Assess and designate Notified Bodies - TOPRA
Assess and designate Notified Bodies - TOPRA
Assess and designate Notified Bodies - TOPRA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
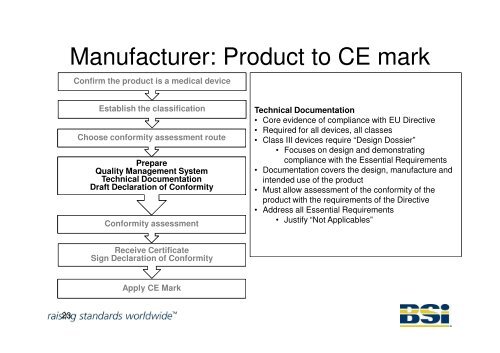
Manufacturer: Product to CE mark<br />
Confirm the product is a medical device<br />
Establish the classification<br />
Choose conformity assessment route<br />
Prepare<br />
Quality Management System<br />
Technical Documentation<br />
Draft Declaration of Conformity<br />
Conformity assessment<br />
Technical Documentation<br />
• Core evidence of compliance with EU Directive<br />
• Required for all devices, all classes<br />
• Class III devices require “Design Dossier”<br />
• Focuses on design <strong>and</strong> demonstrating<br />
compliance with the Essential Requirements<br />
• Documentation covers the design, manufacture <strong>and</strong><br />
intended use of the product<br />
• Must allow assessment of the conformity of the<br />
product with the requirements of the Directive<br />
• Address all Essential Requirements<br />
• Justify “Not Applicables”<br />
Receive Certificate<br />
Sign Declaration of Conformity<br />
Apply CE Mark<br />
23