two issues in the addition of that<br />
enolate to the aldehyde 7: syn vs. anti stereocontrol, and control of the configuration of the newly<br />
formed ternary center on the ring relative to the already-established quaternary center. Inclusion of<br />
Et 3 B in the reaction mixture assured anti aldol formation, but there was only a modest preference<br />
for the desired bond formation trans to the slightly more bulky butenyl group, to give 8.<br />
Medium rings are more strained than are larger rings. The diene 8 was reluctant to close with the<br />
second generation Grubbs catalyst, but the catalyst developed by Professor Hoveyda worked well.<br />
The δ-lactone of 3 was then constructed by acylation of 9 with 10 followed by reductive<br />
cyclization with SmI 2 . Conjugate addition to the derived enone 12 on the outside face of the<br />
medium ring alkene gave the desired 13 (9:1 dr). This reaction may be proceeding via the s-cis<br />
conformer, as the more stable s-trans conformer would have been expected to give the other<br />
diastereomer. Dehydration of 13 then delivered (-)-Clavirolide C (3).
This concise synthesis of the dolabellane 3 showcases the power of the catalytic enantioselective<br />
methods for the construction of both ternary and quaternary, including cyclic quaternary, centers<br />
that Professor Hoveyda has developed. Clearly, asymmetric transformation of inexpensive<br />
prochiral ring precursors such as 1 and 4 will make advanced, high ee intermediates such as 2 and<br />
5 much more readily available than they have been in the past.<br />
26. The Zakarian <strong>Synthesis</strong> of (+)-Pinnatoxin A<br />
(+)-Pinnatoxin A (3), isolated from the shellfish Pinna muricata, is thought to be a calcium<br />
channel activator. A key transformation in the synthesis of 3 reported (J. Am. Chem. Soc. 2008,<br />
130, 3774.) by Armen Zakarian, now at the University of California, Santa Barbara, was the<br />
diastereoselective Claisen rearrangement of 1 to 2.<br />
The alcohol portion of ester 1 was derived from the aldehyde 4, prepared from D-ribose. The<br />
absolute configuration of the secondary allylic alcohol was established by chiral amino alcohol<br />
catalyzed addition of diethyl zinc to the unsaturated aldehyde 5.<br />
The acid portion of the ester 1 was prepared from (S)-citronellic acid, by way of the Evans imide 7.<br />
Methylation proceeded with high diasterocontrol, to give 8. Functional group manipulation<br />
provided the imide 9. Alkylation then led to 10, again with high diastereocontrol. In each case,<br />
care had to be taken in the further processing of the α-chiral acyl oxazolidinones. Direct NaBH 4
eduction of 8 delivered the primary alcohol. To prepare the acid 10, the alkylated acyl<br />
oxazolidinone was hydrolyzed with alkaline hydrogen peroxide.<br />
On exposure of the ester 1 to the enantiomerically-pure base 11, rearrangement proceeded with<br />
high diastereocontrol, to give the acid 2. This outcome suggests that deprotonation proceeded to<br />
give the single geometric form of the enolate, that was then trapped to give specifically the ketene<br />
silyl acetal 12. This elegant approach is dependent on both the ester 1 and the base 11 being<br />
enantiomerically pure.<br />
The carbocyclic ring of pinnatoxin A (3) was assembled by intramolecular aldol condensation of<br />
the dialdehyde 11. This outcome was remarkable, in that 11 is readily epimerizable, and might<br />
also be susceptible to β-elimination. Note that the while the diol corresponding to 11 could be<br />
readily oxidized to 11 under Swern conditions, attempts to oxidize the corresponding hydroxy<br />
aldehyde were not fruitful.<br />
27. The Paquette <strong>Synthesis</strong> of Fomannosin<br />
The compact sesquiterpene (+)-fomannosin (3), isolated from the pathogenic fungus Fomes<br />
annonsus, presents an interesting set of challenges for the organic synthesis chemist, ranging from<br />
the strained cyclobutene to the easily epimerized cyclopentanone. In the synthesis of 3 developed<br />
(J. Org. Chem. 2008, 73, 4548.) by Leo A. Paquette of Ohio State University, the cyclopentane
was constructed by ring-closing metathesis of 1. The real challenge of the synthesis was the<br />
enantiospecific preparation of 1 from D-glucose.<br />
The starting point for the preparation of 1 was the glucose derivative 4. Selective acetonide<br />
hydrolysis followed by oxidative cleavage gave the ester 5, which on base treatment followed by<br />
hydrogenation delivered the endo ester 6. Condensation of the enolate of 6 with formaldehyde<br />
proceeded with high diastereoselectivity, to give, after protection, the ester 7. Conversion of the<br />
ester to the vinyl group, exposure to methanolic acid and ether formation completed the<br />
preparation of 9.<br />
The construction of the cyclobutane of 1 was effected by an interesting application of the Negishi<br />
reagent (Cp 2 ZrCl 2 /2 x BuLi). Complexation of Cp 2 Zr with the alkene followed by elimination<br />
generated an allylic organometallic 11, which added to the released aldehyde to give the<br />
cyclobutanes 12 and 13 in a 2.4:1 diastereomeric ratio.<br />
Homologation of the aldehyde 13 and subsequent oxidation were straightforward, but subsequent<br />
methylenation of the hindered carbonyl was not. At last, it was found that Peterson olefination<br />
worked well. Metathesis then delivered the cyclopentene 2. The last carbons of the skeleton were<br />
added by intramolecular aldol cyclization of the thioester 16.
The seemingly simple task of converting the alkene of 17 into a ketone proved challenging.<br />
Eventually, dihydroxylation followed by oxidation, and then SmI 2 reduction, completed the<br />
transformation. This still left the challenge of controlling the cyclopentane stereogenic center.<br />
Remarkably, dehydration and epimerization led to (+)-Fomannosin (3) as a single dominant<br />
diastereomer.<br />
28. The Wood <strong>Synthesis</strong> of Welwitindolinone A Isonitrile<br />
Welwitindolinone A Isonitrile (3) is the first of a family of oxindole natural products isolated from<br />
the cyanobacteria Hapalosiphon welwischii and Westiella intricate on the basis of their activity for<br />
reversing multiple drug resistance (MDR). A key transformation in the total synthesis of 3<br />
reported (J. Am. Chem. Soc. 2008, 130, 2087) by John L. Wood, now at Colorado State University,<br />
was the chlorination of 1, that in one step established both the axial secondary chloro substituent<br />
and the flanking chiral quaternary center.<br />
The starting material for the synthesis of 3 was the diene acetonide 5, readily prepared from the<br />
Birch reduction product 4. Intermolecular ketene cycloaddition proceeded with high regio- and<br />
diastereoselectivity, to give the bicyclooctenone 6.
The triazene-bearing Grignard reagent 7 added to the ketone 6 with the anticipated high<br />
diastereocontrol, to give, after reduction and protection, the cyclic urethane 8. Selective oxidation<br />
of the diol derived from 8 followed by silylation delivered the enone 9. Conjugate addition of<br />
hydride followed by enolate trapping gave the triflate 10. Pd-catalyzed methoxycarbonylation<br />
established the methyl ester 11. Addition of CH 3 MgBr to 11 gave 1, setting the stage for the<br />
establishment of the two key stereogenic centers of 2 and so of 3.<br />
The transformation of 1 to 2 was envisioned as being initiated by formation of a bridging<br />
chloronium ion. Pinacol-like 1,2-methyl migration then proceeded to form the trans diaxial<br />
product, moving the ketone-bearing branch equatorial. In addition to being an elegant solution of<br />
the problem of how to establish the axial chloro substituent of 3, this strategy might have some<br />
generality for the stereocontrolled construction of other alkylated cyclic quaternary centers.<br />
Reduction of the ketone 2 and dehydration of the resulting alcohol led, after deprotection and<br />
oxidation, to the ketone 12. Protection followed by β-elimination gave the enone 13. Direct<br />
reductive amination of 13 failed, but reduction of the methoxime was successful, giving, after<br />
acylation, the formamide 14. Reductive N-O bond cleavage followed by deprotection and<br />
isonitrile formation then set the stage for the planned intramolecular acylation to complete the<br />
synthesis of Welwitindolinone A Isonitrile (3).<br />
The starting diene 5 used in this synthesis was prochiral, leading to racemic 3. Now that the route<br />
to 3 is established, it would be interesting to devise a method for preparing<br />
enantiomerically-enriched 6. Enantiomerically-pure variants of 5 have been prepared, inter alia by
fermentation of halogenated aromatics. Alternatively, an enantioselective version of the [2+2]<br />
cycloaddition to the prochiral 5 could be developed.<br />
2008<br />
27. The Takayama <strong>Synthesis</strong> of (-)-Cernuine<br />
(-)-Cernuine (3) falls in the subset of the Lycopodium alkaloids that feature a bicyclic aminal core.<br />
There had not been a total synthesis of this class of alkaloids until the recent (Org. Lett. 2008, 10,<br />
1987.) work of Hiromitsu Takayama of Chiba University. The key step in this synthesis was a<br />
diastereoselective intramolecular reductive amination, converting 1 to 2. As is apparent from the<br />
3-D projection, (-)-cernuine (3) has a tricyclic trans-anti-trans aminal core, with an appended<br />
six-membered ring, both branches of which are axial on the core. While the branch that is part of<br />
the aminal could be expected to equilibrate, the other branch had to be deliberately installed.<br />
The synthesis began with (+)-citronellal (4), each enantiomer of which is commercially available<br />
in bulk. After protection and ozonolysis, the first singly-aminated stereogenic center was installed<br />
by enantioselective, and therefore diastereoselective, addition of 5 to the azodicarboxylate 6,<br />
mediated by the organocatalyst 7. Reductive cleavage of the N-N bond followed by acetal<br />
methanolysis converted 8 to 9. Ionization followed by allyl silane addition then delivered 11,<br />
having the requisite axial alkyl branch.<br />
The next two tasks were the assembly of the second of the four rings of 3, and the construction of<br />
the second single-aminated stereogenic center. The ring was assembled by deprotection of 11<br />
followed by acylation with acryloyl chloride, to give 12. Grubbs cyclization followed by<br />
hydrogenation then led to 13. Homologation of 13 to the aldehyde 14 set the stage for<br />
condensation with the camphor-derived tertiary amine 15, following the protocol developed by<br />
Kobayashi. Sequential imine formation, aza-Cope rearrangement, and hydrolysis led to 1 in 94%<br />
de.
One could envision reduction of the lactam carbonyl of 1 to an aldehyde equivalent, that would<br />
then, under acidic conditions, condense to form the desired aminal 2. This approach was, however,<br />
not successful. As an alternative, conditions were developed to convert 1 to the amidine 16.<br />
Reduction then proceeded with the expected high diastereocontrol, to give the cis 1,3-fused aminal<br />
2. This was not isolated, but was directly acylated with acryloyl chloride, to 17.<br />
The synthesis of (-)-cernuine 3 was concluded by Grubbs cyclization of 17 to 3, followed again by<br />
hydrogenation. Note that there was a key difference between this cyclization and the Grubbs<br />
cyclization of 12 that led to 13, in that 17 contained a basic N, while 12 did not. For the<br />
cyclization of 12, the first generation Grubbs catalyst was sufficient, while for the cyclization of<br />
17, the second generation catalyst was required.<br />
This synthesis illustrates the efficacy of the Grubbs cyclization for polycyclic construction. The<br />
approach outlined here also highlights the power of current methods for enantioselective allylation<br />
of imines for the construction of enantiomerically pure, and, in the context of this synthesis,<br />
diastereomerically pure, aminated secondary stereogenic centers.<br />
28. The Roush <strong>Synthesis</strong> of (+)-Superstolide A<br />
(+)-Superstolide A (3), isolated from the New Caledonian sponge Neosiphonia superstes, shows<br />
interesting cytotoxicity against malignant cell lines at ~ 4 ng/mL concentration. The key<br />
transformation in the synthesis of 3 described (J. Am. Chem. Soc. 2008, 130, 2722. ) by William R.<br />
Roush of Scripps Florida was the transannular Diels-Alder cyclization of 2, which established, in<br />
one step with high diastereocontrol, both the cis decalin and the macrolactone of 3.
The octaene 1 was assembled from four stereodefined fragments. The first, the linchpin 6, was<br />
prepared from the stannyl aldehyde 4. Homologation gave the enyne 5, which on hydroboration<br />
and oxidation gave 6.<br />
Earlier, Professor Roush had optimized the crotylation of the protected alaninal 7. In this case, the<br />
Brown reagent 8 delivered the desired Felkin product 9. Protection followed by ozonolysis gave<br />
the aldehyde 10. Crotylation with the Roush-developed tartrate 11 then gave the alkene 12, setting<br />
the stage for conversion to the iodide 13. Coupling of 13 with 6 completed the preparation of 14.<br />
The third component of (+)-superstolide A (3), the phosphonium salt 21, was assembled by Brown<br />
allylation of the aldehyde 15, to give 17. Protecting group interchange followed by ozonolysis<br />
delivered 18, which via Still-Gennari homologation was carried on to 21. Condensation with the<br />
fourth component, the aldehyde 22, and esterification with 14 then gave 1.
Under high dilution Suzuki conditions 1 was converted to 2. Storage in CDCl 3 for five days, or<br />
brief warming, cyclized 2 to a single diastereomer of the transannular Diels-Alder product, that<br />
was carried on to (+)-superstolide A (3). While acyclic trienes comparable to 2 could be induced<br />
to cyclize, the transannular Diels-Alder reaction proceeded with much higher diastereocontrol<br />
29. The Bergman-Ellman <strong>Synthesis</strong> of (-)-Incarvillateine<br />
The monoterpene alkaloid (-)-incarvillateine (3) has interesting symmetry properties. The central<br />
cyclobutane diacid core is not itself chiral, but the appended alkaloids are. The key step in the total<br />
synthesis of 3 recently (J. Am. Chem. Soc. 2008, 130, 6316.) described by Robert G. Bergman and<br />
Jonathan A. Ellman of the University of California, Berkeley was the diastereoselective<br />
Rh-catalyzed cyclization of 1 to 2.<br />
The cyclobutane diacid core 5 was assembled from ferulic acid 4 following the procedure of<br />
Kibayashi (J. Am. Chem. Soc. 2004, 126, 16553.).
The starting point for the preparation of 1 was the commercial aldehyde 6. Enantioselective<br />
allylation followed by silylation delivered 7, which on cross metathesis with methacrolein gave<br />
the diene aldehyde 8. Imine formation then completed the construction of 1.<br />
The cyclization of 1 was effected by warming (45°C, 6 h) with 2.5 mol % [RhCl(coe) 2 ] 2 and 5.5<br />
mol % (DMAPh)Pet 2 ligand. While four diastereomers were possible from the cyclization, only<br />
two were observed, with one predominating. Since the product mixture was easily susceptible to<br />
tautomerization, it was carried on directly to reduction and cyclization, to form the lactam 8.<br />
Hydrogenation of 8 to 9 required high temperature and pressure, but delivered 9 as a single<br />
diastereomer. Reduction and desilylation then set the stage for Mitsunobu coupling with 5, to give<br />
11. Dissolving metal conditions removed the tosyl groups from 5 to give (-)-incarvillateine 3.<br />
It will be interesting to see how general this Rh catalyzed cyclization will be. It will also be<br />
interesting to establish the mechanism. The authors described the cyclization of 1 as proceeding<br />
via initial metalation of the alkene C-H bond, followed by insertion of the ester-bearing alkene<br />
into the C-Rh bond to form a new C-Rh bond, and finally reductive elimination. Their previous<br />
observation of metalation of such an unsaturated imine with maintenance of the alkene geometry<br />
suppported this mechanism. The high diastereocontrol also suggested intramolecular C-C bond<br />
formation. Whatever the mechanism, the enantiomerically-pure cyclopentane 2, having four of its<br />
five carbons functionalized, is a versatile intermediate for further transformation.<br />
30. The Toste <strong>Synthesis</strong> of (+)-Fawcettimine<br />
The tetracyclic Lycopodium alkaloid fawcettimine (3) and its derivatives are of interest as<br />
inhibitors of acetylcholine esterase. F. Dean Toste of the University of California, Berkeley<br />
recently reported (Angew. Chem. Int. Ed. 2007, 46, 7671.) the first enantioselective synthesis of 3.<br />
The key to the synthesis was the rapid assembly of the enantiomerically-enriched hydrindane (2).
The preparation of 2 began with the enantioselective Robinson annulation of the β-keto ester 4<br />
with crotonaldehyde (5), mediated by the organocatalyst 6. In this protocol, originally developed<br />
by Karl Anker Jørgensen, the single stereogenic center was established by conjugate addition,<br />
presumably to the chiral iminium salt generated by the condensation of 5 with 6. Subsequent aldol<br />
(or more likely Mannich) cyclization followed by elimination gave 7. Hydrolysis and<br />
decarboxylation by heating with p-TsOH converted 7 to 1. This procedure was robust enough to<br />
allow preparation of a ten gram batch of 1. This Jørgensen annulation is the current method of<br />
choice for the enantioselective preparation of 2,5-dialkyl cyclohexenones.<br />
Conjugate addition of the propargyl anion equivalent 8 to 1 proceeded with the expected > 95:5<br />
axial diastereoselectivity, to give the silyl enol ether 9. Exposure of the derived iodide 10 to<br />
catalytic [Ph 3 PAu]Cl and AgBF 4 induced smooth cyclization to the cis hydrindane 2.<br />
Before constructing the nine-membered ring amine of fawcettimine (3), it was first necessary to<br />
protect the ketone as the ketal. Pd-mediated coupling of the alkenyl iodide with the organoborane<br />
derived from 11 then proceeded smoothly, as did the subsequent hydroboration of the terminal<br />
alkene.<br />
Neither the mesylate nor the tosylate derived from 12 could be induced to cyclize. In contrast,<br />
intramolecular displacement of the iodide proceeded well, to give 13. Hydroboration followed by<br />
oxidation then gave 15, which on deprotection cyclized to (+)-fawcettimine (3).
Several aspects of this synthesis are attractive. While the stereochemical outcome of the<br />
hydroboration of 14 could not necessarily be predicted with confidence, in fact it did not matter, as<br />
the stereogenic center adjacent to the ketone could be epimerized under the trifluoroacetic acid<br />
deprotection conditions, and only the desired diastereomer would be able to add in an<br />
intramolecular fashion to the cyclohexanone. The construction of 2 from 10 underscores the<br />
importance of the Au-catalyzed cyclizations developed by Professor Toste.<br />
The most important news from this synthesis is the validation in a second research group of the<br />
enantioselective Robinson annulation previously described by Professor Jørgensen. In the<br />
assembly of polycarbocycles, the central challenge is the enantioselective construction of the first<br />
ring. The Jørgensen annulation is a powerful solution to that problem.<br />
31. The Ley <strong>Synthesis</strong> of Rapamycin<br />
Rapamycin (3) is used clinically as an immunosuppressive agent. The synthesis of 3 (Angew.<br />
Chem. Int. Ed. 2007, 46, 591.) by Steven V. Ley of the University of Cambridge was based on the<br />
assembly and subsequent coupling of the iododiene 1 and the stannyl alkene 2.<br />
The lactone of 1 was prepared by Fe-mediated cyclocarbonylation of the alkenyl epoxide 5,<br />
following the protocol developed in the Ley group.<br />
The cyclohexane of 2 was constructed by SnCl 4 -mediated cyclization of the allyl stannane 9, again<br />
employing a procedure developed in the Ley group. Hydroboration delivered the aldehyde 11,<br />
which was crotylated with 12, following the H. C. Brown method. The alcohol so produced (not<br />
illustrated) was used to direct the diastereoselectivity of epoxidation, then removed, to give 13.<br />
Coupling with 14 then led to 2.
Combination of 1 with 2 led to 15, which was condensed with catechol to give the macrocycle 16.<br />
Exposure of 16 to base effected Dieckmann cyclization, to deliver the ring-contracted<br />
macrolactone 17, which was carried on to (-)-rapamycin (3).<br />
32. The Kozmin <strong>Synthesis</strong> of Spirofungin A<br />
Often, 6,6-spiroketals such as Spirofungin A (3) have a strong anomeric bias. Spirofungin A does<br />
not, as the epimer favored by double anomeric stabilization suffers from destabilizing steric<br />
interactions. In his synthesis of 3, Sergey A. Kozmin of the University of Chicago took advantage<br />
(Angew. Chem. Int. Ed. 2007, 46, 8854.) of the normally-destablizing spatial proximity of the two<br />
alkyl branches of 3, joining them with a siloxy linker to assure the anomeric preference of the<br />
spiroketal. The assembly of 1 showcased the power of asymmetric crotylation, and of Professor<br />
Kozmin’s linchpin cyclopropenone ketal cross metathesis.
To achieve the syn relative (and absolute) configuration of 6, commercial cis-2-butene was<br />
metalated, then condensed with the Brown (+)-MeOB(Ipc) 2 auxiliary. The accompanying<br />
Supporting Information, accessible via the online HTML version of the journal article, includes a<br />
succinct but detailed procedure for carrying out this homologation. For the anti relative (and<br />
absolute) configuration of 9, it is more convenient to use the tartrate 8 introduced by Roush.<br />
Driven by the release of the ring strain inherent in 10, ring opening cross metathesis with 6<br />
proceeded to give the 1:1 adduct 11 in near quantitative yield. The derived cross-linked silyl ether<br />
12 underwent smooth ring-closing metathesis to the dienone 1.<br />
On hydogenation, the now-flexible ring system could fold into the spiro ketal. With the primary<br />
and secondary alcohols bridged by the linking silyl ether, only one anomeric form, 2, of the spiro<br />
ketal was energetically accessible.<br />
A remaining challenge was the stereocontrolled construction of the trisubstituted alkene. To this<br />
end, the aldehyde 13 was homologated to the dibromide 14. Pd-mediated coupling of the alkenyl<br />
stannane 15 with 14 was selective for the E bromide. The residual Z bromide was then coupled<br />
with Zn(CH 3 ) 2 to give 16. These steps, and the final steps to complete the construction of<br />
spirofungin A (3), could be carried out without exposure to equilibrating acid, so the carefully<br />
established spiro ketal configuration was maintained.
33. The Burke <strong>Synthesis</strong> of (+)-Didemniserinolipid B<br />
The sulfate (+)-didemniserinolipid B (3), isolated from the tunicate Didemnum sp, has an<br />
intriguing spiroether core. A key step in the synthesis of 3 reported (Org. Lett. 2007, 9, 5357. ) by<br />
Steven D. Burke of the University of Wisconsin was the selective ring-closing metathesis of 1 to<br />
2.<br />
The diol 6 that was used to prepare the ketal 1 was readily prepared from the inexpensive<br />
D-mannitol (4). Many other applications can be envisioned for the enantiomerically-pure diol 6<br />
and for the monoacetate and bis acetate that are precursors to it.<br />
To set up the metathesis, the β,γ-unsaturated ketone 10 was needed. To this end, the keto<br />
phosphonate derived from the addition of the phosphonate anion 8 to the lactone 7 was condensed<br />
with phenyl acetaldehyde 9. The derived enone 10 was a 5:1 mixture of β,γ- and α,βregioisomers.
The diol 6 is C 2 -symmetrical, but formation of the ketal 1 dissolved the symmetry, with one<br />
terminal vinyl group directed toward the styrene double bond, and the other directed away from it.<br />
On exposure to the first generation Grubbs catalyst, ring formation proceeded efficiently, to give 2.<br />
Williamson coupling with the serine-derived alcohol 11 then gave 12.<br />
To establish the secondary alcohol of 13 and so of 3, the more electron rich alkene of 12 was<br />
selectively epoxidized, from the more open face. Diaxial opening with hydride then gave 13.<br />
With 13 in hand, another challenge of selectivity emerged. The plan had been to attach the<br />
ester-bearing sidechain to 13 using alkene metathesis, then hydrogenate. As the sidechain of 3<br />
contained an additional alkene, this had to be present in masked form. To this end, the<br />
α-phenylselenyl ester 14 was prepared. Alkene metathesis with 13 proceeded smoothly, this time<br />
using the second generation Grubbs catalyst. The unwanted alkene was then removed by reduction<br />
with diimide, and the selenide was oxidized to deliver the α,β-unsaturated ester.<br />
34. The Rychnovsky <strong>Synthesis</strong> of Leucascandrolide A
The macrolactone leucascandrolide A (4), isolated from the calcareous sponge L. caveolata, has<br />
both cytotoxic and antifungal activity. The key step in the synthesis of 4 reported (J. Org. Chem.<br />
2007, 72, 5784.) by Scott D. Rychnovsky of the University of California, Irvine, was the<br />
stereoselective condensation of the aldehyde 1 with the allyl vinyl ether 2 to give 3.<br />
The cyclic ether of 1 was assembled from the crotyl addition product 5. Tandem Ru-catalyzed<br />
metathesis / hydrogenation converted 5 to the lactone 6. Reduction of 6 to the lactol followed by<br />
activation as the acetate gave 7, axial-selective condensation of which with the enol ether 8<br />
delivered the enone 9. Diastereoselective Itsuno-Corey reduction of 9 followed by protecting<br />
group exchange and oxidation then gave 1, containing four of the eight stereogenic centers of<br />
leucascandrolide A (4).<br />
The vinyl ether 2 was readily prepared from the corresponding homoallylic alcohol. Condensation<br />
of 1 with 2 involved Lewis acid activation of the aldehyde, addition of the resulting carbocation to<br />
the vinyl ether, and cyclization with trapping by bromide ion. In this process, the other four of the<br />
eight stereogenic centers were assembled. Three of those centers were formed in the course of the<br />
reaction. While stereocontrol was not perfect, the route is pleasingly succinct, so practical<br />
quantities of diastereomerically pure 3 could be prepared.<br />
To complete the synthesis, the secondary alcohol of 3 was methylated. Selective desilyation of the<br />
primary alcohol followed by oxidation and desilylation then set the stage for the Mitsunobu<br />
macrolactonization. The intermediates in the Mitsunobu reaction are such that the lactonization<br />
can proceed with either inversion of absolute configuration at the secondary center, or retention.<br />
While the usually-employed Ph 3 P gave the lactone with retention of absolute configuration, Bu 3 P<br />
led to clean inversion.
The last challenge was the establishment of the (Z) alkene of the side chain. This was<br />
accomplished using the Toru protocol. Coupling of the secondary bromide with the Cs salt 12<br />
proceeded with inversion of absolute configuration, to give 13. The carboxylates of stronger acids<br />
were not sufficiently nucleophilic to displace the bromide. Aldol condensation of 13 with the<br />
aldehyde 14 gave a mixture of diastereomers, exposure of which to MsCl in pyridine delivered the<br />
requisite (Z) alkene 4.<br />
35. The Smith <strong>Synthesis</strong> of (+)-Lyconadin A<br />
The pentacyclic alkaloid (+)-lyconadin A (3), isolated from the club moss Lycopodium<br />
complanatum, showed modest in vitro cytotoxicity. A key step in the first reported (J. Am. Chem.<br />
Soc. 2007, 129, 4148.) total synthesis of 3, by Amos B. Smith III of the University of<br />
Pennsylvania, was the cyclization of 1 to 2.<br />
The pentacyclic skeleton of 3 was constructed around a central organizing piperidine ring 9. This<br />
was prepared from the known (and commercial) enantiomerically-pure lactone 4. The akylated<br />
stereogenic center of 9 was assembled by diastereoselective hydroxy methylation of the acyl<br />
oxazolidinone 5 with s-trioxane, followed by protection. Reduction of the imide to the alcohol led<br />
to the mesylate 7, which on reduction of the azide spontaneously cyclized to give, after protection,<br />
the piperidine 8. Selective desilylation of the primary alcohol then enabled the preparation of 9.
The plan was to assemble the first carbocyclic ring of 3 by intramolecular aldol condensation of<br />
the keto aldehyde 15. The enantiomerically-pure secondary methyl substituent of 15 derived from<br />
the commercial monoester 10. Activation as the acid fluoride followed by selective reduction led<br />
to the volatile lactone 11. Opening of the lactone with H 3 CONHCH . 3 HCl gave, after protection,<br />
the Weinreb amide 12. Alkylation of the derived hydrazone 13, selectively on the methyl group,<br />
led, after deprotection, to 15. The intramolecular aldol condensation of 15 did deliver the unstable<br />
cyclohexenone 1. Under the acidic conditions of the aldol condensation, the enol derived from the<br />
piperidone added in a Michael sense, from the axial direction on the newly-formed ring, to give<br />
the trans-fused bicyclic diketone 2.<br />
To move forward, it was necessary to epimerize 2 to the cis ring fusion, and also to differentiate<br />
the two ketones of 2. These two problems were solved simultaneously by deprotection and<br />
epimerization to the cis-fused hemiaminal 16.<br />
Attempts to deoxygenate the tertiary alcohol of 16 failed, so instead, selective reduction followed<br />
by protection delivered 17. Reduction to the axial alcohol followed by dehydration with the<br />
Martin sulfurane then installed the trisubstituted alkene of 18. The C-N bond was re-established<br />
by exposure of 18 to NIS, leading to the crystalline 19. Activation of the derived ketone with<br />
Mander’s reagent followed by reductive deiodination (Et 3 SiH/Pd) gave 20, Michael addition of<br />
which with 21 led to 3.<br />
36. The Betzer and Ardisson <strong>Synthesis</strong> of (+)-Discodermolide
(+)-Discodermolide (3), a potent anticancer agent that works synergistically with taxol, may yet<br />
prove to be clinically effective. For the synthetic material to be affordable, a highly convergent<br />
synthesis is required. Jean-François Betzer and Janick Ardisson of the Université de<br />
Cergy-Pontoise have described (Angew. Chem. Int. Ed. 2007, 46, 1917.) such a synthesis,<br />
coupling 1 and 2. A central feature of their approach was the repeated application of the inherently<br />
chiral secondary organometallic reagent 5.<br />
The first use of 5 was the addition to the aldehyde 4. The product 6 was ozonized, and the<br />
resulting aldehyde was carried on to the α,β-unsaturated ester. Exposure of the hydroxy ester to<br />
benzaldehyde under basic conditions delivered, by intramolecular Michael addition, the acetal 7.<br />
The next addition of the reagent 5 was to the aldehyde 10. The adduct 11 was deprotonated with<br />
t-BuLi to effect α-elimination, providing, after protection of the alcohol, the alkyne 12. Coupling<br />
of 12 with the amide 7 gave a ketone, enantioselective reduction of which under Itsuno-Corey<br />
conditions led, again after protection of the alcohol, to the alkyne 13. Oxidation followed by<br />
selective hydrogenation and iodine-tin exchange then completed the assembly of 1. Note that PtO 2 ,<br />
not typically used for partial hydrogenation, was the catalyst of choice for this congested alkyne.<br />
The third application of the enantiomerically-pure reagent 5 was addition to the aldehyde that had<br />
been prepared by ozonolysis of 15. Advantage was then taken of another property of the alkenyl<br />
carbamate, Ni-mediated Grignard coupling, to form the next carbon-carbon bond with high<br />
geometric control. Deprotection of the diene 17 so prepared followed by iodination then<br />
completed the synthesis of 2.
The convergent coupling of 1 with 2 was carried out under Suzuki conditions. Reduction of the<br />
iodide of 2 to the corresponding alkyl lithium followed by exchange with B-OMe-9-BBN gave<br />
gave an intermediate organoborane, that smoothly coupled with 1 under Pd catalysis to give 18.<br />
Deprotection and carbamate formation then led to (+)-discodermolide (3).<br />
This synthesis clearly illustrates the power of 5 as an enantiomerically-defined secondary<br />
organometallic reagent, and the synthetic versatility of the product alkenyl carbamates. The ready<br />
availability of the three enantiomerically-pure four-carbon fragments 4, 8, and 14 was also a key<br />
consideration in the design of this synthesis.<br />
37. The Maier <strong>Synthesis</strong> of Cruentaren A<br />
Cruentaren A (3), isolated from the myxobacterium Byssovorax cruenta, is an inhibitor of<br />
mitochondrial F-ATPase. The synthesis of 3 (Org. Lett. 2007, 9, 655,; Angew. Chem. Int. Ed. 2007,<br />
46, 5209.) by Martin E. Maier of the Universität Tübingen illustrates the power of alkyne<br />
metathesis as a tool for the synthesis of complex natural products. Very recently, Alois Fürstner of<br />
the Max-Planck-Institut, Mülheim, reported (Angew. Chem. Int. Ed. 2007, 46, 9275.) an<br />
alternative synthesis, also based on alkyne metathesis, of cruentaren A (3).<br />
The alcohol portion of 1 was prepared by Marshall homologation of 4 with 5, leading to 6.<br />
Homologation of the derived epoxide 7 then gave 8. Note that the homologation of 7 to 8 required<br />
three steps. This might have been accomplished more directly with the Li salt of 1-propyne, easily<br />
prepared from commercial 1- or 2-bromopropene.
Evans auxiliary-controlled homologation of 9 set the relative and absolute configuration of 10,<br />
which was carried on to 12. To effect coupling, the acid of 12 was activated with carbonyl<br />
diimidazole, then condensed with the bis-alcoholate of 8. This acylation was highly regioselective,<br />
giving 1 as the only observed product.<br />
Cruentaren A (3) has two Z alkenes, so the authors chose a bis-alkyne strategy, with a partial<br />
hydrogenation of both alkynes at the end of the synthesis. To this end, alkyne metathesis was<br />
accomplished with the Schrock tungsten carbine catalyst 13. Homologation to 15 followed by<br />
deprotection and hydrogenation then gave enantiomerically pure cruentaren A (3).<br />
38. The Sammakia <strong>Synthesis</strong> of the Macrolide RK-397
The polyene macrolide RK-397 (3), isolated from soil bacteria, has antifungal, antibacterial and<br />
anti-tumor activity. Tarek Sammakia of the University of Colorado has described (Angew. Chem.<br />
Int. Ed. 2007, 46, 1066.) the highly convergent coupling of 1 with 2, leading to 3.<br />
The preparation of 1 depended on the powerful methods that have been developed for acyclic<br />
stereocontrol. Beginning with the allylic alcohol 4, Sharpless asymmetric epoxidation established<br />
the absolute configuration of 5. Following the Jung “non-aldol aldol” protocol, exposure of 5 to<br />
TMSOTf delivered the aldehyde 6 in high de. Condensation of 6 with the lithium enolate of<br />
acetone also proceeded with high de. The resulting alcohol was protected as the MOM ether, to<br />
direct the stereoselectivity of the subsequent aldol condensation with 8. Selective β-elimination<br />
followed by reduction and protecting group exchange then gave 1.<br />
The preparation of 2 took advantage of the power of Brown asymmetric allylation. Allylation of<br />
the symmetrical 11 led to the diol 12. This was desymmetrized by selective acetonide formation,<br />
to give 13. Ozonolysis, reductive work-up, and protection of the newly-formed 1,3-diol gave 14,<br />
setting the stage for oxidation and asymmetric allylation to give 15. Reductive deprotection and<br />
oxidation then delivered the acetonide 2.<br />
The tris acetonide 16 was assembled by addition of the enolate derived from 1 to the aldehyde 2,<br />
followed by reduction and protection. Kinetically-controlled metathesis with 17 established the<br />
triene 18. Phosphonate-mediated homologation to the pentaene 19 followed by hydrolysis and<br />
Yamaguchi macrolactonization then completed the synthesis of the macrolide RK-397 (3).
39. The Clark <strong>Synthesis</strong> of Vigulariol<br />
Vigulariol (3), isolated from the octocoral Vigularia juncea (Pallas), is active against human lung<br />
adenocarcinoma cells (IC 50 = 18 nM). A key step in the synthesis of 3 reported (Angew. Chem. Int.<br />
Ed. 2007, 46, 437.) by J. Stephen Clark of the University of Glasgow was the dipolar<br />
rearrangement of 1 to 2.<br />
The plan for the diastereoselective construction of 1 was based on the known reductive cyclization<br />
of aldehydes such as 7. To this end, the alcohol 6 was prepared and condensed with ethyl<br />
propiolate. As anticipated, the SmI 2 -mediated cyclization of 7 proceeded to give 8 with high<br />
diasterocontrol. The diazo ketone 1 was prepared by reaction of the mixed anhydride (isobutyl<br />
chloroformate) of the acid with diazomethane.<br />
It was anticipated that Cu-catalyzed carbene formation would lead the 1,3-dipole 9, that would<br />
then undergo sigmatropic rearrangement to 2. In the event, the rearrangement was remarkably<br />
efficient, delivering the ten-membered ring carbocycles 10 and the desired 2 in a 1 : 5 ratio.<br />
Exposure of the less stable geometric isomer 10 to catalytic thiyl radical effected equilibration to<br />
2.
Although one might think of medium rings such as that of 2 as being floppy, in fact 2 has a single<br />
preferred conformation, and diastereocontrol for the rest of the synthesis took advantage of that<br />
preferred conformation. The diene 11 derived from 2 has one open face. Addition of methyl vinyl<br />
ketone to that outside face gave a 1 : 2 mixture of the diastereomeric endo and exo cycloadducts.<br />
The mixture was equilibrated to the more stable exo adduct 12. A four-step sequence then<br />
converted 12 into 13.<br />
The completion of the synthesis again depended on the inside-outside bias of the medium ring.<br />
Addition of methyl magnesium chloride to the outside face of the ketone derived from 13<br />
delivered 14 with high diastereocontrol. Epoxidation of 14, again from the outside face, led<br />
directly to vigulariol 3. The intermediate in the MCPBA reaction is the protonated epoxide, and it<br />
may be that the tertiary alcohol poised directly on the opposite face of the alkene opened that<br />
activated intermediate directly.<br />
This synthesis of vigulariol (3) is remarkably concise, with four rings and eight stereogenic<br />
centers being assembled in just twenty steps. All of the stereogenic centers are derived from the<br />
secondary alcohol 6. Use of the known enantiomerically-pure 6 would have delivered vigulariol (3)<br />
in enantiomerically-pure form. This synthesis design once again illustrates the power of<br />
conformational analysis of medium rings.<br />
40. The Trost <strong>Synthesis</strong> of (-)-Terpestacin<br />
(-)-Terpestacin (3), isolated from Arthrinium sp. FA1744, inhibits the formation of syncytia by<br />
HIV-infected T cells. A key step in the total synthesis of 3 reported (J. Am. Chem. Soc. 2007, 129,<br />
4540. ) by Barry M. Trost of Stanford University was the Ru-catalyzed cyclization of 1 to 2. This<br />
synthesis of (-)-terpestacin (3) elegantly illustrates the power of the Trost enantioselective Pd<br />
catalysts.
The preparation of 1 began with the commercially-available diketone 4. With the enol as the<br />
nucleophile, opening of racemic isoprene monoepoxide 5 using the Trost chiral Pd catalyst led to<br />
the ether 6 in high ee. It is impressive that even though it was directed by a quaternary stereogenic<br />
center, the subsequent Claisen rearrangement to 7 proceeded with complete facial selectivity.<br />
Oxidation to the nonenolizable α-diketone 8 then set the stage for the conjugate addition. Again,<br />
even though the directing stereogenic center was quaternary, the addition proceeded with<br />
substantial diastereocontrol. Further study of the elements that govern the facial selectivity of the<br />
Lewis acid-mediated (Sakurai) addition of allyl silanes to enones would certainly be warranted.<br />
The sulfone 11 was prepared in four steps from commericially-available geranyl bromide, with the<br />
absolute configuration being set by Sharpless asymmetric epoxidation. Subsequent to the<br />
alkylation of 11 with the bromide 10, the now-surplus sulfone was removed reductively with<br />
Pd(OAc) 2 and NaBH 4 . It is noteworthy that the enone of 1 was stable to those conditions.<br />
The cyclization of 1 with the second-generation Grubbs catalyst delivered the desired E alkene 2<br />
in 43% yield. As had been observed before by others, the free allylic alcohol was the preferred<br />
substrate for the Ru metathesis catalyst. No cyclization was observed with tetraenes that had the<br />
alcohol protected.<br />
With 2 in hand, it remained to install the side chain. The relative configuration of the pentenyl side<br />
chain of 14 was set by again using chiral Pd catalysis. The Claisen rearrangement proceeded<br />
smoothly, to give, after protection, the ether 15.
The oxidative cleavage of the side chain of 15 initially presented some difficulties. Eventually, it<br />
was found that AD-Mix-α (but not β) could effect selective dihydroxylation. Periodate cleavage<br />
followed by reduction then gave 16, which was deprotected to (-)-terpestacin (3).<br />
Note that both 5 and 13 were racemic. Except for the secondary alcohol on the fifteen-membered<br />
ring, the absolute configuration of every stereogenic center in the (-)-terpestacin (3) prepared by<br />
this synthesis derived from the absolute configuration of the enantiomerically-pure Pd complexes<br />
used as first 5 and then 13 were incorporated selectively into the natural product.<br />
41. The Nakada <strong>Synthesis</strong> of (+)-Digitoxigenin<br />
The total synthesis of steroids such as (+)-digitoxigenin (3) has been studied for more than sixty<br />
years, yet it has never been thought that such studies would lead to a preparative route that would<br />
be competitive with partial synthesis from the abundant plant sterols. The enantioselective<br />
synthesis of 3 recently (Tetrahedron Lett. 2007, 48, 1541.) described by Masahisa Nakada of<br />
Waseda University suggests that the preparative synthesis of even such complex polycarbocycles<br />
may in fact be practical. A key step in the synthesis of 3 was the conjugate addition of the cuprate<br />
derived from 1 to the enone 2.<br />
For this convergent approach to be effective, both 1 and 2 had to first be prepared in<br />
enantiomerically-pure form. The synthesis of 1 started with the bakers’ yeast reduction of the<br />
prochiral diketone 4 to give 5 in high ee. The key problem was the establishment of the ternary<br />
center of 1. This was accomplished by coupling the iodide 8 with the boronic acid 9.<br />
Hydroxyl-directed hydrogenation of 10 then led to the desired 1.
The preparation of 2 began with Cu-catalyzed cyclization of the prochiral diene 12 to give the<br />
crystalline 13. Recrystallization raised the ee of 13 to > 99%. Reductive opening of 13 gave 14,<br />
which was reduced and protected to give 15. Oxidation of 15 with the Ochiai reagent 16 provided<br />
the enone 2. Conjugate addition of 1 proceeded across the more open face of the enone 2, to give<br />
17.<br />
The key step to close the B ring of the steroid was the intramolecular aldol condensation of 18 to<br />
give 19. The β-hydroxy ketone so produced was too sensitive to reduce by direct methods, so the<br />
monoxanthate was prepared from the derived diol, and then reduced under free radical conditions,<br />
leading to the diol 20. Singlet oxygenation of 20 then completed the synthesis of 3.<br />
Clearly, the strength of this synthesis is the elegant construction of the AB enone 2. Even more<br />
important is the demonstration of a general convergent route to the steroids, by addition of an<br />
enantiomerically-pure D ring moiety to an AB fragment. The power of asymmetric synthesis<br />
makes the precursors 1 and 2 readily available in the necessary enantiomerically-pure form.
42. The Pettus <strong>Synthesis</strong> of (+)-Rishirilide B<br />
(+)-Rishirilide (4), an inhibitor of α 2 -macroglobulin, a tetrameric serum glycoprotein that is an<br />
irreversible protease inhibitor, has a deceptively simple structure. Thomas R. R. Pettus of the<br />
University of California at Santa Barbara has reported (J. Am. Chem. Soc. 2006, 128, 15625.) a<br />
concise route to 4, based on the highly regioselective Diels-Alder addition of the<br />
enantiomerically-pure dienophile 2 to the diene derived from 1.<br />
The preparation of 2 was carried out from the aldehyde 5. Selective protection of the less hindered<br />
phenol followed by reduction gave the benzyl alcohol 6. On exposure to excess Grignard reagent 7,<br />
the primary alcohol of 6 apparently underwent elimination to give the o-quinone methide 8,<br />
conjugate addition to which gave 9. Mitsunobu coupling of 9 with the lactic acid amide 10<br />
proceeded with clean inversion, to give 11. The methoxy methyl amide of 11 was required for the<br />
oxidative dearomatization to 2 to proceed efficiently.<br />
The preparation of the masked diene 1 started with the aldehyde 12. Following the Comins<br />
procedure, metalation of the derived hemiaminal alkoxide and subsequent addition of methyl<br />
iodide gave 13. Addition of SO 2 to the photoenol derived from 13 followed by etherification of the<br />
labile secondary alcohol so formed then gave 1.<br />
The dienophile 2 should undergo cycloaddition with high facial selectivity. That is not relevant in<br />
this application, as addition to the intermediate quinone methide 14 was followed by elimination<br />
and oxidative aromatization. The regioselectivity of the addition, delivering 2, was, however,<br />
critical for the success of the synthesis.
With 3 in hand, what remained was a one-carbon homologation. This was accomplished by<br />
selective O-acylation with dimethyl carbamoyl chloride 16. Subsequent addition of an excess of<br />
anion 17 led, via alkoxide-directed addition to the remaining ketone carbonyl followed by<br />
deacylation, to the desired adduct 18. Careful cleavage, to avoid lactone formation, then delivered<br />
4. Note that this synthesis of (+)-rishirilide B (4) would be classified as enantioselective, since the<br />
intial stereogenic center of 2, that set the absolute configuration of the dearomatized ring of 2, was<br />
not included in the final product.<br />
43. The Gin <strong>Synthesis</strong> of Nominine<br />
Retrosynthetic analysis of the heptacyclic ring structure of nominine (3), a representative member<br />
of the hetisine family of alkaloids, is not for the faint of heart. David Y. Gin, now at the<br />
Sloan-Kettering Institute, has presented (J. Am. Chem. Soc. 2006, 128, 8734.) an elegant solution<br />
to this problem. One key step in the synthesis was the intramolecular Diels-Alder cyclization of 1<br />
to 2.<br />
The convergent construction of 1 by dipolar cycloaddition presented some interesting challenges.<br />
One half was prepared from 3-methylcyclohexenone (4). Addition of Et 2 AlCN followed by<br />
triflation gave 5 in regiocontrolled fashion. Reduction followed by Pd-catalyzed coupling of the<br />
enol triflate then led to the aldehyde 6. An intriguing question is how one would prepare 6 in<br />
enantiomerically pure form.
The other half of 1 was prepared from the acetal 7. Lateral metalation followed by acylation with<br />
the Weinreb amide 8 gave the ketone 9. Displacement with azide followed by exposure to acid<br />
gave the bridged acetal 10, which was condensed reductively with 6 to give the oxidopyridinium<br />
betaine 12.<br />
Two products, 13 and 14, could arise from the dipolar cycloaddition of 12. It was not clear at the<br />
outset which would be preferred. In the event, it did not matter. Even though the thermal<br />
equilbrium favored the undesired 13, equilibration was efficient, and the two were easily<br />
separated.<br />
With 14 in hand, the stage was set for the intramolecular Diels-Alder cyclization. In fact, the diene<br />
1 was not isolated. The evidence for the intermediacy of 1 was the observation that on exposure to<br />
pyrollidine in methanol at 60°C, the Birch reduction product 17 smoothly cyclized to 2.<br />
Methylenation followed by kinetic, axial-selective SeO 2 oxidation then completed the synthesis of<br />
3.<br />
The concise elegance of this 17-step synthesis of the heptacyclic hetesine alkaloid nominine 3 is<br />
all the more apparent when it is compared with the only previously-reported synthesis, published<br />
just two years earlier, which took 40 steps
44. The Padwa <strong>Synthesis</strong> of Asphidophytidine<br />
Albert Padwa of Emory University has developed (Org. Lett. 2006, 8, 3275.) a productive<br />
approach to fused indole alkaloids such as asphidophytine (3), based on the dipolar cycloaddition<br />
of the ylide derived by exposure of a diazo ketones such as 1 to a Rh(II) carboxylate catalyst.<br />
The preparation of 1 started with the aniline 4. Ortho iodination followed by N-alkylation with 5<br />
delivered the unsaturated ester 6. Heck cyclization no doubt intially left the alkene still conjugated<br />
with the ester, but traces of acid or base would be expected to easily isomerize this to establish the<br />
aromaticity of the five-membered indole ring. N-methylation followed by saponification then gave<br />
8.<br />
The preparation of 1 continued with the alkylation of 9. Hydrolysis of 10 followed by<br />
homologation and subsequent diazo transfer gave 11, which was coupled with 8 to give 1. The<br />
quaternary center established in the alkylation of 9 was carried through the synthesis, so if<br />
enantiomerically-pure material were desired, an enantioselective route to 10 would have to be<br />
devised.<br />
The push-pull dipole 12 was constructed by exposure of 1 to Rh 2 (OAc) 4 . Loss of N 2 gave the Rh<br />
carbene, which complexed with the nucleophilic amide carbonyl. The dipole 12 was not isolated,<br />
but reacted in situ with the tethered indole to give the hexacyclic adduct 2. Note that two<br />
diastereomers of 2 could have been formed, but only 2, with the bulky t-butyl ester exo, was<br />
observed.
The dipolar cycloadddition established the requisite stereochemical relationship between the three<br />
contiguous quaternary stereogenic centers of 1. It remained to adjust the functional groups around<br />
the newly-formed carbocyclic ring. Exposure to BF 3 .OEt 2 led to ring opening, followed by<br />
trapping of the intermediate carbocation with the t-butyl ester to give the lactone 13, with<br />
concommitant loss of isobutylene. Hydrolysis and decarboxylation gave the alcohol 14, the acetate<br />
of which was removed by reduction with SmI 2 . The derived enol triflate 15 was reduced to the<br />
alkene, which was deoxygenated by way of the thiolactam 16.<br />
The intramolecular dipolar cycloaddition exemplified by the conversion of 12 to 2 is a specific<br />
representative of a general and powerful approach to indole alkaloids, based on cycloaddition of<br />
an intermediate indole to a dipole or a diene. For more recent work along these lines by Professor<br />
Padwa, see Org. Lett. 2007, 9, 279, and Tetrahedron 2007, 63, 5962,<br />
45. The Overman <strong>Synthesis</strong> of (-)-Sarain A<br />
Larry E. Overman of the University of California, Irvine has recently (Angew. Chem. Int. Ed. 2006,<br />
45, 2912. ) described the first total synthesis of the antibiotic and antitumor alkaloid (-)-sarain A<br />
(3). The structure of 3 is particularly challenging, with two tertiary amines, one of them attached<br />
to a quaternary center. Note that although sarain A is drawn as the amino aldehyde, in fact the<br />
aldehyde and the proximal amine interact to form zwitterionic species that are difficult to purify.<br />
A key transformation in the assembly of 3 was the intramolecular Mannich cyclization of 1 to 2.<br />
This C-C bond forming reaction established the single carbocyclic ring of 3, while at the same<br />
time constructing with high diastereocontrol the tetraalkylated quaternary center.<br />
The enantiospecific preparation of 1 began with diethyl tartrate 4. The enolate of the derived<br />
oxazoline 5 added with high diastereocontrol to the Z-α,β-unsaturated ester 6 to give 7. The diester<br />
7 included the three continguous stereogenic centers of 8. The details of the conversion of 7 to the<br />
hemiaminal 8 had previously been reported by Professor Overman (J. Org. Chem. 1998, 63,<br />
8096, ; Tetrahedron Lett. 1999, 40, 1273, ; Org. Lett. 2005, 7, 933, ).
The aldehyde 1 was in fact not isolated. Rather, the intramolecular Mannich condensation was<br />
developed using the silyl enol ether 8 as the starting material. It is not clear whether the striking<br />
diastereocontrol observed is steric in origin, with the small aldehyde tucked into the more<br />
incumbered space, or if there is an attractive interaction between the aldehyde carbonyl and the<br />
polar carbamate. In principle, the Mannich condensation is reversible, so the reaction may be<br />
under thermodynamic, not kinetic, control.<br />
Desulfonylation of 2 followed by reductive alkylation delivered 9, setting the stage for<br />
Ru-mediated (G1) closure of the first macrocyclic ring. Note that from 9 on, a basic tertiary amine<br />
was carried through the remainder of the synthesis, and from 11 on, each intermediate had two<br />
basic amines.<br />
To close the final ring, the amino diol 11 was protected as the hemiaminal, then oxidized to 13.<br />
Diastereoselective (~3-4 : 1) Grignard addition followed by homologation then gave the precursor<br />
15 for Stille coupling to close the last ring. Reduction of the hemiaminal, oxidation with<br />
bicarbonate-buffered Dess-Martin reagent to the aldehyde (zwitterionic!) and a delicate<br />
deprotection then completed the synthesis of (-)-sarain A (3).<br />
46. The Fukuyama <strong>Synthesis</strong> of Morphine
Tohru Fukuyama of the University of Tokyo has recently reported (Org. Lett. 2006, 8, 5311. ) an<br />
elegantly conceived total synthesis of (±)-morphine (3), based on the recognition and reduction to<br />
practice of the highly diastereoselective intramolecular Mannich cyclization of 1 to 2.<br />
The stereocontrolled preparation of 1, a considerable challenge, started with the epoxidation of the<br />
diene 4 to give 6. Although racemic 6 was used for this synthesis of morphine, Professor<br />
Fukuyama had previously demonstrated that high ee 6 could be prepared by lipase resolution of<br />
the intermediate bromohydrin 5. Coupling of 6 with 7 led to 8, by net syn S N 2' displacement. The<br />
secondary alcohol was then inverted, to put the α-H syn to what would be the adjacent C-Pd bond,<br />
to undergo β-hydride elimination in the course of the intramolecular Heck cyclization of 10 to 2.<br />
The intramolecular Mannich cyclization of 1 to 2 takes advantage of the native functionality of<br />
morphine 3. The intermediate in the cyclization is apparently the expected 11, which could be<br />
isolated. Note that under the equilibrating conditions of the cyclization, the desired cis ring fused 2<br />
was the only diastereomer observed.<br />
The Ito-Saegusa procedure was used to oxidize the ketone 2 to the enone 12. It was then necessary<br />
to convert the enone into the allylically-inverted alcohol 14. With the morphine skeleton<br />
assembled, the aromatic ring blocks the bottom face of the enone, so both hydrogen peroxide and<br />
hydride attacked from the top face, to give 13. Conversion of the derived thiocarbamate to the free<br />
radical then led to epoxide cleavage, delivering the desired 14. The last three steps to complete the<br />
total synthesis of (±)-morphine (3), oxidation, addition of hydride to the open top face of the<br />
ketone with concomitant reduction of the urethane protecting group to the N-methyl, and<br />
O-demethylation, then followed literature procedures.
47. The Nicolaou <strong>Synthesis</strong> of Platensimycin<br />
The report last year by scientists at Merck of an antibiotic, platensimycin 3, with a novel<br />
mechanism of action has led to much effort toward the total synthesis of this degraded diterpene.<br />
K. C. Nicolaou of Scripps, La Jolla has now (Angew. Chem. Int. Ed. 2006, 45, 7086.) reported the<br />
first preparation of 3. The key step in the synthesis is the elegantly concise cyclization of 1 to 2.<br />
The preparation of 1 started with iterative Stork-Danheiser alkylation of 4 to give 5. Reduction<br />
followed by hydrolysis unraveled the enol ether to give the enone, which was re-silylated to give 6.<br />
Ru-catalyzed intramolecular ene cyclization of 6 gave the enol ether 7, which was selectively<br />
oxidized to 1. Reductive cyclization of 1 gave 2 as a 2:1 mixture with its diastereomer 8.<br />
The aniline of platensimycin was prepared from 2-nitroresorcinol (9). MOM ether formation<br />
followed by reduction gave 10. Metalation of the protected amine followed by acylation with<br />
Mander’s reagent and thermolysis gave 11.
The cyclization of 2 to 11 proceeded smoothly, as did the alkylation of 11 to 12. The<br />
diastereoselectivity of the alkylation followed from the conformational bias of the ring system.<br />
The conversion of the terminal alkene to the acid 13 initially was troublesome, but a solution was<br />
found in metathesis with vinyl boronate followed by oxidation. Coupling with 11 followed by<br />
deprotection then gave 3.<br />
The absolute configuration of the tricyclic core of 3 was set in the intramolecular ene cyclization<br />
of 6 to 7. There is the possibility that with an enantiomerically-pure catalyst, the product 7 and so<br />
3 could be prepared in high enantiomeric excess.<br />
<strong>Total</strong> syntheses of platensimycin 3 are underway in several other research groups. The diversity of<br />
the approaches being explored will enrich organic synthesis.<br />
48. The Sorensen <strong>Synthesis</strong> of (-)-Guanacastepene E<br />
The guanacastepenes, of which guanacastepene E (3) is representative, initially elicited excitement<br />
because of their activity against drug resistant bacterial strains. Although the systemic toxicity of<br />
the guanacastepenes has cooled that enthusiasm, the guanacastepenes remain as architectural<br />
challenges. In particular, a convergent synthesis plan requires the development of a strategy for<br />
constructing the central 7-membered ring with control of relative and absolute configuration,<br />
particularly of the two angularly methylated quaternary centers. Erik J. Sorensen of Princeton<br />
University (J. Am. Chem. Soc. 2006, 128, 7025.) solved this problem by the intramolecular<br />
photochemical dimerization of 1, which delivered 2 with high diastereocontrol. Directed ring<br />
fragmentation then led to 3.
The enantiomercially-pure cyclopentenone of 1 was prepared from the chiral pool starting material<br />
dihydrocarvone (4). Methylation followed by ozonolytic cleavage gave the aldehyde acid 5, which<br />
was converted into the cyanohydrin lactone 6. Intramolecular acylation of the derived nitrile anion<br />
proceeded smoothly, to give, after fragmentation, the 1,2-diketone 7. Stannylation of the nonaflate<br />
led to 8, which was coupled with enantiomerically-pure 9 to give 1.<br />
Construction of the cyclohexene 9 began with the Diels-Alder cycloaddition of the alkyne 11 to<br />
the diene 10, followed by Baeyer-Villiger cleavage to give 13. This established the quaternary<br />
center, and at the same time set the relative configuration of the secondary alcohol of 9, and thus<br />
of 3. The alcohol 15 so prepared was racemic. Screening of esters led to the mandelate acetate 9,<br />
the diastereomers of which could be separated by open column chromatography. The mandelate<br />
ester also proved to be an effective leaving group for the Pd-mediated coupling of 8 and 9 to give<br />
1.<br />
As anticipated, the intramolecular 2+2 photocyclization was directed by the adjacent isopropyl<br />
group, to give 2 with high diastereocontrol. While opening of carbonyl-activated fused<br />
cyclopropanes is amply precedented, far less was known about the opening of carbonyl-activated<br />
fused cyclobutanes. Happily, double one-electron reduction with excess SmI 2 opened the desired<br />
bond, to give, after selenation and oxidation, the dienenone 16. The acetoxy group adjacent to the<br />
ketone was introduced by Rubottom oxidation. On deprotection of 17, the released primary<br />
alcohol spontaneously added to the enone, to give (-)-guanacastepene E (3) .
49. The Crimmins <strong>Synthesis</strong> of (+)-SCH 351448<br />
The symmetrical macrodiolide (+)-SCH 351448 (4) is the only known selective activator of<br />
transcription from the low density lipoprotein receptor. The highly convergent synthesis of 4 from<br />
1 and 2 reported (Org. Lett. 2006, 8, 2887. ) by Michael T. Crimmins of the University of North<br />
Carolina illustrates the power of the chiral glycidyl anion approach for the preparation of α,α'-bis<br />
chiral ethers.<br />
The upper half of 1 was assembled by Sharpless resolution of the alcohol 5. Allylation of the<br />
enolate derived from 6 then delivered, after reduction and methylenation, the triene 7. The lower<br />
half of 1 was prepared by condensation of the anion derived from 8 with acrolein 9. Methylation<br />
of product 10 led to 11, which was again allylated, to give, after homologation, the aldehyde 12.<br />
Condensation with a chiral acetate equivalent gave 13. The corresponding aldehyde 14 was then<br />
added to the enolate of 15, derived from 7, to arrive at 1.
Although the tandem ring closing metathesis - homologation of 1 with 2 proceeded spectacularly<br />
well, to give 3 in 88% yield, simple dimerization of 3 did not deliver the desired 4. As an<br />
alternative, 15 was cyclized with G1, then acylated with reduced 3 to give 16. Intramolecular<br />
acylation of 17 then worked well, leading to 4.<br />
50. <strong>Synthesis</strong> of (-)-Colombiasin A and (-)-Elisapterosin B<br />
Historically, there have been three methods for assembling enantiomerically-pure carbocycles:<br />
cyclization of an enantiomerically-pure starting material, enantioselective cyclization of a<br />
prochiral substrate, and enantioselective transformation of a prochiral cyclic starting material.<br />
Recently, a fourth strategy has been developed, enantioselective transformation of a racemic<br />
starting material, with only one enantiomer of the starting material going on to the desired product.<br />
This approach is illustrated by the transformation of racemic 1 to enantiomerically (and<br />
diastereomerically) pure 3. The conversion of 1 to 3 is the key step in a general route established<br />
(J. Am. Chem. Soc. 2006, 128, 2485.) by Huw M. L. Davies of the University at Buffalo to the<br />
diterpenes isolated from the gorgonian coral Pseudoterogoria elisabethae, exemplified by<br />
(-)-colombiasin A (4).
The dihydronaphthalene 1 was prepared by Diels-Alder cycloaddition of the diene 5 to the<br />
benzoquinone 6, the preparation of which had been reported by Nicolaou in the course of a<br />
previous synthesis of (-)-colombiasin A (4). Silylation of the cycloadduct followed by selective<br />
hydrolysis gave the ketone 7. The ketone was converted to the enol triflate 8, which was reduced<br />
with triethylsilane to give racemic 1.<br />
Exposure of 1 to 2 in the presence of the DOSP Rh catalyst, designed by Davies, delivered the two<br />
adducts 8 and 9. These were carried together to the alcohols 10 and 11, which were separated. The<br />
overall yield of diasteromerically- and enantiomerically-pure 11 from racemic 1 was 34%. This<br />
was 68% of theoretical, since was derived from only one of the two enantiomers of 1.<br />
Homologation of 11 to the diene followed by desilylation under oxidative conditions led to the<br />
quinone 12. Followed the precedent of Rychnovsky, on heating 12 was converted to the<br />
Diels-Alder adduct 13. Demethylation then gave (-)-colombiasin A (4).<br />
The tetracyclic triketone (-)-elisapterosin B (14), also a Pseudoterogoria elisabethae diterpene, is<br />
the intramolecular [5 + 2] cycloaddition product from 12. Again following a Rychnovsky
procedure, exposure of the diene 12 to BF 3 etherate led directly to 14, accompanied by minor<br />
amounts of the methyl ether 13.<br />
51. The Ready <strong>Synthesis</strong> of (-)-Nigellamine A 2<br />
The nigellamine alkaloids, represented by (-)-nigellamine (3), dolabellane diterpenes isolated from<br />
black cumin, apparently have lipid metabolism promoting activity. Joseph M. Ready of UT<br />
Southwestern Medical Center has described (J. Am. Chem. Soc. 2006, 128, 7428.) the first<br />
synthesis of a nigellamine, (-)-nigellamine A 2 (3). Key steps in the synthesis include an<br />
enantioselective cyclization to prepare 1, and the Cr-mediated cyclization of the aldehyde 2.<br />
(-)-Nigellamine A 2 (3) has an angularly substituted trans ring fusion. The key to the synthesis was<br />
the preparation of the angularly-substituted cis-fused lactone 1. The absolute configuration of 1<br />
was set by the Pd-mediated S N 2' cyclization of the malonate 6, which proceeded in 95% ee.<br />
Equally important was the seemingly mundane iodine-mediated cyclization of 7 to 8. This one<br />
transformation differentiated the two esters of 6, secured the relative configuration of one of the<br />
two secondary alcohols of 3, and established the requisite cis ring fusion of the lactone 1.<br />
Nucleophilic displacement of the iodide 8 failed, but two-carbon homologation to the alkyne could<br />
be accomplished by the free radical Fuchs procedure.<br />
There were intial difficulties with the Negishi methylation/iodination of the terminal alkyne, as the<br />
Cp 2 ZrCl 2 reacted preferentially with the lactol. Remarkably, repeating the reaction in the presence
of water led to clean addition to the alkyne. Homologation followed by, again, wet Negishi<br />
methylation/iodination set the stage for oxidation to the lactone aldehyde 2.<br />
Both the bicyclic skeleton of 2 and the geometry of the two alkenes direct the pendant chain<br />
toward the aldehyde. In fact, Ni-catalyzed cyclization proceeded smoothly. The product alcohol<br />
emerged as a single diastereomer, but unfortunately the wrong one, so an oxidation/reduction<br />
cycle was required to correct the secondary alcohol center.<br />
The final challenge was the selective epoxidation of the triene 12. There are two concerns: facial<br />
selectivity, and chemoselectivity. The facial selectivity is inherent, as the geometry of the medium<br />
ring is such that only the desired face is exposed. Chemoselectivity was more challenging.<br />
MCPBA reacted indiscriminantly with each of the three alkenes. Reasoning that a bulkier<br />
epoxidizing agent might be more selective, they found that the Shi dioxirane (13) delivered a 7:1<br />
ratio of the two trisubstituted epoxides. It is interesting that the enantiomer of 13 gave only a 2:1<br />
ratio.<br />
52. The Leighton <strong>Synthesis</strong> of Dolabelide D<br />
The macrolides dolabelides A-D, isolated from the sea hare Dolabella, are cytotoxic against<br />
HeLa-S3 cells at concentrations of 1.3 - 6.3 μg/mL. The recent (J. Am. Chem. Soc. 2006, 128,<br />
2796. ) synthesis of dolabelide D (3) by James L. Leighton of Columbia University nicely<br />
highlights the powerful reagent-based methods for acyclic stereoselection that they have recently<br />
developed.
Two such reagents, easily prepared on a large scale, are the amino silanes 4 and 5. These were<br />
used to prepare 8 and 12, which were combined to prepare 1. Homologation of the aldehyde 6<br />
with 4 gave 7. Protection followed by Wacker oxidation then delivered 8. To prepare 12,<br />
methacrolein 9 was homologated with ent-5. Hydroformylation followed by in situ acetal<br />
formation gave 11. Diastereroselective hydroboration followed by oxidation and esterification<br />
then led to 12. Aldol condensation of 12 with 8, taking advantage of the inherent chirality of the<br />
alkoxy enolate, proceeded to give, after protection, a 10:1 ratio of 13 and its diastereomer.<br />
The preparation of alkene 2 depended on a third reagent the Leighton group has developed, the<br />
silane 15. Enantioselective condensation of 14 with 15 set the absolute configuration around Si. In<br />
the next step, intramolecular carbonylative silylation followed by intramolecular crotylation of the<br />
transient aldeyde, the absolute configuration at the Si in 16 directed the new stereogenic centers of<br />
17. To achieve the requisite diastereoselectivity in the aldol condensation of the derived ketone 18<br />
with the aldehyde 19, the enolate of 18 was prepared using the chiral director 20.<br />
To complete the synthesis, it was necessary to form the lactone between 1 and 2, and also to effect<br />
alkene metathesis. The esterification proceeded smoothly, to give, after functional group
deprotection, the linear precursor 20. Alkene metathesis was efficient, but proceeded with little<br />
geometric control. It would have been interesting to know what influence the several protecting<br />
groups might have had on the geometry of the metathesis step.<br />
53. <strong>Synthesis</strong> and Absolute Stereochemical Assignment of (-)-Galbulimima Alkaloid 13<br />
The galbulimima alkaloids have intriguing physiological activity, but the absolute configuration of<br />
glabulimima alkaloid 13 (3) had not been assigned. Mohammad Movassaghi of the Massachusetts<br />
Institute of Technology has developed (J. Am. Chem. Soc. 2006, 128, 8126.) an elegant route to 3,<br />
based on the convergent coupling of racemic 1 with the enantiomerically-pure 2 (for a racemic<br />
synthesis of 3:).<br />
The enantiomerically-pure iodide 4 was prepared from L-alaninol. Conjugate addition of the<br />
derived radical to methyl vinyl ketone gave 5, which was hydrolyzed to 2.<br />
The Diels-Alder substrate 11 was prepared from the 1,1-dibromo alkene 6. Pd-mediated coupling<br />
of 6 with 7 gave 8. Further coupling with 9 followed by oxidation and enol ether formation gave<br />
10, which underwent smooth cross-coupling with acrolein, yielding 11. The anticipated<br />
intramolecular Diels-Alder cycloaddition led to (racemic) 1 with the expected high<br />
diastereocontrol.
The enantiomerically-pure imine 2 was readily deprotonated, and the resulting anion was<br />
condensed with the aldehyde 1 to give, after dehydration, the imine 12. The enol ether was<br />
selectively brominated. Free radical reduction led to smooth cyclization, to give 13. On unmasking<br />
of the ketone, spontaneous enamine addition ensued, to give the imine 14. Stereocontrolled<br />
reduction with NaBH 4 and protection then gave the pentacylic 15, accompanied by the<br />
diastereomer resulting from condensation of 2 with the other enantiomer of 1. These two<br />
diastereomers were readily separable by flash chromatography.<br />
To complete the synthesis, the enamide 15 was hydrolyzed by aqueous acid. In situ reduction<br />
carried the liberated ketone on to the enone, which was deprotected to give glabulimima alkaloid<br />
13 (3). Both enantiomers of 3 were prepared this way, beginning with each of the enantiomers of 4.<br />
The enantiomer derived from L-alaninol gave the same rotation as the natural product, allowing<br />
the assignment of the absolute configuration.<br />
The convergent coupling employed here allowed the ready preparation of each of the four<br />
enantiomerically-pure diastereomers of the natural product. There are real advantages to such an<br />
approach, because it provides not just one substance, the natural product, but each of the four, for<br />
further evaluation of physiological activity.<br />
54. The Corey Route to the Dolabellanes: Isoedunol and β-Araneosene
A variety of dolabellanes, some of which show substantial physiological activity, have been<br />
isolated from natural sources. E. J. Corey of Harvard University has introduced (J. Am. Chem. Soc.<br />
2005, 127, 13813. ) a unfied approach to the dolabellanes, represented by isoedunol (3), based on<br />
the designed rearrangement of the mesylate 1 to 2.<br />
The key to this approach was the stereocontrolled construction of the cyclobutane 1. The starting<br />
material was the racemic iodo acetonide 4 derived from farnesol. Alkylation of 5 using the<br />
Seebach protocol followed by hydrolysis led the methylthiomethyl ether 7. The ester was<br />
converted to the hydroxy cyclopropane 8 by the Kulinkovic procedure. On activation with Me 3 Al,<br />
8 was smoothly carried on to the enantiomerically-pure cyclobutanone 9. The ring expansion must<br />
not be proceeding by full ionization, as carbocation formation would have led to the racemic<br />
product. The aldehyde derived from 9 was cyclized with SmI 2 to the trans diol 10.<br />
In medium ring derivatives such as 10, one substituent on a ring carbon will be inside, and the<br />
other will be outside. The conformation of 10 is such that formation of the mesylate from the<br />
secondary alcohol led to migration of the more substituted cyclobutane bond, delivering 11. It<br />
follows that the conformation of the diol 12 will be flipped, to keep the OH outside the ring.<br />
Formation of the mesylate from the secondary alcohol of 12 led cleanly to migration of the less<br />
substituted cyclobutane bond, to give the desired cyclopentanone 2.
Addition of 2-propenyl lithium to the cyclopentanone 2 gave the dolebellane isoedunol (3).<br />
Deoxygenation converted 3 to the β-araneosene ( dolabellane13). This strategy for the<br />
construction of the dolabellanes may open a route for the preparation of the cytotoxic dolabellanes<br />
clavulactone (14) and clavirolide (15).<br />
55. Adventures in Complex Indole <strong>Synthesis</strong>: (-)-Fischerindole I, (+)-Fischerindole G and<br />
(+)-Weltwitindolinone A<br />
Phil S. Baran of Scripps La Jolla has described (J. Am. Chem. Soc. 2005, 127, 15394.) an elegant<br />
entry to the complex indole-derived natural products (-)-fischerindole G (1), (+)-fischerindole I (2)<br />
and (+)-weltwitindolinone A (3).<br />
The starting point for the preparation of 1 and 2 was the epoxide 4 of R-carvone. Enolization<br />
followed by opening with vinyl magnesium bromide 5 delivered the alcohol 6. The yield of this<br />
reaction was modest, but it directly provided 6, with all of the non-indole carbon skeleton of 1,<br />
suitably oxidized, in enantiomerically-pure form.
Remarkably, chlorination of the hindered secondary cyclohexyl alcohol proceeded cleanly, to give<br />
7. Oxidative condensation of the enolate of 7 with indole 8 gave the key intermediate 9. After<br />
some experimentation, it was found that the clay Montmorillonite K-10 would effect the<br />
cyclization to 10. There is not yet a general reductive amination procedure for converting a<br />
cyclohexanone to the equatorial amine, so 10 was reduced to the axial alcohol, which was<br />
converted to the equatorial azide. Selective reduction then delivered the amine 11, the formamide<br />
of which was dehydrated to give 1. The alkaloid 1 so prepared was the enantiomer of the natural<br />
product.<br />
It was not possible to dehydrogenate 1 or the precursor formamide to 2. The ketone 10 was<br />
therefore reduced selectively to the axial amine 12. Exposure of the derived formamide to<br />
t-BuOCl followed by silica gel and Et 3 N installed the desired double bond. Dehydration with the<br />
Burgess reagent then gave 2.<br />
With the absolute configuration of the series established by the preparation of 1, the enantiomer of<br />
the alkaloid 2 was prepared from S-carvone. Both R-carvone and S-carvone are inexpensive and<br />
available in bulk. Exposure of the 2 so prepared to t-BuOCl followed by trifluoroacetic acid<br />
induced oxidative rearrangement to (+)-weltwitindoline A (3), identical, including sign of rotation,<br />
with the natural product.<br />
These three syntheses, which remarkably proceed without resort to functional group protection,<br />
underline the power of the oxidative indole coupling illustrated by the union of 7 and 8 to give 9.<br />
The authors suggest that the facile conversion of 12 to 2, contrasted to the difficulties encountered<br />
in attempting to prepare 2 from 11, could indicate that it is in fact the axial amine 12 or a<br />
derivative that is the biosynthetic precursor to 2.<br />
56. <strong>Synthesis</strong> of Erythronolide A<br />
Erythronolide A (4), with its array of ten stereogenic centers, is the parent of several classic<br />
antibiotics, including erythromycin. The key step in the total synthesis of 4 recently reported<br />
(Angew. Chem. Int. Ed. 2005, 44, 4036. ) by Erick M. Carreira of the ETH Hönggerberg is the<br />
activation of 1 and subsequent diastereoselective 1,3-dipolar cycloaddition to 2 to give 3.
The synthesis of 1 started with two simple chirons, the alcohol 2, prepared by Noyori reduction of<br />
the acetylenic ketone followed by semi-hydrogenation, and the inexpensive (< one USD/gram)<br />
Roche alcohol (5). Functional group manipulation led to 6, which underwent smooth Mg-mediated<br />
cycloaddition to the enantiomerically-pure alcohol 2, to give 7. Addition of the Grignard reagent 8,<br />
also derived from the Roche ester (5), to the derived methyl ketone also proceeded with high<br />
diastereocontrol, to give the tertiary alcohol. Reduction of 9 and subsequent hydrolysis liberated<br />
the hydroxy ketone, which was reduced selectively to the syn diol. It is a tribute to the stability of<br />
tertiary triethylsilyl ethers that the TES protecting group put on at this stage survived all the way<br />
to the end of the synthesis.<br />
While the diastereoselectivity of 1,3-dipolar cycloaddition was well-precedented in simpler<br />
systems, it was not clear that the diastereoselectivity would be maintained with such a complex<br />
substrate as 1. In fact, addition to 2 proceeded with > 99:1 dr. Wittig homologation of the derived<br />
methyl ketone proceeded with remarkable (33:1) geometric control, setting the stage for<br />
asymmetric dihydroxylation to put in place the last two stereocenters.
Controlled desilylation of 11 followed by selective oxidation delivered the seco acid 12. It had<br />
previously been shown by others that some cyclic protecting groups facilitate macrolactone<br />
formation, while others do not. Fortunately, the two cyclic protecting groups of 12 served well,<br />
and the macrolactonization proceeded efficiently. Protecting group removal and reductive<br />
unmasking of the hydroxy ketone then delivered erythronolide A (4).<br />
Overall, this elegant synthesis is a showcase for the iterative use of the 1,3-dipolar cycloaddition<br />
of enantiomercially-pure allylic alcohols for the preparation of extended arrays of acyclic<br />
stereogenic centers.<br />
57. The Boger Route to (-)-Vindoline<br />
The Vinca-derived vinblastine (2a) and vincristine (2b) are still mainstays of cancer chemotherapy.<br />
The more complex half of these dimeric alkaloids, vindoline (1), has in the past presented a<br />
formidable challenge for total synthesis. Dale L. Boger of Scripps, La Jolla has developed (Org.<br />
Lett. 2005, 7, 4539.) a strikingly simple solution to this problem, based on sequential<br />
cycloaddition.<br />
The starting point for the synthesis was N-methyl 6-methoxytryptamine (3), an improved<br />
preparation of which is described by the authors. This was extended to 4, which was then cyclized<br />
to 5, and acylated with 6 to give 7. On heating, 7 cyclized to 8, which lost N 2 to give the<br />
zwitterion 9. Addition of the intermediate 9 to the indole then gave 10. In one reaction, the entire
ing system of vindoline, appropriately oxygenated, was assembled! The precursor 7 was flat, so it<br />
offered no opportunity for chiral synthesis. Fortunately, 10 and ent-10 proved to be very easy to<br />
resolve by chiral chromatography.<br />
To complete the synthesis, the δ-lactam 11 was oxygenated to 12. Conditions for desulfurization<br />
of the derived thiolactam also effected debenzylation, to give, after acetylation, the ether 13.<br />
Pt-mediated hydrogenolysis gave 14, which was dehydrated to vindoline (1).<br />
A complementary synthetic route, based on the E isomer of 6, and so leading through the ether 15,<br />
is also described. Although slightly longer, this approach was about as efficient as the route via<br />
11.
58. The Stork <strong>Synthesis</strong> of (-)-Reserpine<br />
The history of reserpine (3), isolated from the Indian shrub Rauwolfia serpentina, is storied. The<br />
anxiolytic activity of reserpine, the first tranquilizer, pointed the way to the development by<br />
Hoffmann-LaRoche of the blockbuster drugs Librium and Valium.<br />
The spectacular first total synthesis of reserpine was achieved by R.B. Woodward in 1956. Several<br />
alternative approaches have been been published since that time. The most recent (J. Am. Chem.<br />
Soc. 2005, 127, 16255. ), by Gilbert Stork of Columbia University, centering on the aldehyde<br />
tosylate 2, illustrates the power of chiral induction for the kinetic establishment of distal<br />
stereocenters. Condensation of 2 with 6-methoxytryptamine (1) led to reserpine (3).<br />
The preparation of 2 started with the previously-reported enantioselective addition of acrylate to<br />
butadiene, to give the acid 6. Iodolactone formation followed by reduction gave the diol 7. Under<br />
the conditions of benzyl ether formation, the iodohydrin cyclized to the epoxide, giving 8. Phenyl<br />
selenide added to 8 to give the expected diaxial product 9, which on oxidation gave 10 in high<br />
enantiomeric excess.<br />
The key reaction in the assembly of 2 was the addition of the kinetic lithium enolate of 10 to the<br />
silyl acrylate 11 to give 12. This reaction is seen as involving two sequential Michael additions,<br />
but the stereochemical outcome is the same as would be expected from a concerted Diels-Alder<br />
cycloaddition. Exposure to TBAF converted the furyl silane to the fluorosilane, which was<br />
debenzylated and carried on to the tosylate 13. On exposure to two equivalents of hydrogen<br />
peroxide, the ketone underwent Baeyer-Villiger oxidation with high regioselectivity. The silane<br />
was also oxidized, delivering 14. Methylation followed by Dibal reduction then gave 2.
An additional stereogenic center is created when 1 and 2 are combined. Initial attempts to carry<br />
out the condensation gave the wrong stereochemical outcome, as Pictet-Spengler condensation<br />
preceded tosylate displacement. To work around this problem, 1 and 2 were combined in the<br />
presence of cyanide ion, to give 15. Heating of 15 gave cyclization, but again to the wrong<br />
diastereomer, perhaps because in the intermediate ion pair from cyanide ionization, the cyanide<br />
ion was blocking one face of the intermediate cation. Fortunately, on stirring at room temperature<br />
in aqueous HCl, 15 did cyclize to the correct diastereomer, providing, after acylation, reserpine<br />
(3).<br />
59. The Overman Route to Gelsemine<br />
Gelsemine 3 has no particular biological activity that recommends it, but its challenging<br />
architecture has been a motivation to a generation of organic synthesis chemists. Larry E.<br />
Overman of the University of California at Irvine has described (J. Am. Chem. Soc. 2005, 127,<br />
18046, 18054,) his total synthesis of gelsemine, the key step of which was the acid-mediated<br />
cyclization of 1 to 2.<br />
The synthesis of 1 started with the preparation of the diene 4 from 3-methyl anisole. Diels-Alder<br />
cycloaddition with methyl acrylate gave the endo adduct 7. The methyl group was converted to the<br />
vinyl group of the natural product by allylic oxidation followed by methylenation. Hydrolysis<br />
followed by inversion and trapping with p-methoxybenzyl alcohol gave the protected amine 7.<br />
This underwent smooth aza-oxy-Cope rearrangement, to give, after protection and bromination,<br />
the ketone 1.
The acid-mediated cyclization of 1 to 2 proceeds by way of the protonated enol 9. It seems likely<br />
that kinetic protonation at the indicated carbon would have led to the other diastereomer of the<br />
bromide. With that diastereomer, however, the Br would be inside in the transition state leading to<br />
cyclization. That may slow down the cyclization sufficiently that epimerization can intervene,<br />
leading to the more easily cyclized diastereomer 9 and thus to 2.<br />
There were several problems to solve in the conversion of 2 to 3. The key step was the<br />
intramolecular Heck cyclization of 11 to 12. There was some concern that such a cyclization<br />
would not proceed with a tetrasubstituted alkene. In fact, Moriarty methoxylation of the ketone 2<br />
followed by generation of the enol triflate delivered 10. Pd-mediated carbonylation followed by<br />
coupling with o-iodoaniline and protection gave 11, which underwent smooth intramolecular Heck<br />
cyclization, to give, after hydrolysis, predominantly the unnatural diastereomer 12.<br />
The completion of the synthesis, including the addition of the last carbon, proved elusive. Early on,<br />
it was found to be important to reduce and protect the 1,3-dicarbonyl system, to give the equatorial<br />
ether 13. Attempted displacement to introduce the last carbon led instead to the aziridine 14.<br />
Taking advantage of this, the aziridine was quaternized, then opened at the less-hindered<br />
secondary site. Deprotection followed by warming with base led, via retro-aldol epimerization of<br />
two stereogenic centers, to the long-sought lactone 16. Deprotection followed by reduction of the<br />
lactone then gave 3.
60. <strong>Synthesis</strong> of the Potent FBBP12 Ligand Antascomicin B<br />
The FK binding protein ligands that suppress the immune response, such as FK506 and rapamycin,<br />
have been thoroughly studied. FKBP ligands have also been shown to promote the regrowth of<br />
damaged neurons, both peripherally and in the central nervous system. To differentiate these two<br />
disparate activities, it is important to develop potent FKBP ligands that are not immune<br />
suppressive. The antascomicins, represented by antascomicin B (2), are such a class of natural<br />
products. Steven V. Ley of the University of Cambridge has described (Angew. Chem. Int. Ed.<br />
2005, 44, 2732. ) an elegant synthesis of 2, the key step of which was the ring-contracting<br />
transannular Dieckmann cyclization of 1.<br />
The enantiomerically-pure fragments 8, 12 and 17 were coupled to prepare the macrolide 1. The<br />
preparation of 8 started with the commercially-available enantiomerically-pure bromide 3.<br />
Protection and halide exchange set the stage for homologation with allylmagnesium chloride.<br />
Ozonolysis followed by condensation with the acyl oxazolidinone 6 set the last two stereogenic<br />
centers of 8.<br />
The starting point for 12 was the commercially-available enantiomerically-pure Roche ester (9).<br />
Protection, reduction and oxidation gave 10, which was homologated to the ester 11. Reduction to<br />
the allylic alcohol followed by conversion to the chloride and coupling with TMS acetylene led to<br />
12. Addition to 8 of the alkenyl zirconium derived from 12 then gave 13.<br />
Professor Ley used his elegant tartrate method to assemble the carbocyclic fragment 17.<br />
Condensation of two inexpensive components, dimethyl D-tartrate and biacetyl, followed by
eduction and monoprotection delivered the aldehyde 14. Diastereoselective addition of the allyl<br />
stannane 15 led to diene 16, setting the stage for cyclization with the Grubbs second-generation<br />
Ru catalyst.<br />
The sulfone anion derived from 13 added smoothly to 17, to give, after reduction and acylation,<br />
the ester 1. The templating effect of the arene ring of 1 facilitated macrolide formation. The<br />
Dieckmann cyclization then could proceed via a 6-membered ring transition state, leading to the<br />
ring-contracted product 18. In addition to establishing the β-keto amide, this cyclization left<br />
residual oxygenation at the α-carbon, allowing direct elaboration of the 1,2,3-tricarbonyl system of<br />
2.<br />
61. <strong>Synthesis</strong> of the Potent FBBP12 Ligand Antascomicin B<br />
The FK binding protein ligands that suppress the immune response, such as FK506 and rapamycin,<br />
have been thoroughly studied. FKBP ligands have also been shown to promote the regrowth of<br />
damaged neurons, both peripherally and in the central nervous system. To differentiate these two<br />
disparate activities, it is important to develop potent FKBP ligands that are not immune<br />
suppressive. The antascomicins, represented by antascomicin B (2), are such a class of natural<br />
products. Steven V. Ley of the University of Cambridge has described (Angew. Chem. Int. Ed.<br />
2005, 44, 2732.) an elegant synthesis of 2, the key step of which was the ring-contracting<br />
transannular Dieckmann cyclization of 1.
The enantiomerically-pure fragments 8, 12 and 17 were coupled to prepare the macrolide 1. The<br />
preparation of 8 started with the commercially-available enantiomerically-pure bromide 3.<br />
Protection and halide exchange set the stage for homologation with allylmagnesium chloride.<br />
Ozonolysis followed by condensation with the acyl oxazolidinone 6 set the last two stereogenic<br />
centers of 8.<br />
The starting point for 12 was the commercially-available enantiomerically-pure Roche ester (9).<br />
Protection, reduction and oxidation gave 10, which was homologated to the ester 11. Reduction to<br />
the allylic alcohol followed by conversion to the chloride and coupling with TMS acetylene led to<br />
12. Addition to 8 of the alkenyl zirconium derived from 12 then gave 13.<br />
Professor Ley used his elegant tartrate method to assemble the carbocyclic fragment 17.<br />
Condensation of two inexpensive components, dimethyl D-tartrate and biacetyl, followed by<br />
reduction and monoprotection delivered the aldehyde 14. Diastereoselective addition of the allyl<br />
stannane 15 led to diene 16, setting the stage for cyclization with the Grubbs second-generation<br />
Ru catalyst.<br />
The sulfone anion derived from 13 added smoothly to 17, to give, after reduction and acylation,<br />
the ester 1. The templating effect of the arene ring of 1 facilitated macrolide formation. The<br />
Dieckmann cyclization then could proceed via a 6-membered ring transition state, leading to the<br />
ring-contracted product 18. In addition to establishing the β-keto amide, this cyclization left<br />
residual oxygenation at the α-carbon, allowing direct elaboration of the 1,2,3-tricarbonyl system of<br />
2.
62. <strong>Synthesis</strong> of (-)-Avrainvillamide and (+)-Stephacidin B<br />
The dimeric alkaloid stephacidin B (1) was recently isolated from a fungus culture. The<br />
“monomer” avrainvillamide (2) had previously been described. Andrew G. Myers of Harvard<br />
University has reported (J. Am. Chem. Soc. 2005, 127, 5342.) the enantioselective total synthesis<br />
of 2, and the dimerization of 2 to 1. The key intermediate in the synthesis was the tetracyclic<br />
amide 3.<br />
The absolute configuration of the target natural products was set by enantioselective reduction of<br />
the enone 6. Usually, catalytic Itsuno-Corey reduction of cyclohexenones without an α-substituent<br />
is not selective. In this case, advantage was taken of that lack of induction from the alkene side,<br />
with the steric bulk on the other side of the ketone directing the reduction. Alkylation of 8 with 9<br />
proceeded to give the expected axial product 10. Cyanation proceeded with remarkable<br />
diastereocontrol, to give, after epimerization and hydrolysis, the amide 11. Conjugate addition of<br />
thiophenol followed by spontaneous cyclization and dehydration led to the amide 12. With the<br />
phenylthio enamide in place, the stage was set for the elegant final cyclization: hydrogen atom<br />
abstraction from the dihydroaromatic followed by fragmentation delivered the formamide radical,<br />
that cyclized efficiently to give the tetracyle 13. Oxidation and iodination of the enone then gave<br />
3.
Ullman coupling of 3 with the aryl iodide 4 to give 5 proved to be more effective than the<br />
alternative coupling with the areneboronic acid. Reduction of 5 with activated zinc powder<br />
converted the nitro group to the N-OH, which spontaneously cyclized to the nitrone 2. While 2 so<br />
preparared gave a 13 C spectrum that was congruent with that of natural avrainvillamide, authentic<br />
material was not available, so a direct comparison could not be made.<br />
In Et 3 N and CH 3 CN, 2 spontaneously dimerized to 1. As 2 is levorotatory (-35°) and 1 is<br />
dextrorotatory (91°), this ready interconversion of the monomer and the dimer will make it<br />
difficult to assign the absolute configuration of either natural product solely by comparison of<br />
rotations.<br />
63. <strong>Synthesis</strong> of (-)-Littoralisone<br />
The loss of mental function associated with aging is thought to be due at least in part to the<br />
degradation of neurite connections between neurons. Natural products such as (-)-littoralisone (3)<br />
that promote neurite outgrowth in cell culture are therefore interesting lead compounds for<br />
pharmaceutical discovery. The synthesis of 3 recently reported (J. Am. Chem. Soc. 2005, 127,<br />
3696. ) by David W. C. MacMillan of Caltech uses organocatalysis to assemble 1, to control the<br />
relative configuration of the ring system of 2, and to assemble the glucose component of 3.<br />
The preparation of 1 began with commercial enantiomerically-pure citronellol 4. Ozonolysis of<br />
the ester delivered the aldehyde 5, which was hydroxylated with high diastereocontrol
(enantiocontrol), using the proline-catalyzed procedure developed by MacMillan (Org. Chem.<br />
<strong>Highlights</strong> 2004, January 26.). Protection and homologation of 6 then gave 1.<br />
With most catalysts the dialdehyde 1 cyclized predominantly to the trans-fused product. With the<br />
correct enantiomer of the organocatalyst, however, the cyclization could be directed toward the<br />
desired cis-fused 2. Vilsmeier homologation of the electron-rich alkene followed by oxidation and<br />
lactonization then delivered 8.<br />
A glucose derivative such as 12 would usually be prepared over several protection and<br />
deprotection steps from a commercially-available form of glucose. The authors took a different<br />
approach, using the method for protected glucose synthesis they have developed (Org. Chem.<br />
<strong>Highlights</strong> 2005, March 21.). Thus, proline-catalyzed dimerization of 9 gave 10, which after<br />
condensation with 11 and benzylation gave 12.<br />
Condensation of 12 with 8 gave the exo glycoside 13. Brief exposure of 13 to 350 nm irradiation<br />
followed by debenzylation then gave 3. It is interesting to note that the photocyclization of 13<br />
proceeded slowly even under ambient laboratory light, suggesting that this step in the biosynthesis<br />
of 3 need not be enzyme-mediated.<br />
64. <strong>Total</strong> <strong>Synthesis</strong> of (-)-Sordaricin
Sordaricin (2) is the aglycone of sordarin (3), the parent of a family of clinically-effective<br />
antifungal agents. Lewis N. Mander of the Australian National University has published (J. Org.<br />
Chem. 2005, 70, 1654.) a full account of their enantioconvergent synthesis of 2 (see below), based<br />
on the intramolecular Diels-Alder cyclization of the triene 1. For a complementary total synthesis<br />
by Narasaka of sordaricin (2), see Org. Chem. <strong>Highlights</strong> 2005, April 4.<br />
To prepare 1, two enantiomerically-pure pieces were needed, 10 and 11. As it developed, 10 was<br />
prepared from one enantiomer of 4, and 11 was prepared from the other enantiomer of 4. In both<br />
the synthesis of 10 and the synthesis of 11, the folded nature of the tricyclic ring system was used<br />
to control relative (and absolute) configuration around the cyclopentane ring. Conjugate addition<br />
to (+)-4 followed by alkylation led to the cis dialkyl 5. The retro Diels-Alder unmasking of the<br />
enone was effected by heating to reflux in 1,2-dichlorobenzene while a stream of nitrogen was<br />
bubbled through the solution. Conjugate addition to 6 proceeded to the more open face of the<br />
enone, to give 7. Removal of the carbonyl under conditions that did not epimerize the adjacent<br />
methyl group followed by oxidation of the isopropenyl group then led to 10.<br />
With the iodide 10 in hand, the preparation of the triene 15 could proceed. The single quaternary<br />
center of 15 was constructed by deprotonation of the nitrile 11, followed by alkylation with 10.<br />
Again, bond formation proceeded on the outside face of the tricyclic ketone, to give 12. Because it<br />
was thought (correctly, as it developed) that the Diels-Alder cyclization would proceed with<br />
higher selectivity at lower temperature, 13 was cracked to give the enone 14.<br />
Methoxycarbonylation with the Mander reagent followed by enol triflate formation and<br />
Cu-mediated coupling then delivered the triene 15.
Deprotection of 15 followed by selective oxidation gave the aldehyde 1. In the event, cyclization<br />
of 1 proceeded smoothly, albeit a little slowly, near room temperature, to give exclusively the<br />
desired regioisomer. Demethylation then gave (-)-sordaricin (2).<br />
It is interesting that on heating 1 or the cycloadduct above 150°C, the predominant product (3~4:1)<br />
from the cycloaddition is the undesired regioisomer 16. So, even though the transition state in the<br />
intramolecular Diels-Alder cyclization is product-like, the more stable transition state for the<br />
cyclization of 1 in fact leads to the thermodynamically less stable regioisomer 2.<br />
65. <strong>Total</strong> <strong>Synthesis</strong> of the Tetracyclines<br />
Although the tetracycline antibiotics have been mainstays of antibacterial chemotherapy for<br />
decades, they had eluded efficient total synthesis. In a landmark accomplishment, Andrew G.<br />
Myers of Harvard University recently reported (Science 2005, 308, 395,; J. Am. Chem. Soc. 2005,<br />
127, 8292,) the first such syntheses.<br />
In the Science paper, which was published first, total syntheses of the clinically-important<br />
6-deoxytetracyclines, including doxycycline (9), are described. The starting point for the synthesis<br />
was the enantiomerically-pure ester 2, prepared by fermentation of benzoic acid (1) to the<br />
1,2-dihydrodiol, followed by epoxidation, rearrangement and silylation. Acylation of 3 with 2<br />
gave the ketone 4, which on exposure to LiOTf underwent a very interesting, and<br />
diastereoselective, carbon-carbon bond forming reaction to give, after selective desilylation with<br />
TFA, the alcohol 5. The authors speculate that this reaction is proceeding by initial S N 2´epoxide<br />
opening by the N, followed by ylide formation and 2,3-rearrangement.
The alcohol 5 was the common intermediate for both syntheses. For the deoxy series, 5 was<br />
carried on to the enone 6. Conjugate addition of the anion 7 proceeded with remarkable<br />
diastereoselectivity, to give, after intramolecular acylation and deprotection, doxycycline (9).<br />
The JACS paper describes the total synthesis of the more highly oxygenated (-)-tetracycline (16).<br />
To this end, the alcohol 5 was carried on to the enone 10. Opening of the cyclobutane 11 to the<br />
o-quinone methide followed by Diels-Alder cycloaddition to 10 delivered the endo adduct 12.<br />
Deprotection and oxidation of 12 gave 13, which was further oxidized to the sulfoxide.<br />
Elimination of the sulfoxide gave the naphthalene derivative 14, which underwent spontaneous<br />
oxidation to 15. Reductive deprotection then gave tetracycline (16). The diastereoselectivity of the<br />
air and light-mediated oxidation is remarkable.<br />
66. <strong>Synthesis</strong> of Erythronolide A<br />
Erythronolide A (4), with its array of ten stereogenic centers, is the parent of several classic<br />
antibiotics, including erythromycin. The key step in the total synthesis of 4 recently reported<br />
(Angew. Chem. Int. Ed. 2005, 44, 4036. ) by Erick M. Carreira of the ETH Hönggerberg is the<br />
activation of 1 and subsequent diastereoselective 1,3-dipolar cycloaddition to 2 to give 3.
The synthesis of 1 started with two simple chirons, the alcohol 2, prepared by Noyori reduction of<br />
the acetylenic ketone followed by semi-hydrogenation, and the inexpensive (< one USD/gram)<br />
Roche alcohol (5). Functional group manipulation led to 6, which underwent smooth Mg-mediated<br />
cycloaddition to the enantiomerically-pure alcohol 2, to give 7. Addition of the Grignard reagent 8,<br />
also derived from the Roche ester (5), to the derived methyl ketone also proceeded with high<br />
diastereocontrol, to give the tertiary alcohol. Reduction of 9 and subsequent hydrolysis liberated<br />
the hydroxy ketone, which was reduced selectively to the syn diol. It is a tribute to the stability of<br />
tertiary triethylsilyl ethers that the TES protecting group put on at this stage survived all the way<br />
to the end of the synthesis.<br />
While the diastereoselectivity of 1,3-dipolar cycloaddition was well-precedented in simpler<br />
systems, it was not clear that the diastereoselectivity would be maintained with such a complex<br />
substrate as 1. In fact, addition to 2 proceeded with > 99:1 dr. Wittig homologation of the derived<br />
methyl ketone proceeded with remarkable (33:1) geometric control, setting the stage for<br />
asymmetric dihydroxylation to put in place the last two stereocenters.<br />
Controlled desilylation of 11 followed by selective oxidation delivered the seco acid 12. It had<br />
previously been shown by others that some cyclic protecting groups facilitate macrolactone<br />
formation, while others do not. Fortunately, the two cyclic protecting groups of 12 served well,<br />
and the macrolactonization proceeded efficiently. Protecting group removal and reductive<br />
unmasking of the hydroxy ketone then delivered erythronolide A (4).
Overall, this elegant synthesis is a showcase for the iterative use of the 1,3-dipolar cycloaddition<br />
of enantiomercially-pure allylic alcohols for the preparation of extended arrays of acyclic<br />
stereogenic centers.<br />
67. <strong>Synthesis</strong> of (+)-Cyanthiwigin U<br />
The cyanthin diterpenes show physiological activity ranging from cytotoxicity to nerve-growth<br />
factor stimulation. Andrew J. Phillips of the University of Colorado recently described (J. Am.<br />
Chem. Soc. 2005, 127, 5334.) a concise enantioselective synthesis of cyanthiwigin U (3), based on<br />
the metathesis conversion of 1 to 2, using the second generation Grubbs catalyst.<br />
It was clear that 1 would be derived from a Diels-Alder adduct. There has been a great deal of<br />
work in recent years around the development of enantioselective catalysts for the Diels-Alder<br />
reaction, but the catalysts that have been developed to date only work with activated<br />
dienophile-diene combinations. For less reactive dienes, it is still necessary to use chiral auxiliary<br />
control. One of the more effective of those was the known camphor-derived tertiary alcohol, so<br />
that was used in this project. Diels-Alder cycloaddition of the diene 4 with the<br />
enantiomerically-pure enone 5 led to the adduct 6 with high diastereocontrol. Oxidative cleavage<br />
led to the acid 7, which was carried on to the bis-enone 1.<br />
The two-directional tandem metathesis of 1 to 2 proceeded smoothly using 20 mol % of the<br />
second generation Grubbs catalyst under an atmosphere of ethylene. The conversion of 2 to 3 took<br />
advantage of the differing reactivity of the two ketones. Addition of hydride to 2 from the less<br />
hindered face of the less hindered ketone delivered 4. Addition of isopropyl lithium to the<br />
surviving ketone followed by oxidative rearrangement of the resulting tertiary allylic alcohol and<br />
concomitant oxidation of the secondary allylic alcohol gave the diketone 10. Selective addition of<br />
methyl lithium to the less hindered of the two ketones, again from the more open face, then gave<br />
3.
The elegantly concise strategy displayed here for the enantioselective and diastereoselective<br />
construction of the tricyclic enone 3, by two-directional tandem methathesis of the Diels-Alder<br />
derived diketone 1, should have some generality<br />
67. Enantioselective <strong>Synthesis</strong> of (-)-Epoxomycin<br />
For many classes of physiologically-important natural products, total synthesis requires the<br />
construction of an extended array of acyclic stereogenic centers, with control of relative and<br />
absolute configuration. In the course of a synthesis (J. Am. Chem. Soc. 2004, 126, 15348.) of the<br />
proteasome inhibitor (-)-epoxomycin (3), Lawrence J. Williams of Rutgers University has<br />
developed an elegant and potentially powerful solution to this problem. It was apparent that the<br />
preparation of 3 reduced to the stereocontrolled synthesis of 2, since the balance of the natural<br />
product is made up of readily-available amino acids. The oxygenated quaternary center of 2<br />
appeared to be a particular challenge. The key insight of the synthesis was that both centers could<br />
be established in a single step by selective nucelophilic opening of the enantiomerically-enriched<br />
spiro bis epoxide 1.<br />
The left hand fragment of epoxymycin 3 was assembled from the previously-described amino acid<br />
derivatives 4, 5, and 7, using standard coupling techniques.<br />
The preparation of the allene bis-epoxide 1 started with isovaleraldehyde 9. Addition of the<br />
protected propargyl alcohol 10 under the Carreira conditions led to 11 in > 95% ee. Mesylation<br />
followed by displacement with methyl cuprate provided the allene without loss of enantiomeric<br />
excess. Oxidation of the allene 12 with dimethyldioxirane could have led to any of the four<br />
diastereomers of the spiro bis epoxide. In the event, only two diastereomers were observed, as a
3:1 mixture. That 1 was the major diastereomer followed from its conversion to 3. The<br />
configuration of the minor diastereromer was not noted. Exposure of 1 to nucleophilic azide then<br />
gave the easily-purified 2.<br />
The spirodiepoxide 1 is an intriguing new approach to relative and absolute stereocontrol. It will<br />
be interesting to see what other nucleophiles can be used in the opening. It is possible that a chiral<br />
oxygen transfer reagent, such as the dioxirane prepared by the Shi protocol, would convert 12 to 1<br />
with improved diastereoselectivity (double diastereoselection)<br />
68. Enantioselective <strong>Synthesis</strong> of the Polyene Antiobiotic Aglycone Rimocidinolide Methyl<br />
Ester<br />
The complex polyene macrolide antibiotics are clinically effective as antifungal agents. Scott<br />
Rychnovsky of the University of California at Irvine has reported (Angew. Chem. Int. Ed. 2004, 43,<br />
2822. ) the first synthesis of rimocidinolide methyl ester (4), the aglycone of rimocidin (1). The<br />
key step in the synthesis is the condensation of the aldehyde 2 with the phosphonate 3, leading to<br />
4.<br />
Preparation of the Aldehyde 2: The absolute configuration of the triene aldehyde 2 was set by<br />
Noyori hydrogenation of ethyl butyrylacetate 5. Silylation and Dibal reduction then gave the<br />
aldehyde 6. Reduction of the homologated ester gave the alcohol, which was oxidized to the
desired aldehyde 7 by the Swern procedure. Condensation of 7 with the Wollenberg stannyl diene<br />
followed by deprotection then gave the unstable aldehyde 2.<br />
The power of convergent synthesis is illustrated by the preparation of the acid 3. The three<br />
components 9, 12, and 17 were each prepared in enantiomerically-pure form using<br />
readily-available chiral reagents, followed by functional group manipulation. One of the more<br />
remarkable transformations was the homologation of the Weinreb amide 15 to give the unstable<br />
allyl ketone, which was then reduced with high diastereoselectivity to give the diol 16.<br />
Convergent coupling of 17 with 9, followed by functional group manipulation, gave the iodide 18,<br />
which was then homologated with 12 to give 19. Although the two monosubstituted alkenes of 19<br />
appear to be similar, dihydroxylation with OsO 4 was remarkably selective, leading to the aldehyde<br />
20.<br />
To complete the synthesis of 1, the acid 3 derived from 20 was converted to the mixed anhydride<br />
(Yamaguchi coupling), then esterified with 2. Exposure to K 2 CO 3 /18-crown-6 gave the all-E<br />
tetraene, which was deprotected to give the aglycone 4<br />
69. <strong>Synthesis</strong> of (+)-Brasilenyne<br />
(+)-Brasilenyne (3), isolated from the digestive gland of the sea hare Aplysia brasiliana, shows<br />
significant antifeedant activity. Scott Denmark of the University of Illinois has described (J. Am.<br />
Chem. Soc. 2004, 126, 12432.) an elegant synthesis of 3, the key step of which is the Pd-mediated<br />
intramolecular coupling of 1 to give 2.
The enantiomerically-pure intermediate 1 was prepared from the dioxolanone 4, available in three<br />
steps from L-malic acid. Lewis acid-mediated homologation converted 4, a 4:1 mixture of<br />
diastereomers, into 5 as a single diastereomer. After establishment of the alkenyl iodide, it was<br />
necessary to maintain the lactone in its open form. A solution was found in the formation of the<br />
Weinreb amide. The final stereogenic center was established by Brown allylation of the derived<br />
aldehyde. The alkene metathesis to form 1 was carried out with the commercially-available<br />
Schrock Mo catalyst. The authors did not comment on the relative efficacy of alternative alkene<br />
metathesis catalysts.<br />
With 1 in hand, the stage was set for the proposed Pd-mediated coupling. The authors were<br />
pleased to observe that the coupling conditions previously developed in the group worked<br />
efficiently with this much more complex substrate, leading to 2 as a single geometric isomer.<br />
After protection-deprotection and oxidation, homologation using the Corey protocol gave 10.<br />
Formation of the chloride proceeded with the expected clean inversion of absolute configuration,<br />
to give 3.<br />
The least precedented transformation in this synthesis is the homologation of 4 to 5. This appears<br />
to be a general solution to a longstanding challenge, the construction of secondary-secondary<br />
ethers with absolute stereocontrol.<br />
70. <strong>Synthesis</strong> of (-)-Norzoanthamine
The marine alkaloid (-)-norzoanthamine (3) suppresses bone loss in ovariectomized mice, and so<br />
is of interest as a lead to antiosteoporotic drugs. A substantial challenge in the assembly of 3 is the<br />
stereocontrolled construction of the C ring, with its three all-carbon quaternary centers. In the<br />
synthesis of 3 by Masaaki Mayashita of Hokkaido University (Science 2004, 305, 495. DOI), the<br />
B and C rings were built and two of the three needed quaternary centers were set by the<br />
intramolecular Diels-Alder cyclization of 1 to 2.<br />
The triene 1 was prepared from the enone 4, available in enantiomerically-pure form over several<br />
steps from pulegone. Triply-convergent coupling with the cuprate 5 and the aldehyde 6 led to the<br />
furan 7. Functional group manipulation then gave 1, setting the stage for the intramolecular<br />
Diels-Alder cyclization.<br />
The cyclization of 1 proceeded with 72: 28 diastereoselectivity, leading, after hydrolysis, to the<br />
crystalline diketone 8 as the major product. Reduction of the ketones to the axial alcohols was<br />
followed by spontaneous lactonization, allowing easy differentiation of the several functional<br />
groups. Homologation to 10 followed by condensation with methyl carbonate and subsequent<br />
O-methylation then gave 11. C-Methylation of 11 then set the third quaternary center of the C ring.<br />
The deuteriums were introduced to minimize an unwanted intramolecular hydride transfer in a<br />
later step.<br />
The EFG ring carbons were attached by condensing 13, derived from 12, with the aldehyde 14,<br />
followed by oxidation. Hydrogenation followed by deprotection and oxidation then led to the<br />
triketone 16. Selective enolization of the A-ring ketone allowed facile oxidation to the alkene.
Deprotection and acid-catalyzed cyclization then gave 3. Note that the original stereocenter in 4<br />
was used to set seven of the nine stereocenters of 3.<br />
71. <strong>Synthesis</strong> of (-)-Tetrodotoxin<br />
Tetrodotoxin 3, the toxic principle of pufferfish poison, is a formidable challenge for synthesis,<br />
with each carbon of the cyclohexane functionalized. Minoru Isobe of Nagoya University recently<br />
reported (Angew. Chem. Int. Ed. 2004, 43, 4782.) a second-generation synthesis of<br />
enantiomerically-pure tetrodotoxin. This synthesis features the rapid construction of the<br />
cyclohexene by Diels-Alder cycloaddition using an enantiomerically-pure dienopile, the early<br />
introduction of the aminated quaternary center, and the use of that center to direct the relative<br />
configuration of further functionalization around the ring.<br />
The synthesis started with levoglucosenone 4, available by the pyrolysis of cellulose, e.g. old<br />
newspapers. Bromination-dehydrobromination gave the enantiomerically-pure Diels-Alder<br />
dienophile 5, which was combined with isoprene to give predominantly the crystalline adduct 1.<br />
Hydrolysis and acetylation led to 6, which was carried on to the geometrically-defined allylic<br />
alcohol 7 via reduction with Zn-Cu couple. Overman rearrangement of 7 proceeded with high<br />
facial control, to give 8.
The next stage of the synthesis was ring oxygenation, to convert 8 into 13. The key to this<br />
transformation was the observation that the amide oxygen of 8 participated in the solvolysis of the<br />
allylic bromide, setting, after hydrolysis, the new secondary stereocenter of 9. Hydroxyl-directed<br />
epoxidation gave 10, which was rearranged with Ti(O-i-Pr) 4 to 11. After some experimentation, it<br />
was found that the derived dione 12 could be reduced to the desired cis diol 13 with LiBr and<br />
LiAlH(O-t-Bu) 3 followed by NaBH 4 /CeCl 3 .<br />
Silylation followed by selenium dioxide oxidation converted 13 into 14. Epoxidation of the<br />
derived TES ether proceeded by addition of oxygen to the more open face of the alkene, leading to<br />
15. Ozonolysis followed by diastereoselective one-carbon homologation provided 17. This set the<br />
stage for intramolecular epoxide opening by the carboxylate, to give 2, in which all of the<br />
stereogenic centers of tetrodotoxin have been established.<br />
Justin Du Bois of Stanford University has put forward (J. Am. Chem. Soc. 2003, 125, 11510. ) a<br />
quite different total synthesis of tetrodotoxin, including an elegant late-stage introduction of the<br />
nitrogen
72. <strong>Total</strong> <strong>Synthesis</strong> of (±)-Sordaricin<br />
Carbohydrate derivatives of sordaricin 3 are clinically-effective antifungal agents, but<br />
development efforts were halted when a suffficient supply of 3 could not be established. Koichi<br />
Narasaka of the University of Tokyo recently reported (Chem. Lett. 2004, 33, 942.) a total<br />
synthesis of 3, based on the elegant Pd-mediated cyclization of 1 to 2.<br />
The 5-7-5 skeleton of 1 was assembled from cyclohexenone 4 by conjugate addition followed by<br />
enolate trapping. Simmons-Smith cyclopropanation of 5 led to the cyclopropyl alcohol 6.<br />
Generation of the oxy radical from 6 led to fragmentation to give 7, which further cyclized to give<br />
8. Note that the seven-membered ring is flexible enough that the radical cyclization delivers the<br />
required trans ring fusion. Annulation to 9 followed by kinetically-controlled conjugate addition<br />
then gave 10.<br />
The β-keto ester was protected as the enol acetate, then the ketone 12 was homologated to the<br />
carbonate 1. Despite the strain in the [2.2.1] system being formed, the Pd-mediated cyclization of<br />
1 to 2 proceeded smoothly.
To complete the synthesis, the ketone of 2 was homologated to the alkene 12. Selective oxidative<br />
cleavage of the two vinyl groups followed by reduction provided the diol, the less encumbered<br />
alcohol of which was protected to deliver the fully-differentiated ester 13. Oxidation followed by<br />
ester cleavage then gave 3<br />
73. <strong>Synthesis</strong> of (-)-Hamigeran B<br />
Of all the ring-forming reactions of organic synthesis, diastereoselective intramolecular<br />
Diels-Alder reactions are among the most powerful. Often, as illustrated by the cyclization of 1 to<br />
2, a single stereogenic center can set the relative and absolute configuration of two rings. The<br />
cyclization of 1 to 2 is the key step in the total synthesis of (-)-hamigeran B recently reported (J.<br />
Am. Chem. Soc. 2004, 126, 613. ) by K.C. Nicolaou of the Scripps Research Institute.<br />
The preparation of 1 started with the addition of lithiated 4 to the enantiomerically-pure epoxide 5,<br />
which was prepared from the racemate using the Jacobsen protocol. Reduction followed by<br />
selective protection of the primary alcohol gave the monosilyl ether, which was further protected<br />
with MOM chloride to give 7. Pd-mediated oxidation to the methyl ketone followed by<br />
condensation with the Horner-Emmons reagent gave the unsaturated ester 8 as an inconsequential<br />
mixture of geometric isomers. Oxidation then set the stage for the crucial cyclization.<br />
On irradiation, the aldehyde 1 underwent photoenolization to give the quinone methide 9.<br />
Intramolecular Diels-Alder cyclization then proceeded with high diastereocontrol to give 2 as a<br />
mixture of epimeric esters.
The ester 2 has a trans 6-5 ring fusion, whereas in the desired 3 the ring fusion is cis. This was<br />
easily corrected, as the 6-5 ring fusion is more stable cis. Acid-mediated dehydration to give the<br />
alkene proceeded with concomitant removal of the MOM protection. Osmylation followed by<br />
acetonide formation and subsequent oxidation gave the ketone, which was readily epimerized to<br />
10. The acetonide was designed to block H addition to the bottom side, so hydrogenation of 11<br />
would give the isopropyl group endo. In fact, hydrogenation led to the undesired exo isopropyl,<br />
but hydroboration proceeded from the exo face, leading to the desired 12.<br />
Hydrolysis of the acetonide followed by oxidation and bromination provided the ketone 13, which<br />
is itself a natural product, hamigeran A. Hydrolysis under aerobic conditions led first to<br />
decarboxylation, then to autooxidation, to give(-)-hamigeran B 3<br />
74. <strong>Synthesis</strong> of the Dendrobatid Alkaloid 251F<br />
The Dendrobatid “poison arrow” frogs of Central and South America exude a potent mixture of<br />
alkaloids from their skins. It was originally thought that the frogs biosynthesized these alkaloids,<br />
but it has since been shown that they are of dietary origin. The skin exudate of the Colombian frog<br />
Minyobates bombetes causes severe locomotor difficulties, muscle spasms and convulsions upon<br />
injection in mice. The major component of the alkaloid mixture is 251F 3. Jeff Aubé of the<br />
University of Kansas recently described (J. Am. Chem. Soc. 2004, 126, 5475.) the enantioselective<br />
total synthesis of 3. The key step in the synthesis was the cyclization of the keto azide 2.<br />
The ketone 1, with four ternary stereogenic centers in one cyclopentane ring, was a significant<br />
challenge for synthesis. A clever solution flowed from the idea of coupling a chiral Diels-Alder<br />
reaction with alkene metathesis. The relative and absolute configuration of 1 were set by the<br />
Diels-Alder cycloaddition of the acyl oxazolidine 4 to cyclopentadiene, to give 5. Homologation<br />
of 5 to the enone 6 set the stage for alkene metathesis, mediated by the Grubbs catalyst 7, to give<br />
1.
Conjugate addition to 1 proceeded across the open face of the bicyclic system to give an enolate,<br />
condensation of which with the enantiomerically-pure aldehyde 8 gave the enone 9. Conjugate<br />
reduction of the enone also removed the benzyl ether, to give the alcohol. Conversion of the<br />
alcohol to the azide gave 10. Ozonolysis followed by selective reduction then gave 2.<br />
The acid-mediated intramolecular addition of the azide to the ketone proceeds by way of addition<br />
followed by a pinacol-type rearrangement, to give the amide 12. The regioselectivity of the bond<br />
migration is remarkable. Reduction of the amide then gave 3.<br />
75. Enantioselective <strong>Synthesis</strong> of (+)-Tricycloclavulone<br />
Triclavulone 3, recently isolated from Clavularia vulgaris, is one of the most complex of the<br />
family of about forty structurally-related fatty acid-derived marine prostanoids described from this<br />
species. Hisanaka Ito and Kazuo Iguchi of the Tokyo University of Pharmacy and Life Science<br />
recently reported (J. Am. Chem. Soc. 2004, 126, 4520. ) the total synthesis of 3, starting with the<br />
preparation of the enantiomerically-enriched bicyclic ketone 1.
The enantioselective Cu-catalyzed cycloaddition of the reactive enone 4 to the alkyne 5 proceeded<br />
efficiently, but with only modest enantiomeric excess. This was improved at a later point in the<br />
synthesis, by recrystallization of 2.<br />
The folded geometry of 1 directed the Grignard addition, to give, after protection, the ester 7.<br />
Homologation to the allylic alcohol 8 set the stage for Grubbs ring closure, to give, after oxidation,<br />
the tricyclic sulfone 9, having the skeleton of triclavulone 3.<br />
There were two more stereocenters to set. It was expected that cuprates would add to the open face<br />
of the strained cyclobutene. The control of the other stereocenter was more problematic. One<br />
solution was to prepare an α-sulfonyl lactone. To this end, the ketone was converted to the<br />
secondary carbonate. As hoped, conjugate addition was followed by intramolecular acylation, but<br />
the reaction continued to full acyl transfer, to give 10. Fortunately, desilylation of 10 proceeded<br />
with concomitant lactonization. Desulfonylation then gave 2, which could be brought to high ee<br />
by recrystallization.<br />
With 2 in hand, the rest of the synthesis proceeded smoothly. Reprotection followed by reduction<br />
and oxidation gave the keto aldehyde 11. Condensation of the keto phosphonate 12 with 11 gave<br />
the enone 13. Enantioselective transfer hydrogenation of 13 gave the allylic alcohol 14 with 11:1<br />
diastereoselectivity. Protecting group interchange then gave triclavulone 3, identical in every<br />
respect with natural material.
76. <strong>Synthesis</strong> of Amphidinolide T1<br />
The amphidinolides are a class of structurally diverse and physiologically potent natural products.<br />
The key step in the total synthesis of enantiomerically-pure amphidinolide T1 3 recently reported<br />
(J. Am. Chem. Soc. 2004, 126, 998. ) by Timothy Jamison of MIT, the Ni-mediated cyclization of<br />
1 to 2, clearly illustrates the power of organometallic C-C bond formation in organic synthesis.<br />
The alcohol fragment of 1 was prepared by Evans alkylation of 4 to give, after reduction and<br />
protection, the alkyne 5. Ni-mediated coupling of the alkyne 5 with the enantiomerically-pure<br />
epoxide 6, following the procedures developed by the Jamison group, led to the alcohol 7 with<br />
high regio- and geometric control.<br />
The acid portion of 1 was assembled by enantio- and diastereocontrolled addition of Z-crotyl<br />
borane to the aldehyde 8, following the Brown protocol. Hydroboration and oxidation led to 9,<br />
which was condensed with the allenyl silane 10 to give 11 with high diastereocontrol. Conversion<br />
of the alcohol to the iodide followed by three-carbon homologation by the Myers procedure then<br />
led to 1, which was cyclized with > 10:1 regio- and diasterocontrol to give 12. Ozonolysis and<br />
methylenation of the less hindered ketone then delivered 3.<br />
In both of the Ni-mediated steps in this synthesis, the Ni-alkyne complex is acting as an acyl anion,<br />
in one case opening an epoxide and in the other case adding to the aldehyde in an intramolecular<br />
sense. Such Ni-reduced phenylalkynes are among the easiest to prepare and least expensive of<br />
acyl anion equivalents
77. Catalytic Asymmetric <strong>Synthesis</strong> of Quinine and Quinidine<br />
The tetracyclic alkaloid quinine 1 and the diastereomeric alkaloid quinidine 2 share a storied<br />
history. Eric Jacobsen of Harvard recently completed (J. Am. Chem. Soc. 2004, 126, 706.)<br />
syntheses of enantiomerically-pure 1 and of 2. For each synthesis, the key reaction for establishing<br />
the asymmetry of the target molecule was the enantioselective conjugate addition developed by<br />
the Jacobsen group.<br />
For both 1 and 2, the synthesis started with the alkenyl amide 3. Salen-mediated conjugate<br />
addition proceeded with remarkable induction, to give 5 in 92% ee as a mixture of diastereomers.<br />
Reduction and cyclization followed by deprotonation and kinetic quench delivered the<br />
enantiomerically-enriched cis dialkyl piperidine 6. Homologation of the two sidechains then gave<br />
the alkenyl boronic ester 8.<br />
The quinoline portion of the target alkaloids was prepared by condensing p-anisidine 9 with ethyl<br />
propiolate, followed by bromination. Coupling of 10 with the boronic ester 8 proceeded to give 11,<br />
the intermediate for the synthesis of both 1 and 2. Selective direct epoxidation of 11 using the<br />
usual reagents failed, but Sharpless asymmetric dihydroxylation was successful, providing the diol<br />
in > 96:4 diastereoselectivity, with only traces of the tetraol and of the product from<br />
dihydroxylation of the terminal vinyl group. The diol could be converted cleanly to the desired<br />
epoxide 11. Deprotection followed by cyclization led to quinine 1. Preparation of the<br />
diastereomeric epoxide, using AD-mix-α, followed by cyclization gave quinidine 2. The brevity of<br />
the preparation of these two classical alkaloids is a testament to the power of reagent-controlled<br />
synthesis.
78. <strong>Synthesis</strong> of Deacetoxyalcyonin Acetate<br />
Deacetoxyalcyonin acetate 1 and euncellin 2 are representative members of the eunicellin class of<br />
diterpenes. The synthesis of deacetoxyalcyonin acetate 2 by Gary Molander of the University of<br />
Pennsylvania (J. Am. Chem. Soc. 2004, 126, 1642.) illustrates the power of intramolecular<br />
organometallic carbonyl addition for ring construction.<br />
The six-membered ring of 1 was commerically available in enantiomerically-pure form as<br />
α-phellandrene 3. The challenge was to stitch the highly-substituted ten-membered ring of 1 onto<br />
the disubstituted alkene of 3. The strategy that was conceived was to first construct the<br />
seven-membered ring of 8, then effect three-carbon ring expansion to give 11.<br />
The plan for seven-membered ring construction was to effect stepwise 4 + 3 cycloaddition of 6 to<br />
the protected dialdehyde 5. The preparation of 5 began began with 2+2 cycloaddition between 3<br />
and methoxy ketene, to give 4 with high regio- and diastereocontrol. Photochemical cleavage then<br />
gave 5. The acid-mediated 4 + 3 proceeded via initial addition to the less congested ionized<br />
aldehyde, to give the β-keto ester 7. Alkylation of the dianion of 7 followed by ester hydrolysis<br />
and selenation /oxidation established the enone 8.
Three-carbon ring expansion was carried out in two stages. First, two-carbon homologation of the<br />
exo methylene ketone 8 followed by trapping of the intermediate enolate as the triflate led to 9.<br />
Nozaki-Hiyama-Kishi coupling followed by acetylation smoothly converted 9 into 10.<br />
The trisubstituted alkene of 10 was more readily oxidized than was the congested tetrasubstituted<br />
alkene, so the more reactive alkene was temporarily epoxidized. After ozonolysis, the epoxide was<br />
reduced off using the Sharpless protocol. It is a tribute to the specificity of this reagent that the<br />
easily-reduced α-acetoxy ketone is not affected. Selective silylation of the more accessible ketone<br />
followed by methylenation, hydrolysis and addition of methyl lithium to the outside face of the<br />
previously protected carbonyl then delivered 1.<br />
79. <strong>Synthesis</strong> of (-)-Podophyllotoxin<br />
(-)-Podophyllotoxin 1 and its derivative etoposide 2, derived from natural sources, are in current<br />
clinical use. Michael Sherburn of Australian National University reports (J. Am. Chem. Soc. 2003,<br />
125, 12108. DOI: 10.1021/ja0376588) complementary total syntheses of 1 and of its enantiomer.<br />
The key step in each of these syntheses is a spectacular intramolecular alkene arylation,<br />
exemplified by the conversion of 4 to 5.
The absolute configuration of 1 was set by conjugate addition to the oxazoline 3 followed by<br />
trapping of the product anion, following the precedent of Meyers. Reduction of the oxazoline to<br />
the alcohol followed by thionocarbonate formation then set the stage for the key aryl transfer<br />
reaction.<br />
The aryl transfer is thought to be initiated by addition of the silyl radical to the thiocarbonyl. The<br />
radical so formed adds to the alkene to generate the benzylic radical 7. This radical adds to the<br />
arene to give 8, which fragments to 5.<br />
Oxidation of the silyl group of 5 to the ketone followed by Pd-mediated decarboxylation led to the<br />
ketone 9. The conversion of 9 to podophyllotoxin 1 followed the literature precedent.
80. <strong>Synthesis</strong> of (-)-Strychnine<br />
The total synthesis of (-)-strychnine 3 reported (J. Am. Chem. Soc. 2003, 125, 9801.) by Miwako<br />
Mori of Hokkaido University is a tour de force of selective organopalladium couplings.<br />
The absolute configuration of the final product was established at the outset, by Pd-catalyzed<br />
de-racemizing coupling of 4 with the o-bromoaniline derivative 5. Using the inexpensive<br />
Binol-derived ligand (S)-BINAPO 6 , a model coupling was carried out on several cyclohexenol<br />
derivatives having different one-carbon substituents at C-2. The best ee's were observed with the<br />
silyloxymethyl group. Several alcohol derivatives were then tried, and it was found that the allylic<br />
phosphate gave the best rates and ee's. Using the optimized 4, the coupling with 5 to give 7<br />
proceeded in 84% ee.<br />
The sidechain of 7 was extended by one carbon, to give the nitrile 8. A second organopalladium<br />
step then was used to cyclize 8 to 2. Using Ag 2 CO 3 as the base suppressed unwanted alkene<br />
migration. The reaction ran more slowly in DMSO than in DMF, but byproduct formation was<br />
suppressed.<br />
The crystalline nitrile 2 (99% ee from EtOH) was reduced and protected to give the carbamate 9,<br />
setting the stage for another Pd-catalyzed ring-forming step. Allylic oxidation of 9 gave the enone<br />
only in unacceptably low yield. Pd-mediated cyclization, by contrast, proceeded efficiently to give<br />
the alkene 10. Hydroboration followed by oxidation then gave the ketone 11, a useful intermediate<br />
for the construction of a variety of Strychnos alkaloids.
For strychnine 3, the ketone 11 was converted to the alkene 12 by reduction of the enol triflate<br />
derived from the more stable enolate. Deprotection and acylation gave 13, which was cyclized<br />
with Pd to give, after equilibration, the diene 14. Alkylation, to give 15, followed by Pd-mediated<br />
cyclization then gave 16, which was reduced and cyclized to (-)-strychnine 3.<br />
81. Enantioselective <strong>Total</strong> <strong>Synthesis</strong> of (+)-Amphidinolide T1<br />
Amphdinolide T1 1 is representative of a family of macrolides, isolated from the Amphidinium<br />
marine dinoflagellates, that show significant antitumor properties. Arun Ghosh of the University<br />
of Illinois at Chicago recently completed (J. Am. Chem. Soc. 2003, 125, 2374.) a total synthesis of<br />
1, based conceptually on the convergent coupling of the enantiomerically-pure fragments 2 and 3.<br />
For each of the two fragments, a key component was assembled by the syn selective aldol<br />
condensation developed by Ghosh. For 2, addition to 3-benzyloxypropionaldehyde gave 4, which<br />
was carried on to the protected lactol 6. Homologation to 7 allowed Grubbs coupling with the<br />
fragment 8, leading to 9. Activation of the lactol by condensation with benzenesulfinic acid then<br />
gave 2.
The enantiomerically-pure aldehyde 14was prepared by adding dithiane to the<br />
commercially-available glycidyl tosylate 10. For the other half of 3, another syn-selective aldol<br />
condensation gave 12, which was carried on to the iodide 13. Reduction with t-butyl lithium,<br />
addition of the resulting organolithium to 11 and oxidation then gave the coupled ketone, which<br />
was homologated using the Petasis procedure to give 14.<br />
In fact, the sensitive disubstituted alkene of 14 turned out to not be stable to the subsequent AlCl 3<br />
coupling conditions, so the alkene and the secondary alcohol were protected together as the<br />
bromoether 15. Condensation of the derived enol ether 16 with the sulfone 2 in the presence of<br />
DTBMP (2,6-di-t-butyl-4-methylpyridine) then gave 17. Yamaguchi lactonization followed by<br />
regeneration of the alkene by zinc reduction completed the synthesis of 1.<br />
82. <strong>Synthesis</strong> of (+)-4,5-Deoxyneodolabelline<br />
The dolabellanes, represented by 3-hydroxydolabella-4(16), 7, 11(12)-triene-3,13-dione 1 and the<br />
neodolabellanes, represented by (+)-4,5-deoxyneodolabelline 2, are isolated from both terrestrial<br />
and marine sources. They show cytotoxic, antibiotic and antiviral activity. The recent synthesis of<br />
(+)-4,5-deoxyneodolabelline 2 by David Williams of Indiana University (J. Am. Chem. Soc., 2003,<br />
125, 1843.) highlights both the strengths and the challenges of the current state of the art in<br />
asymmetric synthesis.
The synthetic plan was to assemble both the dihydropyran 3 and the cyclopentane 4 in<br />
enantiomerically-pure form, then to effect Lewis acid-mediated coupling of the allyl silane of 4<br />
with the anomeric ether of 3 to form a new stereogenic center on the heterocyclic ring. A critical<br />
question was not just the efficiency of this step, but whether or not the desired stereocontrol could<br />
be achieved at C-3.<br />
The construction of the heterocycle 3 started with enantiomerically-pure ethyl lactate. Protection,<br />
reduction and oxidation led to the known aldehyde 6. Chelation-controlled allylation gave the<br />
monoprotected-diol 7. Formation of the mixed acetal with methacrolein followed by<br />
intramolecular Grubbs condensation then gave 3. The dihydropyran 3 so prepared was a 1:1<br />
mixture at the anomeric center.<br />
The preparation of the cyclopentane 4 proved to be more of a challenge. Rather than attempt an<br />
enantioselective synthesis, racemic 11 was prepared in straightforward fashion from<br />
commercially-available 2-methylcyclopentenone, by conjugate addition followed by alkylation of<br />
the regenerated ketone enolate. Ozonolysis followed by selective reduction then led to 11.<br />
Resolution was accomplished by enantioselective reduction of the racemic ketone, to give a 1:1<br />
mixture of separable diastereomers. Reoxidation of one of the diastereromers gave ketone 11,<br />
which was determined to be a 96:4 mixture of enantiomers. Homologation followed by allylic<br />
silylation then gave 4 as an inconsequential mixture of diastereomers.<br />
Condensation of the allyl silane 4 with 3 proceeded to give exclusively the desired trans<br />
dihydropyran 5. McMurry coupling of the derived keto aldehyde gave the diol 13 as a mixture of<br />
diastereomers. Oxidation of the mixture gave 2 and its C-8 diastereomer in a ratio of 8:1.
83. <strong>Synthesis</strong> of (+)-Phomactin A<br />
The diterpene (+)-Phomactin A 4 is an antagonist of platelet activating factor. The preparation of 4<br />
recently reported (J. Am. Chem. Soc. 2003, 125, 1712.) by Randall Halcomb of the University of<br />
Colorado elegantly illustrates the use of readily-available natural products as starting materials for<br />
natural product synthesis.<br />
The synthetic plan called for a late-stage intramolecular reductive coupling of the iododiene 3 to<br />
establish the macrocyclic ring of 4. The iododiene 3 was to be assembled by condensation of the<br />
highly-substituted cyclohexene 1 with the aldehyde 2.<br />
The aldehyde 2 was prepared from the inexpensive geraniol ether 5. Selective ozonolysis followed<br />
by Wittig homologation gave the bromodiene, which was converted via dehydrobromination and<br />
alkylation to the alkyne 6. Regioselective hydridozirconation followed by iodination of the C-Zr<br />
bond gave the alkenyl iodide 7 with high geometric control. The two stereogenic centers of 2 were<br />
then established by Sharpless asymmetric epoxidation.<br />
The preparation of the cyclohexene 1 began with pulegone 8, available commercially in high<br />
enantiomeric purity. Methylation followed by retro aldol condensation to remove the unwanted<br />
isopropylidene group gave 2,3-dimethylcyclohexanone, which on<br />
bromination-dehydrobromination gave 9. Vinylation followed by alkylative enone transposition
gave 11, which was brominated over several steps to give 12. Conditions to reduce the ketone 11<br />
directly to the axial alcohol were unavailing, so the dominant pseudoequatorial alcohol from<br />
NaBH 4 reduction was inverted, to give 1.<br />
Condensation of 1 with 2 led to 3, setting the stage for the key macro ring closure. Happily,<br />
conditions could be developed to effect this important transformation, a B-alkyl Suzuki coupling.<br />
The ligand dppf is 1,1'-bisdiphenylphosphinoferrocene. The use of AsPh 3 , rather than a phosphine,<br />
as the supporting ligand was important, as was the use of the thallium base.<br />
84. <strong>Synthesis</strong> of the Mesotricyclic Diterpenoids Jatrophatrione and Citlalitrione<br />
Leo Paquette of Ohio State recently reported (J. Am. Chem. Soc. 2003, 125, 1567.) the total<br />
synthesis of jatrophatrione 1 and citlalitrione 2. These diterpenes, which share a central<br />
highly-subsituted 5-9-5 core, show remarkable tumor-inhibitory activity.<br />
The 5-9-5 skeleton was assembled by the addition of the alkenyl cerate derived from 6 with the<br />
ketone 4, to give 7. Oxy-Cope rearrangement then gave the 5-9-5 enolate, which was quenched<br />
with methyl iodide to give 8. The ketone 8 underwent spontaneous intramolecular ene cyclization,<br />
to give 9.
The transient 5-9-5 ketone 8 has two cis-fused rings. To invert the ring stereochemistry, the alkene<br />
9 was oxidized to the enone 10. After some experimentation, it was found that a CuH preparation<br />
would reduce the enone to give predominantly the trans-fused ketone. Monomesylation of the<br />
derived diol set the stage for Grob fragmentation to reopen the nine-membered ring, providing,<br />
after reduction, the alcohol 12.<br />
At this point, there were two problems in selective alkene functionalization to be addressed.<br />
Although all attempts at oxidation of the cyclopentene failed, intramolecular hydrosilylation<br />
proceeded smoothly, to give 13. On exposure of the derived cyclic carbonate to ,<br />
the cyclononene then underwent allylic oxidation, to give 14.<br />
Attempts to functionalize the homoallylic alcohol 15 quickly revealed that this product of an<br />
intramolecular aldol condensation was sensitive to base. Fortunately, heating with<br />
thiocarbonyldiimidazole effected clean dehydration to give predominantly the desired regioisomer<br />
of the diene. Methanolysis followed by oxidation then gave the triketone 1, which on epoxidation<br />
with MCPBA gave 2 as the minor component of a 3:1 mixture.<br />
Further Information:<br />
J. Yang, Ph.D. Thesis, Ohio State University, 2003.
85. <strong>Total</strong> <strong>Synthesis</strong> of Ingenol<br />
The total synthesis of the tetracyclic Euphorbia tetraol ingenol 3 reported by Keiji Tanino of<br />
Hokkaido University (J. Am. Chem. Soc. 2003, 125, 1498.) illustrates the power of<br />
diastereoselective carbocationic rearrangements, as exemplified by the conversion of 1 to 2.<br />
The construction of the tricyclic epoxide depended on several highly diastereoselective<br />
transformations. The addition of lithio t-butyl acetate to ketone 4 proceeded to give 5 as a single<br />
diastereomer, even though the ketone is flanked by a quaternary center. The authors speculate that<br />
lithium chelation with the methyl ether directed addition. Even more spectacular was the<br />
cyclization of the propargylic acetate 7 to 10. The Co complex activated the acetate for ionization,<br />
while at the same time establishing the proper geometric relationship for bond formation.<br />
Dissolving metal reduction of the Co complex then gave the alkene.<br />
The elegant pinacol rearrangement of 1 to 2, mediated by (ArO) 2 AlCH 3 , exposed a ketone that<br />
might usually need to be protected. In this case, however, the ketone is so buried in the<br />
inside-outside ingenol skeleton that it is unreactive. After several further manipulations, a<br />
spectacular osmylation of the diene 12 led to ingenol 3, in an overall 45-step sequence.<br />
The ingenol 3 prepared by this route was racemic. It is interesting to speculate how one might<br />
efficiently prepare 4 or its precursors in enantiomerically-pure form.
86. <strong>Total</strong> <strong>Synthesis</strong> of the Galbulimima Alkaloid GB 13<br />
Lew Mander of the Australian National University recently reported (J. Am. Chem. Soc. 2003, 125,<br />
2400. ) the total synthesis of the pentacyclic alkaloid GB 13 3, which had been isolated from the<br />
bark of the rain forest tree Galbulimima belgraveana. In the course of the synthesis, he took full<br />
advantage of benzene precursors, while at the same time carefully establishing each of the eight<br />
stereogenic centers of 3.<br />
The core tricyclic ketone 1 was assembled by Birch reduction of 2,5-dimethoxybenzoic acid,<br />
followed by alkylation with 3-methoxybenzyl bromide, to give 4. Acid-catalyzed electrophilic<br />
cyclization of 4 gave the tricyclic ketone 5, which on decarboxylation and protection gave 1.<br />
Diazo transfer to 1 followed by irradiation in the presence of bis-(trimethylsilyl)amide led to ring<br />
contraction with concomitant carbonyl extrusion, to give 7. Dehydration to the nitrile followed by<br />
selenation then set the stage for a highly diastereoselective ytterbium-catalyzed Diels-Alder<br />
reaction, to give, after reduction and protection, the pentacyclic intermediate 2.<br />
Intermediate 2 appears to have two extraneous carbons, the red nitrile and the blue carbon in the<br />
aromatic ring. In fact, the blue carbon was carried all the way through, to appear as the α-methyl<br />
group on the piperidine ring. Birch reduction of 2 deleted the now-superfluous nitrile, and reduced<br />
the aromatic ring, to give, after hydrolysis, the enone 10. Eschenmoser fragmentation of the<br />
intermediate epoxy ketone then gave the keto alkyne 11. The subsequent condensation with<br />
hydroxylamine followed by reduction proceeded with spectacular (but anticipated) stereocontrol,<br />
to establish the three stereogenic centers of the trisubstituted piperidine ring. Oxidation of 12 then<br />
gave the enone 3.