A. Carbon sp3 hybridization - Mathematical operation to create four ...
A. Carbon sp3 hybridization - Mathematical operation to create four ...
A. Carbon sp3 hybridization - Mathematical operation to create four ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
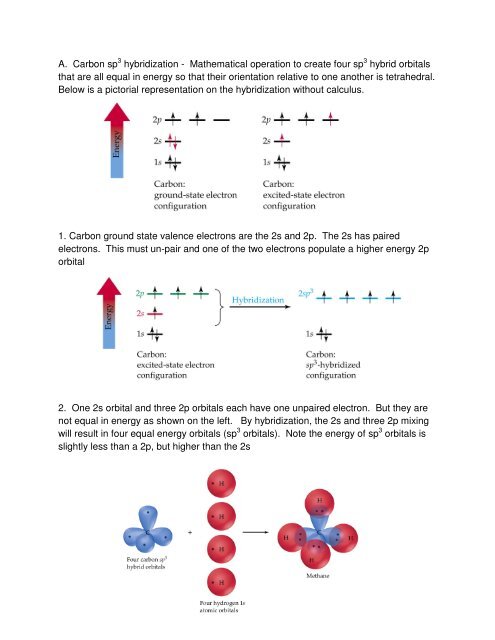
A. <strong>Carbon</strong> sp 3 <strong>hybridization</strong> - <strong>Mathematical</strong> <strong>operation</strong> <strong>to</strong> <strong>create</strong> <strong>four</strong> sp 3 hybrid orbitals<br />
that are all equal in energy so that their orientation relative <strong>to</strong> one another is tetrahedral.<br />
Below is a pic<strong>to</strong>rial representation on the <strong>hybridization</strong> without calculus.<br />
1. <strong>Carbon</strong> ground state valence electrons are the 2s and 2p. The 2s has paired<br />
electrons. This must un-pair and one of the two electrons populate a higher energy 2p<br />
orbital<br />
2. One 2s orbital and three 2p orbitals each have one unpaired electron. But they are<br />
not equal in energy as shown on the left. By <strong>hybridization</strong>, the 2s and three 2p mixing<br />
will result in <strong>four</strong> equal energy orbitals (sp 3 orbitals). Note the energy of sp 3 orbitals is<br />
slightly less than a 2p, but higher than the 2s
Similarly, the C three 2sp 2 orbitals are equal in energy and must occupy a trigonal<br />
planar arrangement. Note, one 2p orbital remains pure (not hybridized.)<br />
Types of overlaps shown below
3. Similarly, the Csp orbital require <strong>hybridization</strong> of one 2s and one 2p orbital <strong>to</strong> give<br />
two 2sp hybrid orbitals (blue) that are arranged linearly. This leaves two 2p orbitals for<br />
pi bonding (green)