Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Ch 3-6 Small Book Practice Test<br />
Atomic, <strong>Mole</strong>cular, and Formula <strong>Mass</strong><br />
<strong>Mole</strong>s, % Composition & Empirical Formulas<br />
<strong>Review</strong> Sheet <strong>Answers</strong><br />
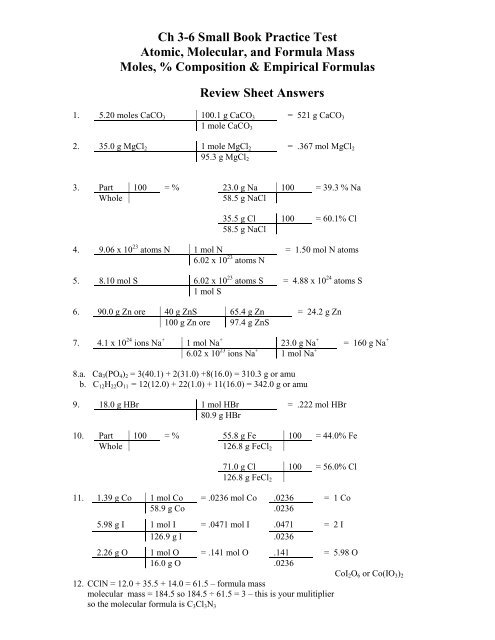
1. 5.20 moles CaCO 3 100.1 g CaCO 3 = 521 g CaCO 3<br />
1 mole CaCO 3<br />
2. 35.0 g MgCl 2 1 mole MgCl 2 = .367 mol MgCl 2<br />
95.3 g MgCl 2<br />
3. Part 100 = % 23.0 g Na 100 = 39.3 % Na<br />
Whole<br />
58.5 g NaCl<br />
35.5 g Cl 100 = 60.1% Cl<br />
58.5 g NaCl<br />
4. 9.06 x 10 23 atoms N 1 mol N = 1.50 mol N atoms<br />
6.02 x 10 23 atoms N<br />
5. 8.10 mol S 6.02 x 10 23 atoms S = 4.88 x 10 24 atoms S<br />
1 mol S<br />
6. 90.0 g Zn ore 40 g ZnS 65.4 g Zn = 24.2 g Zn<br />
100 g Zn ore 97.4 g ZnS<br />
7. 4.1 x 10 24 ions Na + 1 mol Na + 23.0 g Na + = 160 g Na +<br />
6.02 x 10 23 ions Na + 1 mol Na +<br />
8.a. Ca 3 (PO 4 ) 2 = 3(40.1) + 2(31.0) +8(16.0) = 310.3 g or amu<br />
b. C 12 H 22 O 11 = 12(12.0) + 22(1.0) + 11(16.0) = 342.0 g or amu<br />
9. 18.0 g HBr 1 mol HBr = .222 mol HBr<br />
80.9 g HBr<br />
10. Part 100 = % 55.8 g Fe 100 = 44.0% Fe<br />
Whole 126.8 g FeCl 2<br />
71.0 g Cl 100 = 56.0% Cl<br />
126.8 g FeCl 2<br />
11. 1.39 g Co 1 mol Co = .0236 mol Co .0236 = 1 Co<br />
58.9 g Co .0236<br />
5.98 g I 1 mol I = .0471 mol I .0471 = 2 I<br />
126.9 g I .0236<br />
2.26 g O 1 mol O = .141 mol O .141 = 5.98 O<br />
16.0 g O .0236<br />
CoI 2 O 6 or Co(IO 3 ) 2<br />
12. CClN = 12.0 + 35.5 + 14.0 = 61.5 – formula mass<br />
molecular mass = 184.5 so 184.5 ÷ 61.5 = 3 – this is your mulitiplier<br />
so the molecular formula is C 3 Cl 3 N 3
13. 18.2 g Co 1 mol Ca = .456 mol Ca .456 = 1 Ca<br />
40.1 g Ca .456<br />
32.37 g Cl 1 mol Cl = .912 mol Cl .912 = 2 Cl<br />
35.5 g Cl .456<br />
49.3 g H 2 O 1 mol H 2 O = 2.74 mol H 2 O 2.74 = 6.01 H 2 O<br />
18.0 g H 2 O .456<br />
CaCl 2 * 6 H 2 O<br />
14. Fe(NO 3 ) 2 contains 1 mol Fe atoms 15. 3 HC 2 H 3 O 2 contains 3 mol hydrogen (3 H)<br />
2 mol N atoms 9 mol hydrogen (3 H 3 )<br />
6 mol O atoms = 12 mol hydrogen<br />
= 9 mol atoms