wave worksheet - Avon Chemistry
wave worksheet - Avon Chemistry
wave worksheet - Avon Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ATOMIC STRUCTURE WORKSHEET:<br />
Name: _____________________________________ p: _____<br />
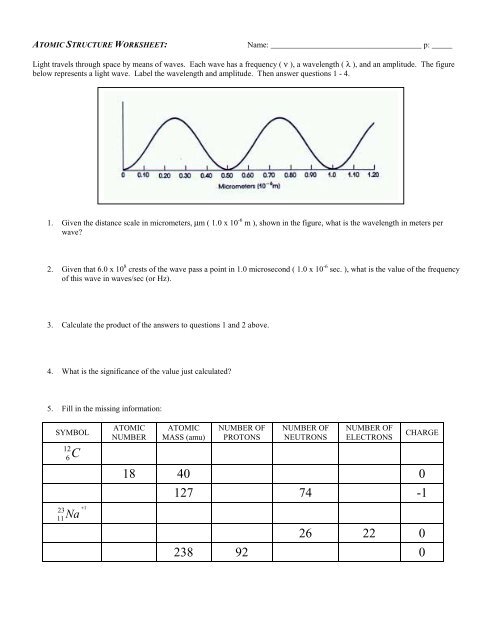
Light travels through space by means of <strong>wave</strong>s. Each <strong>wave</strong> has a frequency ( ν ), a <strong>wave</strong>length ( λ ), and an amplitude. The figure<br />
below represents a light <strong>wave</strong>. Label the <strong>wave</strong>length and amplitude. Then answer questions 1 - 4.<br />
1. Given the distance scale in micrometers, µm ( 1.0 x 10 -6 m ), shown in the figure, what is the <strong>wave</strong>length in meters per<br />
<strong>wave</strong><br />
2. Given that 6.0 x 10 8 crests of the <strong>wave</strong> pass a point in 1.0 microsecond ( 1.0 x 10 -6 sec. ), what is the value of the frequency<br />
of this <strong>wave</strong> in <strong>wave</strong>s/sec (or Hz).<br />
3. Calculate the product of the answers to questions 1 and 2 above.<br />
4. What is the significance of the value just calculated<br />
5. Fill in the missing information:<br />
SYMBOL<br />
23<br />
11<br />
12<br />
6<br />
C<br />
Na<br />
+ 1<br />
ATOMIC<br />
NUMBER<br />
ATOMIC<br />
MASS (amu)<br />
NUMBER OF<br />
PROTONS<br />
NUMBER OF<br />
NEUTRONS<br />
NUMBER OF<br />
ELECTRONS<br />
CHARGE<br />
18 40 0<br />
127 74 -1<br />
26 22 0<br />
238 92 0
6. What is the energy of a quantum of light with a frequency of 6.17 x 10 14 Hz<br />
7. Solar panels rely on light's ability to remove electrons from the surface of the solar cell. The energy required to release an<br />
electron from atoms on the surface of the cell is 6.7 x 10 -19 J. What <strong>wave</strong>length of light would be necessary for electrons to<br />
leave the cell's surface and return to the surface to produce the energy (used as electricity)<br />
8. A certain <strong>wave</strong>length of blue light has a frequency of 6.91 x 10 14 Hz. What is the <strong>wave</strong>length of this blue light<br />
9. Find the average mass of Tungsten if the relative amounts are as follows:<br />
Isotopic Mass:<br />
Percentage:<br />
181.27 amu 10.5 %<br />
182.69 amu 19.6 %<br />
184.73 amu 67.3 %<br />
185.34 amu 2.6 %