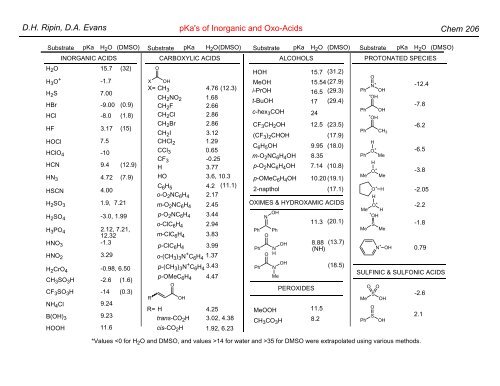
D.H. Ripin, D.A. Evans pKa's of Inorganic and Oxo-Acids Chem 206
D.H. Ripin, D.A. Evans pKa's of Inorganic and Oxo-Acids Chem 206
D.H. Ripin, D.A. Evans pKa's of Inorganic and Oxo-Acids Chem 206
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
D.H. <strong>Ripin</strong>, D.A. <strong>Evans</strong><br />
<strong>pKa's</strong> <strong>of</strong> <strong>Inorganic</strong> <strong>and</strong> <strong>Oxo</strong>-<strong>Acids</strong><br />
<strong>Chem</strong> <strong>206</strong><br />
Substrate<br />
pKa<br />
H 2 O<br />
(DMSO)<br />
Substrate<br />
pKa H 2 O(DMSO)<br />
Substrate<br />
pKa<br />
H 2 O<br />
(DMSO)<br />
Substrate<br />
pKa<br />
H 2 O<br />
(DMSO)<br />
INORGANIC ACIDS<br />
CARBOXYLIC ACIDS<br />
ALCOHOLS<br />
PROTONATED SPECIES<br />
H 2 O<br />
H 3 O +<br />
HOOH<br />
15.7<br />
-1.7<br />
11.6<br />
(32)<br />
H 2 S<br />
7.00<br />
H 3 PO 4 2.12, 7.21,<br />
12.32<br />
HBr<br />
-9.00 (0.9)<br />
HCl<br />
-8.0 (1.8)<br />
HF<br />
3.17 (15)<br />
HOCl<br />
7.5<br />
HClO 4<br />
-10<br />
HCN<br />
9.4 (12.9)<br />
HNO 2 3.29<br />
HN 3 4.72 (7.9)<br />
HSCN<br />
4.00<br />
H 2 SO 3 1.9, 7.21<br />
H 2 SO 4 -3.0, 1.99<br />
HNO 3 -1.3<br />
H 2 CrO 4<br />
-0.98, 6.50<br />
CH 3 SO 3 H<br />
CF 3 SO 3 H<br />
-2.6<br />
-14<br />
(1.6)<br />
(0.3)<br />
9.24<br />
NH 4 Cl<br />
B(OH) 3<br />
9.23<br />
X<br />
O<br />
OH<br />
X= CH 3 4.76 (12.3)<br />
CH 2 NO 2<br />
CH 2 F<br />
CH 2 Cl<br />
CH 2 Br<br />
cis-CO 2 H<br />
1.68<br />
2.66<br />
2.86<br />
2.86<br />
1.29<br />
C 6 H 5<br />
2.17<br />
CH 2 I<br />
3.12<br />
CHCl 2<br />
CCl 3 0.65<br />
CF 3 -0.25<br />
H<br />
3.77<br />
HO<br />
3.6, 10.3<br />
4.2 (11.1)<br />
o-O 2 NC 6 H 4<br />
m-ClC 6 H 4 3.83<br />
m-O 2 NC 6 H 4 2.45<br />
p-O 2 NC 6 H 4 3.44<br />
o-ClC 6 H 4 2.94<br />
p-ClC 6 H 4 3.99<br />
o-(CH 3 ) 3 N + C 6 H 4 1.37<br />
p-(CH 3 ) 3 N + C 6 H 4 3.43<br />
p-OMeC 6 H 4 4.47<br />
O<br />
R<br />
OH<br />
R= H<br />
4.25<br />
trans-CO 2 H 3.02, 4.38<br />
1.92, 6.23<br />
HOH<br />
CF 3 CH 2 OH<br />
(CF 3 ) 2 CHOH<br />
MeOOH<br />
CH 3 CO 3 H<br />
PEROXIDES<br />
15.7<br />
MeOH<br />
15.54 (27.9)<br />
i-PrOH<br />
16.5 (29.3)<br />
t-BuOH<br />
17 (29.4)<br />
c-hex 3 COH 24<br />
C 6 H 5 OH<br />
m-O 2 NC 6 H 4 OH<br />
p-O 2 NC 6 H 4 OH<br />
p-OMeC 6 H 4 OH<br />
12.5<br />
8.35<br />
7.14<br />
11.5<br />
8.2<br />
(31.2)<br />
(23.5)<br />
(17.9)<br />
9.95 (18.0)<br />
(10.8)<br />
10.20 (19.1)<br />
2-napthol (17.1)<br />
OXIMES & HYDROXAMIC ACIDS<br />
Ph<br />
Ph<br />
Ph<br />
N<br />
O<br />
O<br />
OH<br />
Ph<br />
N<br />
H<br />
N<br />
Me<br />
OH<br />
OH<br />
11.3 (20.1)<br />
8.88<br />
(NH)<br />
(13.7)<br />
(18.5)<br />
O<br />
N +<br />
Ph OH<br />
+<br />
OH<br />
Ph OH<br />
+<br />
OH<br />
Ph CH 3<br />
H<br />
O +<br />
Ph Me<br />
H<br />
Me<br />
O+ Me<br />
O + H<br />
H<br />
O +<br />
Me H<br />
+<br />
OH<br />
S<br />
Me Me<br />
N + OH<br />
-12.4<br />
-7.8<br />
-6.2<br />
-6.5<br />
-3.8<br />
-2.05<br />
-2.2<br />
-1.8<br />
0.79<br />
SULFINIC & SULFONIC ACIDS<br />
O O<br />
Me S OH<br />
O<br />
Ph S OH<br />
-2.6<br />
2.1<br />
*Values 14 for water <strong>and</strong> >35 for DMSO were extrapolated using various methods.
D.H. <strong>Ripin</strong>, D.A. <strong>Evans</strong><br />
<strong>pKa's</strong> <strong>of</strong> Nitrogen <strong>Acids</strong><br />
<strong>Chem</strong> <strong>206</strong><br />
Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO)<br />
+ 9.2 (10.5) HN 3 4.7 (7.9)<br />
N H 4<br />
EtN + NH<br />
H 3<br />
38 (41)<br />
3<br />
10.6<br />
i-Pr 2 N + i-Pr 2 NH (36 THF))<br />
H 2<br />
11.05<br />
TMS 2 NH 26(THF) (30)<br />
Et 3 N + H<br />
10.75 (9.00) PhNH 2<br />
(30.6)<br />
PhN + H 3<br />
4.6 (3.6) Ph 2 NH<br />
(25.0)<br />
Ph 2 N + H 2 0.78<br />
NH<br />
(44)<br />
2-napthal-N + Me<br />
H 3 4.16<br />
Me<br />
PhN + (Me) 2 H 5.20 (2.50) NCNH 2 (16.9)<br />
H 2 NN + H 3<br />
8.12<br />
HON + H 3 5.96<br />
Quinuclidine 11.0 (9.80)<br />
Morpholine O 8.36<br />
N-Me morpholine 7.38<br />
O 2 N<br />
PROTONATED NITROGEN<br />
DABCO<br />
H<br />
+ NH 3<br />
+ NH 3<br />
H 3 N + + NH 3<br />
Proton Sponge<br />
NO 2<br />
+<br />
N + H<br />
N +<br />
N + H N + H 2<br />
NO 2<br />
NH 3<br />
-9.3<br />
2.97, 8.82<br />
(2.97, 8.93)<br />
6.90, 9.95<br />
-9.0, 12.0<br />
(--, 7.50)<br />
TMP<br />
H 2 N<br />
R= H<br />
CH 3<br />
Ph<br />
CF 3<br />
NH 2 (urea)<br />
OEt<br />
(24.1)<br />
AMINES<br />
15.1<br />
(37)<br />
(26.5)<br />
AMIDES & CARBAMATES<br />
R NH 2<br />
Et<br />
O<br />
O<br />
O<br />
N<br />
H<br />
NH<br />
Ph<br />
N<br />
NH<br />
Me<br />
Me<br />
O<br />
O<br />
(23.5)<br />
(25.5)<br />
(23.3)<br />
(17.2)<br />
(26.9)<br />
(24.8)<br />
(21.6)<br />
12 (20.5)<br />
NH<br />
IMIDES<br />
Ac 2 NH (17.9)<br />
8.88 (13.7)<br />
(17.0)<br />
PhCN + OH<br />
H -10<br />
Ph N<br />
(NH)<br />
Bn<br />
N O<br />
N<br />
H<br />
H<br />
*Values 14 for water <strong>and</strong> >35 for DMSO were extrapolated using various methods.<br />
8.30<br />
SULFONAMIDE<br />
(14.7)<br />
MeSO 2 NH 2<br />
(17.5)<br />
CF 3 SO 2 NH 2 6.3 (9.7)<br />
PhSO 2 NH 2<br />
(16.1)<br />
MeSO 2 NHPh (12.9)<br />
GUANIDINIUM,<br />
HYRDAZONES,- IDES, & -INES<br />
(13.6)<br />
(21.6)<br />
Ph Me<br />
O<br />
(18.9)<br />
Ph NHNH 2<br />
PhSO 2 NHNH 2 (17.2)<br />
PhNHNHPh (26.1)<br />
O<br />
O<br />
NH<br />
O<br />
N + H 2<br />
Me 2 N NMe 2<br />
NNH 2<br />
O<br />
NH<br />
O<br />
HYDROXAMIC ACID<br />
R<br />
R=<br />
DBU<br />
DMAP<br />
R<br />
+<br />
NH<br />
AMIDINES<br />
R= Me (17.3)<br />
Ph (15.0)<br />
PROTONATED HETEROCYCLES<br />
Me 2 N<br />
NSO 2 Ph<br />
R<br />
+<br />
NH<br />
+<br />
N<br />
H<br />
9.2<br />
(12) (estimate)<br />
+<br />
NH<br />
6.95<br />
H (PPTS) 5.21 (3.4)<br />
t-Bu<br />
4.95 (0.90)<br />
Me 6.75 (4.46)<br />
Cl, H 0.72<br />
NH<br />
NH 2<br />
HETEROCYCLES<br />
H<br />
N<br />
(23.0)<br />
N<br />
HN<br />
HN<br />
(20.95)<br />
N<br />
(18.6)<br />
1,2,3 triazole<br />
N<br />
NH<br />
(13.9)
D.H. <strong>Ripin</strong>, D.A. <strong>Evans</strong><br />
<strong>pKa's</strong> <strong>of</strong> CH bonds in Hydrocarbons <strong>and</strong> Carbonyl Compounds<br />
<strong>Chem</strong> <strong>206</strong><br />
Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO)<br />
CH 2 =CH 2<br />
CH 4<br />
CH 2 =CHCH 3<br />
PhH<br />
PhCH 3<br />
Ph 2 CH 2<br />
Ph 3 CH<br />
HCCH<br />
24<br />
PhCCH 23 (28.8)<br />
H 2<br />
HYDROCARBONS<br />
(Me) 3 CH 53<br />
(Me) 2 CH 2 51<br />
XC 6 H 4 CH 3<br />
X= p-CN<br />
p-NO 2<br />
Me<br />
Me<br />
p-COPh<br />
Me<br />
Me<br />
50<br />
48<br />
46<br />
43<br />
43<br />
41<br />
33.5<br />
31.5<br />
20<br />
15<br />
~36<br />
(56)<br />
(44)<br />
(43)<br />
(32.2)<br />
(30.6)<br />
(30.8)<br />
(20.4)<br />
(26.9)<br />
(26.1)<br />
(20.1)<br />
(18.0)<br />
t-BuO<br />
O<br />
LiO<br />
O<br />
O<br />
t-BuO Me O<br />
Ph<br />
EtO<br />
EtO<br />
MeO<br />
MeO<br />
Me 2 N<br />
O<br />
O<br />
O<br />
Me 2 N<br />
O<br />
Et 2 N<br />
Me 2 N<br />
Me 2 N<br />
N<br />
O<br />
O<br />
O<br />
S<br />
O<br />
S<br />
ESTERS<br />
N + Me 3<br />
O<br />
Ph<br />
Me<br />
Me<br />
O<br />
S<br />
AMIDES<br />
Ph<br />
SPh<br />
OMe<br />
N + Me 3<br />
O<br />
CN<br />
Me<br />
24.5<br />
11<br />
13<br />
(30.3)<br />
(23.6)<br />
(20.0)<br />
(14.2)<br />
(15.7)<br />
(20.9)<br />
[30.2 (THF)]<br />
(26.6)<br />
(25.9)<br />
(24.9)<br />
(17.2)<br />
(18.2)<br />
(25.7)<br />
X= H<br />
Ph<br />
SPh<br />
COCH 3<br />
SO 2 Ph<br />
KETONES<br />
9<br />
(26.5)<br />
(19.8)<br />
(18.7)<br />
(13.3)<br />
(15.1)<br />
19-20 (27.1)<br />
*Values 14 for water <strong>and</strong> >35 for DMSO were extrapolated using various methods.<br />
Me<br />
Et<br />
i-Pr<br />
O<br />
O<br />
Et<br />
O<br />
O<br />
t-Bu O<br />
Ph<br />
Ph<br />
O<br />
X<br />
i-Pr<br />
Me<br />
i-Pr<br />
X<br />
(28.3)<br />
(27.7)<br />
(26.3)<br />
X= H<br />
CH 3<br />
Ph<br />
COCH 3<br />
COPh<br />
CO 2 Et<br />
CN<br />
F<br />
OMe<br />
(24.7)<br />
(24.4)<br />
(17.7)<br />
(12.7)<br />
(13.3)<br />
(22.7)<br />
(10.2)<br />
(21.6)<br />
(22.85)<br />
OPh<br />
SPh<br />
SePh<br />
NPh 2<br />
(21.1)<br />
(16.9)<br />
(18.6)<br />
(20.3)<br />
N + Me 3<br />
NO 2<br />
(14.6)<br />
(7.7)<br />
SO 2 Ph<br />
(11.4)<br />
X<br />
X= H<br />
OMe<br />
NMe 2<br />
Br<br />
CN<br />
O<br />
n<br />
n=<br />
Me<br />
4<br />
5<br />
6<br />
7<br />
8<br />
O<br />
O<br />
O<br />
O<br />
Me<br />
O<br />
Me<br />
(24.7)<br />
(25.7)<br />
(27.5)<br />
(23.8)<br />
(22.0)<br />
(25.1)<br />
(25.8)<br />
(26.4)<br />
(27.7)<br />
(27.4)<br />
(28.1)<br />
(29.0)<br />
(25.5)<br />
(32.4)
D.H. <strong>Ripin</strong>, D.A. <strong>Evans</strong><br />
<strong>pKa's</strong> <strong>of</strong> CH bonds at Nitrile, Heteroaromatic, <strong>and</strong> Sulfur Substituted Carbon<br />
<strong>Chem</strong> <strong>206</strong><br />
Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO)<br />
NC<br />
X= H<br />
CH 3<br />
Ph<br />
COPh<br />
CONR 2<br />
CO 2 Et<br />
CN<br />
OPh<br />
N + Me 3<br />
SPh<br />
SO 2 Ph<br />
N<br />
N<br />
N<br />
N +<br />
O -<br />
O<br />
S<br />
X<br />
NITRILES<br />
11<br />
HETERO-AROMATICS<br />
Ph<br />
Ph<br />
Ph<br />
Ph<br />
Ph<br />
Ph<br />
(31.3)<br />
(32.5)<br />
(21.9)<br />
(10.2)<br />
(17.1)<br />
(13.1)<br />
(11.1)<br />
(28.1)<br />
(20.6)<br />
(20.8)<br />
(12.0)<br />
(28.2)<br />
(30.1)<br />
(26.7)<br />
(25.2)<br />
(30.2)<br />
(30.0)<br />
PhSCH 2 X<br />
X= Ph<br />
CN<br />
COCH 3<br />
COPh<br />
NO 2<br />
SPh<br />
SO 2 Ph<br />
SO 2 CF 3<br />
POPh 2<br />
MeSCH 2 SO 2 Ph<br />
PhSCHPh 2 (26.7)<br />
(PhS) 3 CH<br />
(22.8)<br />
(PrS) 3 CH<br />
(31.3)<br />
Me<br />
S<br />
(30.5)<br />
S S H<br />
(PhS) 2 CHPh<br />
(23.0)<br />
X=<br />
S<br />
S<br />
X<br />
Ph<br />
CO 2 Me<br />
CN<br />
RSCH 2 CN<br />
R=<br />
Me<br />
Et<br />
i-Pr<br />
t-Bu<br />
SULFIDES<br />
(30.8)<br />
(20.8)<br />
(18.7)<br />
(16.9)<br />
(11.8)<br />
(30.8)<br />
(20.3)<br />
(11.0)<br />
(24.9)<br />
(23.4)<br />
(30.7)<br />
(20.8)<br />
(19.1)<br />
(24.3)<br />
(24.0)<br />
(23.6)<br />
(22.9)<br />
PhSCH=CHCH 2 SPh (26.3)<br />
BuSH<br />
10-11 (17.0)<br />
PhSH<br />
≈7 (10.3)<br />
X= H (35.1)<br />
Ph<br />
(29.0)<br />
SPh (29.0)<br />
H<br />
Ph<br />
SOPh<br />
Me 3 S + =O<br />
SULFONIUM<br />
(33)<br />
(27.2)<br />
(18.2)<br />
*Values 14 for water <strong>and</strong> >35 for DMSO were extrapolated using various methods.<br />
Me<br />
Ph S X<br />
X=<br />
Ph<br />
Ph<br />
O<br />
S<br />
O<br />
O<br />
S<br />
CHPh 2<br />
SULFOXIDES<br />
X<br />
(24.5)<br />
(18.2)<br />
(16.3)<br />
SULFIMIDES & SULFOXIMINES<br />
NTs<br />
S<br />
Ph R<br />
Ph S Me<br />
R= Me<br />
i-Pr<br />
O NTs<br />
O NMe<br />
S<br />
Ph Me<br />
O N + Me 2<br />
Ph S Me<br />
O NTs<br />
Ph<br />
Me<br />
S+ CH 2 Ph<br />
S<br />
CH 2 Cl<br />
(27.6)<br />
(30.7)<br />
(24.5)<br />
(33)<br />
(14.4)<br />
(20.7)<br />
Ph<br />
O<br />
S<br />
O<br />
SULFONES<br />
X<br />
X= H<br />
(29.0)<br />
CH 3<br />
(31.0)<br />
t-Bu<br />
(31.2)<br />
Ph<br />
(23.4)<br />
CH=CH 2<br />
(22.5)<br />
CH=CHPh<br />
(20.2)<br />
CCH<br />
(22.1)<br />
CCPh<br />
(17.8)<br />
COPh<br />
(11.4)<br />
COMe<br />
(12.5)<br />
OPh<br />
(27.9)<br />
N + Me 3<br />
(19.4)<br />
CN<br />
(12.0)<br />
NO 2<br />
(7.1)<br />
SMe<br />
(23.5)<br />
SPh<br />
(20.5)<br />
SO 2 Ph<br />
(12.2)<br />
PPh 2 (20.2)<br />
O<br />
O<br />
Ph S CHPh 2<br />
O O<br />
S<br />
Me<br />
O<br />
Me<br />
O<br />
S<br />
CF 3 Me<br />
O O<br />
S<br />
CF 3 i-Pr<br />
O O<br />
CF 3<br />
Et<br />
O<br />
S<br />
S<br />
O<br />
Et<br />
(PhSO 2 ) 2 CH 2 Me<br />
(22.3)<br />
(31.1)<br />
(18.8)<br />
(21.8)<br />
(26.6)<br />
(32.8)<br />
(14.3)
D. H. <strong>Ripin</strong>, D. A. <strong>Evans</strong><br />
<strong>pKa's</strong> <strong>of</strong> CH bonds at Heteroatom Substituted Carbon & References<br />
<strong>Chem</strong> <strong>206</strong><br />
Substrate pKa H 2 O (DMSO) Substrate pKa H 2 O (DMSO) Substrate pKa<br />
CH 3 OPh<br />
MeOCH 2 SO 2 Ph<br />
PhOCH 2 SO 2 Ph<br />
PhOCH 2 CN<br />
MeO<br />
X=<br />
CN<br />
O<br />
O<br />
COPh<br />
CO 2 Et<br />
Ph<br />
Me 3 N + CH 2 X<br />
SO 2 Ph<br />
CONEt 2<br />
ETHERS<br />
SELENIDES<br />
AMMONIUM<br />
(49)<br />
(30.7)<br />
(27.9)<br />
(28.1)<br />
(21.1)<br />
PhSe<br />
(18.6)<br />
Ph<br />
PhSeCHPh 2<br />
(27.5)<br />
(PhSe) 2 CH 2 (31.3)<br />
PhSeCH 2 Ph<br />
PhSeCH=CHCH 2 SePh<br />
(31.0)<br />
(27.2)<br />
(20.6)<br />
(19.4)<br />
(14.6)<br />
(20.6)<br />
(24.9)<br />
P + H 4<br />
-14<br />
MeP + H 3 2.7<br />
Et 3 P + H<br />
9.1<br />
Ph 3 P + CH 3 (22.4)<br />
Ph 3 P + i-Pr<br />
(21.2)<br />
Ph 3 P + CH 2 COPh<br />
Ph 3 P + CH 2 CN<br />
(EtO) 2 P<br />
X=<br />
O<br />
Ph 2 P<br />
PHOSPONATES &<br />
PHOSPHINE OXIDES<br />
O<br />
Ph<br />
CN<br />
CO 2 Et<br />
Cl<br />
PHOSPHONIUM<br />
SiMe 3<br />
X<br />
X<br />
X= SPh<br />
(24.9)<br />
CN<br />
(16.9)<br />
Ph 2 PCH 2 PPh 2<br />
PHOSPHINES<br />
Ph 2 PCH 2 SO 2 Ph<br />
(6.2)<br />
(7.0)<br />
(27.6)<br />
(16.4)<br />
(18.6)<br />
(26.2)<br />
(28.8)<br />
(29.9)<br />
(20.3)<br />
RNO 2<br />
R=<br />
O 2 N<br />
n=<br />
Ph<br />
CH 3<br />
CH 2 Me<br />
CHMe 2<br />
CH 2 Ph<br />
CH 2 Bn<br />
CH 2 SPh<br />
CH 2 SO 2 Ph<br />
CH 2 COPh<br />
N<br />
3<br />
4<br />
5<br />
6<br />
7<br />
n<br />
Ph<br />
Ph<br />
NITRO<br />
IMINES<br />
H 2 O (DMSO)<br />
≈10<br />
(17.2)<br />
(16.7)<br />
(16.9)<br />
(12.2)<br />
(16.2)<br />
(11.8)<br />
(7.1)<br />
(7.7)<br />
(26.9)<br />
(17.8)<br />
(16.0)<br />
(17.9)<br />
(15.8)<br />
(24.3)<br />
Oxime ethers are ~ 10 pka units less<br />
acidic than their ketone counterparts<br />
Streitwieser, JOC 1991, 56, 1989<br />
DMSO:<br />
REFERENCES<br />
JACS 97, 7007 (1975)<br />
JACS 97, 7160 (1975)<br />
JACS 97, 442 (1975)<br />
JACS 105, 6188 (1983)<br />
JOC 41, 1883 (1976)<br />
JOC 41, 1885 (1976)<br />
JOC 41, 2786 (1976)<br />
JOC 41, 2508 (1976)<br />
JOC 42, 1817 (1977)<br />
JOC 42, 321 (1977)<br />
JOC 42, 326 (1977)<br />
JOC 43, 3113 (1978)<br />
JOC 43, 3095 (1978)<br />
JOC 43, 1764 (1978)<br />
JOC 45, 3325 (1980)<br />
JOC 45, 3305 (1980)<br />
JOC 45, 3884 (1980)<br />
JOC 46, 4327 (1981)<br />
JOC 46, 632 (1981)<br />
JOC 47, 3224 (1982)<br />
JOC 47, 2504 (1982)<br />
Acc. <strong>Chem</strong>. Res. 21, 456 (1988)<br />
Unpublished results <strong>of</strong> F. Bordwell<br />
Water:<br />
Advanced Org. <strong>Chem</strong>., 3rd Ed.<br />
J. March (1985)<br />
Unpublished results <strong>of</strong> W. P. Jencks<br />
THF:<br />
JACS 110, 5705 (1988)<br />
*Values 14 for water <strong>and</strong> >35 for DMSO were extrapolated using various methods.