Carpentier-Edwards PERIMOUNT THEON Pericardial Bioprostheses
Carpentier-Edwards PERIMOUNT THEON Pericardial Bioprostheses
Carpentier-Edwards PERIMOUNT THEON Pericardial Bioprostheses
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CARPENTIER-EDWARDS<br />
<strong>PERIMOUNT</strong> <strong>THEON</strong><br />
PERICARDIAL BIOPROSTHESES<br />
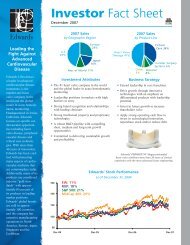
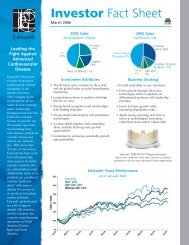
ECLIPSING PERFORMANCE
CARPENTIER-EDWARDS<br />
<strong>PERIMOUNT</strong> <strong>THEON</strong><br />
PERICARDIAL BIOPROSTHESES<br />
Putting performance out front<br />
<strong>Carpentier</strong>-<strong>Edwards</strong> <strong>PERIMOUNT</strong> <strong>THEON</strong> pericardial bioprostheses balance ease of implantation<br />
with a 20-year <strong>PERIMOUNT</strong> tissue valve legacy of proven long-term durability. Now, by adding<br />
the <strong>Carpentier</strong>-<strong>Edwards</strong> ThermaFix process * —the only tissue treatment to confront both major<br />
causes of calcification—<strong>PERIMOUNT</strong> <strong>THEON</strong> pericardial bioprostheses put long-term performance<br />
and confidence in a whole new light. 1,2<br />
The ThermaFix process<br />
Untreated leaflet tissue.<br />
Treated with the ThermaFix process.<br />
Lasting<br />
Performance<br />
Longevity<br />
The <strong>PERIMOUNT</strong> platform<br />
track record of uncomprom<br />
mitral position. 5<br />
Mechanism of dual-action performance<br />
1: Residual glutaraldehyde subtraction.<br />
2: Phospholipid extraction.<br />
Dual-Action<br />
Performance<br />
The <strong>Carpentier</strong>-<strong>Edwards</strong> ThermaFix<br />
Current science indicates that confronting<br />
phospholipids and glutaraldehyde residual<br />
necessary to mitigate the effects of SVD. 1
Streamlined<br />
Performance<br />
Proven<br />
Performance<br />
Implantability<br />
Designed for ease of use, <strong>PERIMOUNT</strong> <strong>THEON</strong> bioprostheses provide<br />
exceptional annular conformity, accessible suturing areas, low profiles,<br />
and efficient deliverability to challenging anatomy. 3<br />
Hemodynamics<br />
<strong>PERIMOUNT</strong> <strong>THEON</strong> bioprostheses are supported by 17 years of proven hemodynamic stability, 6<br />
as well as industry-leading performance data on EOAs and transvalvular gradients. 3,7<br />
of pericardial tissue valves delivers robust performance with a proven<br />
ised durability up to 20 years in the aortic position 4 and 16 years in the<br />
process *<br />
the two potential calcium binding sites in bioprosthetic tissue —<br />
s 2 —is critical to achieving the long-term structural integrity<br />
The ThermaFix process is the<br />
only dual-action tissue treatment<br />
that confronts both major<br />
calcium binding sites and only<br />
<strong>PERIMOUNT</strong> valves have the<br />
ThermaFix process. 3
Magenta keyline does not print. Indicates pocket.<br />
<strong>Carpentier</strong>-<strong>Edwards</strong> <strong>PERIMOUNT</strong> <strong>THEON</strong> <strong>Pericardial</strong> <strong>Bioprostheses</strong><br />
Aortic nominal specifications (mm) Model 2700TFX Model 2800TFX (RSR) †<br />
B<br />
A<br />
D<br />
C<br />
Size 19 mm 21mm 23 mm 25 mm 27mm 29 mm 19 mm 21mm 23 mm 25 mm 27mm 29 mm<br />
A. Mounting Diameter (Annulus) 19 21 23 25 27 29 19 21 23 25 27 29<br />
B. Internal Diameter (Stent I.D.) 18 20 22 24 26 28 18 20 22 24 26 28<br />
C. Profile Height 13 14 15 16 17 18 14 15 16 17 18 19<br />
D. External Sewing Ring Diameter 28 31 33 35 38 40 26 28 31 32 35 37<br />
Note: Valve size is denoted by Dimension A, Mounting Diameter (Annulus)<br />
†Reduced sewing ring<br />
Mitral nominal specifications (mm) model 6900PTFX<br />
Size 25 mm 27mm 29 mm 31 mm 33 mm<br />
A. Stent Diameter (Wireform) 25 27 29 31 31<br />
B. External Stent Post Diameter (Base) 28.0 29.5 31.5 33.5 33.5<br />
C. External Stent Post Diameter (Tip) 29 31 34 35 35<br />
D. External Sewing Ring Diameter 34 36 39 41 43<br />
E. Ventricular Projection 12 13 14 14 15<br />
F. Total Profile Height 17 18 19 20 20<br />
Aortic accessories<br />
• Sizer model 1127 available in sizes 19-29 mm<br />
(for use with 2700TFX).<br />
• Sizer model 1161 available in sizes 19-29 mm<br />
(for use with 2800TFX).<br />
• Reusable handle, model 1111.<br />
Mitral accessories<br />
• <strong>Edwards</strong> Tricentrix holder system.<br />
• Flexible mitral handle, model 1117.<br />
• Performance-based sizers with EOA identification,<br />
model 1169HP.<br />
• Longer reusable handle, model 1117.<br />
• Longer single-use handle, model 1126.<br />
*No clinical data are available which evaluate the long-term impact of the <strong>Edwards</strong> Lifesciences tissue treatment in patients.<br />
References<br />
1. Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: Progress toward understanding and prevention.<br />
Ann Thorac Surg 2005;79: 1072-80.<br />
2. Data on file. <strong>Edwards</strong> Lifesciences. RD 127, RD 128, RD 784, RD 795.<br />
3. <strong>Carpentier</strong>-<strong>Edwards</strong> <strong>PERIMOUNT</strong> Theon pericardial bioprostheses, models 2700TFX, 2800TFX, and 6900PTFX product inserts–<br />
Directions for use–2006. 2007.<br />
4. <strong>Carpentier</strong>-<strong>Edwards</strong> <strong>PERIMOUNT</strong> aortic pericardial bioprosthesis 20-year Results. Data on file at <strong>Edwards</strong> Lifesciences, 2003.<br />
5. <strong>Carpentier</strong>-<strong>Edwards</strong> <strong>PERIMOUNT</strong> mitral pericardial bioprosthesis 16-year Results. Data on file at <strong>Edwards</strong> Lifesciences, 2003.<br />
6. Banbury, et al. Hemodynamic stability during 17 years of the <strong>Carpentier</strong>-<strong>Edwards</strong> aortic pericardial bioprosthesis.<br />
Ann Thorac Surg 2002; 73: 1460-5.<br />
7. Dalmau, et al. The <strong>Carpentier</strong>-<strong>Edwards</strong> Perimount Magna aortic xenograft: a new design with an improved hemodynamic performance.<br />
Interactive Card and Thorac Surg 5 2006; 263-267.<br />
Rx only. See instructions for use for full prescribing information.<br />
<strong>Edwards</strong>, <strong>PERIMOUNT</strong> <strong>THEON</strong> and ThermaFix are trademarks<br />
of <strong>Edwards</strong> Lifesciences Corporation. <strong>Edwards</strong> Lifesciences, the<br />
stylized E logo, <strong>Carpentier</strong>-<strong>Edwards</strong>, <strong>PERIMOUNT</strong> and Tricentrix<br />
are trademarks of <strong>Edwards</strong> Lifesciences Corporation and are<br />
registered in the United States Patent and Trademark Office.<br />
© 2007 <strong>Edwards</strong> Lifesciences LLC.<br />
All rights reserved. AR02235<br />
<strong>Edwards</strong> Lifesciences LLC • One <strong>Edwards</strong> Way • Irvine, CA 92614 USA • 949.250.2500 • 800.424.3278 • www.edwards.com