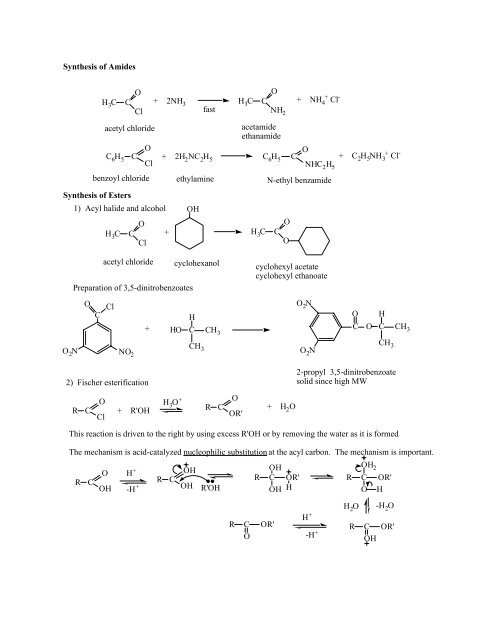
Synthesis of Amides H3C C O Cl + 2NH3 H3C C O NH2 + NH4 fast ...
Synthesis of Amides H3C C O Cl + 2NH3 H3C C O NH2 + NH4 fast ...
Synthesis of Amides H3C C O Cl + 2NH3 H3C C O NH2 + NH4 fast ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Synthesis</strong> <strong>of</strong> <strong>Amides</strong><br />
H 3 C<br />
C 6 H 5<br />
C<br />
C<br />
O<br />
<strong>Cl</strong><br />
acetyl chloride<br />
O<br />
<strong>Cl</strong><br />
O<br />
+<br />
+ 2NH 3 H 3 C C + NH 4 <strong>Cl</strong> -<br />
<strong>fast</strong><br />
NH 2<br />
acetamide<br />
ethanamide<br />
+ 2H 2 NC 2 H 5 C 6 H 5 C O NHC 2 H 5<br />
+ C 2 H 5 NH 3<br />
+<br />
<strong>Cl</strong> -<br />
benzoyl chloride ethylamine N-ethyl benzamide<br />
<strong>Synthesis</strong> <strong>of</strong> Esters<br />
1) Acyl halide and alcohol<br />
O<br />
H 3 C C +<br />
<strong>Cl</strong><br />
OH<br />
H 3 C<br />
C<br />
O<br />
O<br />
O 2 N<br />
acetyl chloride<br />
Preparation <strong>of</strong> 3,5-dinitrobenzoates<br />
O<br />
C<br />
<strong>Cl</strong><br />
NO 2<br />
cyclohexanol<br />
cyclohexyl acetate<br />
cyclohexyl ethanoate<br />
O 2 N<br />
H<br />
O H<br />
+ HO C CH<br />
C O C CH 3<br />
3<br />
CH<br />
CH 3 3<br />
O 2 N<br />
2) Fischer esterification<br />
2-propyl 3,5-dinitrobenzoate<br />
solid since high MW<br />
R<br />
C<br />
O<br />
<strong>Cl</strong><br />
+ R'OH<br />
H 3 O +<br />
R<br />
C<br />
O<br />
OR'<br />
+ H 2 O<br />
This reaction is driven to the right by using excess R'OH or by removing the water as it is formed<br />
The mechanism is acid-catalyzed nucleophilic substitution at the acyl carbon. The mechanism is important.<br />
R<br />
C<br />
O<br />
OH<br />
H + R C<br />
-H +<br />
OH<br />
OH<br />
R C<br />
OH R'OH OH<br />
OR'<br />
H<br />
R<br />
OH 2<br />
C OR'<br />
O H<br />
R<br />
C<br />
O<br />
OR'<br />
H +<br />
-H +<br />
H 2 O<br />
R<br />
C<br />
OH<br />
-H 2 O<br />
OR'
Reactions <strong>of</strong> Esters<br />
1) Reduction<br />
O 1) LiAlH 4 /ether<br />
R C<br />
RCH 2 OH + R'OH<br />
OR'<br />
2) H 2 O<br />
esters reduce more readily than carboxylic acids<br />
O<br />
e.g. 1) LiAlH 4 /ether<br />
C6 H 5 C<br />
C 6 H 5 CH 2 OH + CH 3 OH<br />
OCH 2) H<br />
3<br />
2 O<br />
2) Grignard reaction<br />
O 1) R"MgX/ether OH<br />
R C<br />
OR' 2) H 3 O + R C R" + R'OH<br />
R"<br />
tertiary alcohol is formed<br />
in which two <strong>of</strong> the R groups<br />
are the same. They both come<br />
from the Grignard reagent<br />
Mechanism<br />
R<br />
C<br />
O<br />
OR'<br />
OMgX<br />
O<br />
+ R"MgX R C OR' R C R" + R'OMgX<br />
R"<br />
R"MgX<br />
H 3 CH 2 C<br />
C<br />
O<br />
OC 2 H 5<br />
OH<br />
R C R"<br />
R"<br />
1) 2CH 3 MgBr<br />
H 3 O +<br />
2) H 3 O + H 3 CH 2 C C CH 3<br />
CH 3<br />
1) 2C 6 H 5 MgBr<br />
OH<br />
OH<br />
OMgX<br />
R C R"<br />
R"<br />
3) Ester Hydrolysis<br />
O<br />
R C<br />
OR'<br />
OH -<br />
H 3 O +<br />
heat<br />
2) H 3 O + H 3 CH 2 C C C 6 H 5<br />
C 6 H 5<br />
R<br />
R<br />
C<br />
C<br />
O<br />
O<br />
O -<br />
OH<br />
+ HOR'<br />
+ HOR'<br />
base hydrolysis is non-reversible so is<br />
preferred method<br />
reverse <strong>of</strong> Fischer esterification<br />
read mechanism backwards
Condensation Polymers - formed by the removal <strong>of</strong> a small molecule<br />
1) Terylene, Fortrel, Dacron, Mylar film<br />
H 3 CO<br />
O<br />
C<br />
O<br />
C OCH 3<br />
+ HOCH 2 CH 2 OH<br />
dimethyl terephthalate<br />
- CH 3 OH<br />
ethylene glycol<br />
O<br />
O<br />
O<br />
C<br />
C<br />
H O<br />
O<br />
2 CH 2 C CH 2 CH 2 O C<br />
repeating unit<br />
n<br />
O<br />
C<br />
O<br />
CH 2 CH 2 O<br />
2) Nylon<br />
O<br />
C (CH 2 ) 6<br />
<strong>Cl</strong><br />
C O <strong>Cl</strong><br />
+<br />
H<br />
N (CH 2 ) 6<br />
H<br />
N<br />
H<br />
H<br />
- H<strong>Cl</strong><br />
N<br />
H<br />
O<br />
O<br />
C (CH 2 ) 6 C<br />
(CH 2 ) 6<br />
N N<br />
H H<br />
repeating unit<br />
Nylon 66<br />
O<br />
C<br />
(CH 2 ) 6<br />
C<br />
O<br />
N<br />
(CH 2 ) 6<br />
N<br />
H<br />
peptide link<br />
H<br />
present in proteins<br />
also amide group<br />
Fats and Oils - found in nature<br />
These materials are esters <strong>of</strong> glycerol and are long-chain fatty acids<br />
e.g. trimyristin, the fat from nutmeg<br />
O<br />
H 2 C O C<br />
O (CH 2 ) 12 CH 3 it is not usual to have all <strong>of</strong> the acids attached to glycerol<br />
the same; we usually have a mixed ester<br />
HC O C (CH 2 ) 12 CH 3<br />
H 2 C O C (CH 2 ) 12 CH 3<br />
O<br />
fats are defined as solids at room temperature and are usually from animals<br />
oils are liquids at room temperature and are from the seeds <strong>of</strong> plants<br />
When fats are treated with base they hydrolyse to the free fatty acids and glycerol.