Electrophilic Aromatic Substitution Worksheet - ChemConnections
Electrophilic Aromatic Substitution Worksheet - ChemConnections
Electrophilic Aromatic Substitution Worksheet - ChemConnections
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
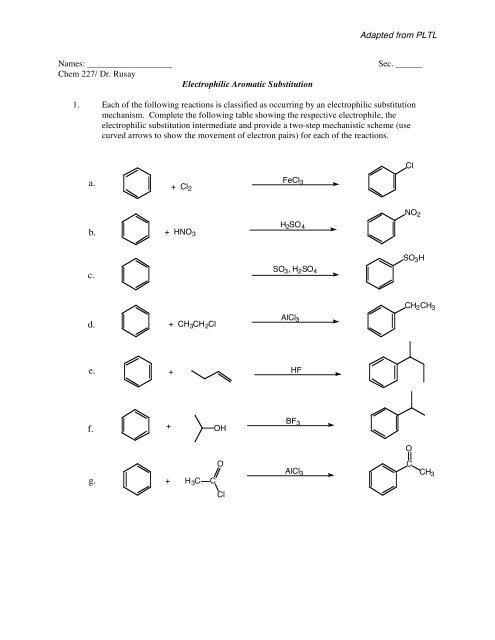
Adapted from PLTL<br />
Names: ___________________<br />
Chem 227/ Dr. Rusay<br />
<strong>Electrophilic</strong> <strong>Aromatic</strong> <strong>Substitution</strong><br />
Sec. ______<br />
1. Each of the following reactions is classified as occurring by an electrophilic substitution<br />
mechanism. Complete the following table showing the respective electrophile, the<br />
electrophilic substitution intermediate and provide a two-step mechanistic scheme (use<br />
curved arrows to show the movement of electron pairs) for each of the reactions.<br />
Cl<br />
a.<br />
+ Cl 2<br />
FeCl 3<br />
NO 2<br />
b.<br />
+ HNO 3<br />
H 2 SO 4<br />
SO 3 H<br />
c.<br />
SO 3 , H 2 SO 4<br />
CH 2 CH 3<br />
d.<br />
+ CH 3 CH 2 Cl<br />
AlCl 3<br />
e.<br />
+<br />
HF<br />
f.<br />
+<br />
OH<br />
BF 3<br />
O<br />
g.<br />
+<br />
H 3 C<br />
C<br />
O<br />
AlCl 3<br />
C<br />
CH3<br />
Cl
Electrophile Intermediate Mechanism<br />
a.<br />
b.<br />
c.<br />
d.<br />
e.<br />
f.<br />
g.
2. Consider the following reactions; a) explain the differences in reactivity between A, B, and C. b)<br />
Which reaction is faster C or D Circle one. Explain your answer, and why B does not react.<br />
A<br />
.<br />
B<br />
.<br />
C<br />
.<br />
D<br />
.<br />
H 3 C<br />
H<br />
H<br />
C<br />
C<br />
H<br />
H<br />
+ Br 2<br />
CH 2 Cl 2<br />
BrCH 2 CH 2 Br<br />
+ Br 2<br />
CH 2 Cl 2<br />
no reaction<br />
Br<br />
+ Br 2<br />
FeBr 3<br />
CH 2 Cl 2<br />
Br<br />
CH 3<br />
H 3 C<br />
+ HBr<br />
CH 3<br />
+ HBr<br />
CH 3<br />
CH 3<br />
a)<br />
b) C or D. Circle one.<br />
Explanation:
3. A DVC Chem 227 team of students ran the following two reactions and obtained the products<br />
noted. Which reacts faster Circle your choice. Explain the reasons for your choice and why<br />
the products differ in their respective substitution patterns.<br />
NH 2<br />
NH 2<br />
A<br />
.<br />
H 2 SO 4<br />
versus<br />
SO 3 H<br />
(major product)<br />
O<br />
NHCCH 3<br />
O<br />
NHCCH 3<br />
B<br />
.<br />
H 2 SO 4<br />
SO 3 H
4. Consider the products of the following reactions.<br />
OCH 3<br />
OCH 3<br />
A<br />
.<br />
HNO 3 , H 2 SO 4<br />
versus<br />
NO 2<br />
B<br />
.<br />
OCH<br />
OCH 3 3<br />
HNO 3 , H 2 SO 4<br />
O 2 N<br />
Show a detailed mechanism for each reaction taking care to show all important resonance<br />
structures. Provide energy diagrams (reaction coordinate diagrams) for the two mechanisms.<br />
A: B:
5. Complete the table that follows with reactions & reagents for preparing each of the following<br />
compounds from benzene. (More than one reaction step may be needed to prepare a product.)<br />
CH 2 CN<br />
Br<br />
A<br />
.<br />
COOH<br />
SO 3 H<br />
Br<br />
H<br />
.<br />
B<br />
.<br />
COOH<br />
G<br />
.<br />
C<br />
.<br />
Br<br />
D<br />
.<br />
O<br />
C<br />
O<br />
H C H<br />
CH 3<br />
E<br />
.<br />
NO 2<br />
CO 2 H<br />
F<br />
.<br />
SO 3 H
A<br />
B<br />
C<br />
D<br />
E<br />
F<br />
G<br />
H