Assess and designate Notified Bodies - TOPRA
Assess and designate Notified Bodies - TOPRA
Assess and designate Notified Bodies - TOPRA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
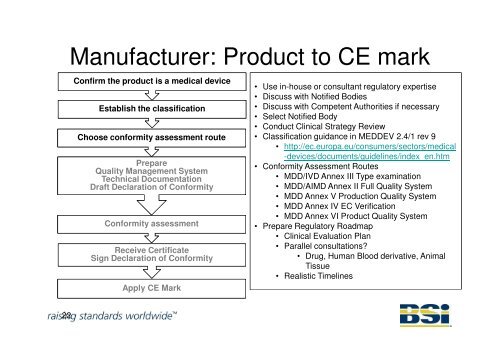
Manufacturer: Product to CE mark<br />
Confirm the product is a medical device<br />
Establish the classification<br />
Choose conformity assessment route<br />
Prepare<br />
Quality Management System<br />
Technical Documentation<br />
Draft Declaration of Conformity<br />
Conformity assessment<br />
Receive Certificate<br />
Sign Declaration of Conformity<br />
Apply CE Mark<br />
• Use in-house or consultant regulatory expertise<br />
• Discuss with <strong>Notified</strong> <strong>Bodies</strong><br />
• Discuss with Competent Authorities if necessary<br />
• Select <strong>Notified</strong> Body<br />
• Conduct Clinical Strategy Review<br />
• Classification guidance in MEDDEV 2.4/1 rev 9<br />
• http://ec.europa.eu/consumers/sectors/medical<br />
-devices/documents/guidelines/index_en.htm<br />
• Conformity <strong>Assess</strong>ment Routes<br />
• MDD/IVD Annex III Type examination<br />
• MDD/AIMD Annex II Full Quality System<br />
• MDD Annex V Production Quality System<br />
• MDD Annex IV EC Verification<br />
• MDD Annex VI Product Quality System<br />
• Prepare Regulatory Roadmap<br />
• Clinical Evaluation Plan<br />
• Parallel consultations?<br />
• Drug, Human Blood derivative, Animal<br />
Tissue<br />
• Realistic Timelines<br />
22