Biowave PRO
Biowave PRO
Biowave PRO
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
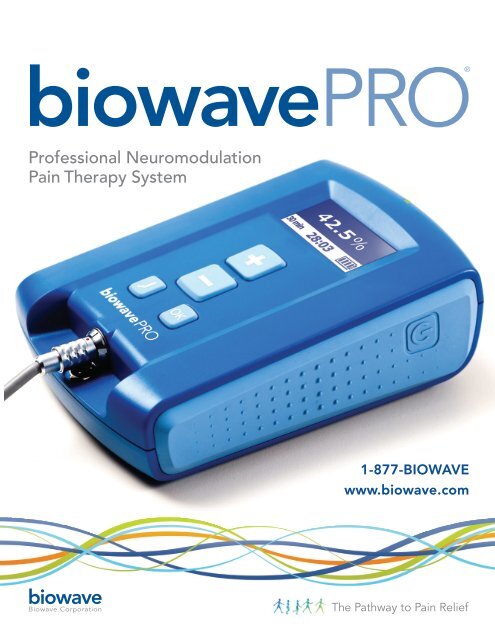
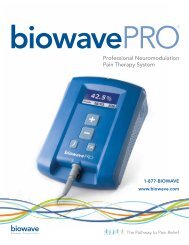
iowave<strong>PRO</strong>®<br />
Professional Neuromodulation<br />
Pain Therapy System<br />
1-877-BIOWAVE<br />
www.biowave.com<br />
biowave<br />
<strong>Biowave</strong> Corporation<br />
The Pathway to Pain Relief
Electrode Placements<br />
<strong>Biowave</strong><strong>PRO</strong> electrode placements are<br />
different from conventional electrical<br />
stimulation. The mixing of the proprietary<br />
therapeutic signals occurs in a 2 - 3 inch<br />
hemisphere beneath each electrode, not along<br />
the surface of the skin between the electrodes.<br />
As a result, to treat two equal points of pain or<br />
to cover pain over a large area, two same sized<br />
electrodes may be used and are placed directly<br />
over the points of pain. To treat two unequal<br />
points of pain, the small round electrode is<br />
placed directly over the primary pain site. The<br />
larger rectangular electrode is placed over a<br />
secondary pain site or if there is no secondary<br />
pain site, over a bony prominence near the<br />
region being treated.<br />
<strong>Biowave</strong><strong>PRO</strong> ®<br />
Key Features<br />
• Advanced Deep Tissue Signal Technology—<br />
optimized to pass through the skin<br />
into deep tissue and to prevent action<br />
potential propagation along pain fibers<br />
• Long Carryover Effect — provides up<br />
to 48 hours of pain relief and functional<br />
improvement<br />
• Hypoesthesia — remains at the location<br />
of the pain site for up to 20 minutes<br />
B<br />
B<br />
B<br />
B<br />
• Portable — hand held and easily used in<br />
physical therapy during range of motion<br />
or exercise therapy, in training rooms,<br />
on the sidelines, in meeting rooms, on<br />
airplanes, buses or at home<br />
Bilateral Low Back Pain<br />
Unilateral Low Back<br />
Pain Focused on one<br />
Side of Spine<br />
Unilateral Low Back<br />
Pain Focused Over a<br />
Facet Joint<br />
Low Back Pain Focused<br />
Over the Spine<br />
• Easy to Use — patient controlled<br />
and no programming - the signals are<br />
optimized for penetrating the skin,<br />
blocking pain and improving function<br />
• Comfortable — feels like a deep smooth<br />
continuous sensation, not the noxious<br />
electrical twitching sensation common<br />
with other types of electrical stimulation<br />
U<br />
Hip Pain (or Other Pain<br />
in One Location in the<br />
Mid Torso Region)<br />
E<br />
Patellar Tendinitis<br />
E<br />
Medial Knee Pain<br />
(e.g. MCL Sprain, Bursitis,<br />
Osteoarthritis)<br />
E<br />
Lateral Knee Pain<br />
(e.g. LCL Sprain, Bursitis,<br />
Osteoarthritis, IT Band)<br />
Facilitate and Accelerate<br />
Rehabilitation<br />
As part of a rehabilitation regimen,<br />
<strong>Biowave</strong><strong>PRO</strong> should be used first, for 8-10<br />
minutes in place of heat. The patient obtains<br />
a significant long acting period of pain<br />
relief as well as a functional improvement<br />
including an increase in range of motion.<br />
E<br />
Low Ankle or Foot Sprain<br />
with Pain in One Location<br />
E<br />
Plantar Fasciitis<br />
E<br />
Cervical or Neck Pain in One<br />
Location with Secondary<br />
Pain in the Trapezius<br />
E<br />
Shoulder Pain at AC Joint<br />
(e.g. AC Sprain or pain<br />
throughout the shoulder)<br />
<strong>Biowave</strong><strong>PRO</strong> therapy then continues during<br />
active or passive range of motion, exercise<br />
or stretching therapy allowing patients to<br />
control more resistance through a greater<br />
range of motion with significantly less pain.<br />
<strong>Biowave</strong><strong>PRO</strong> is portable and hand held so<br />
it is easy to move around the clinic with the<br />
patient. <strong>Biowave</strong><strong>PRO</strong> is an excellent tool<br />
to facilitate motion, allowing the patient to<br />
comfortably complete treatment protocols<br />
set up by their physical therapist.<br />
E<br />
Anterior Shoulder Pain<br />
(e.g. Biceps Tendinitis)<br />
E<br />
Lateral Elbow Pain<br />
(e.g. Lateral Epicondylitis)<br />
E<br />
Medial Elbow Pain<br />
(e.g. Medial Epicondylitis)<br />
E<br />
Anterior Wrist Pain<br />
(e.g. Sprains, Strains,<br />
Tendinosis)<br />
Additionally, because of <strong>Biowave</strong><strong>PRO</strong>’s<br />
long carryover effect following the<br />
treatment, the patient has a significant<br />
reduction in post exercise soreness and is<br />
often comfortable into the following day.<br />
B Bilateral E Extremities U Unilateral<br />
WARNING: Electrodes must not touch each other.<br />
See back cover for electrode descriptions.
®<br />
biowave<strong>PRO</strong><br />
The Future of Pain Relief Has Arrived.<br />
The <strong>Biowave</strong><strong>PRO</strong> ®<br />
Neuromodulation Pain Therapy System is a first course<br />
therapy for treating chronic, acute or postoperative musculoskeletal pain.<br />
<strong>Biowave</strong><strong>PRO</strong> utilizes a unique signal mixing<br />
technology to deliver electrical signals through<br />
the skin into deep tissue for inhibiting pain<br />
transmission and improving function. The signal<br />
technology is covered by numerous U.S. and<br />
international patents.<br />
Clinical studies have shown that <strong>Biowave</strong><strong>PRO</strong><br />
can be used to reduce pain and improve function<br />
including an increase in range of motion,<br />
decrease in stiffness and reduction of muscle<br />
spasm for up to 48 hours following a single<br />
30-minute treatment.<br />
How is <strong>Biowave</strong><strong>PRO</strong> ®<br />
Background<br />
Low frequency signals (1-180Hz in frequency)<br />
are required to inhibit transmission of pain<br />
signals along nerve fibers in the body. However,<br />
electrical signals in this frequency range cannot<br />
pass through the skin because of the skin’s<br />
impedance and capacitance. High frequency<br />
signals (greater than 1000Hz in frequency) easily<br />
pass through the skin. However, these signals<br />
used individually do not elicit an electrical or<br />
mechanical response from either muscle tissue<br />
or nerve fibers to inhibit pain transmission.<br />
TENS<br />
TENS devices deliver pulsed low frequency<br />
signals directly to the skin between two surface<br />
electrodes placed on either side of the painful<br />
area. The result is a surface effect and the patient<br />
feels a noxious twitching, electrical sensation<br />
between the electrodes. The sensation<br />
produced by TENS may act as a distraction from<br />
the pain while the device is on, however, there is<br />
little residual benefit or functional improvement<br />
once the therapy session is over.<br />
<strong>Biowave</strong><strong>PRO</strong> can be used to treat numerous<br />
locations on the body including the lower back,<br />
cervical spine, hip, groin, knee, shoulder, ankle, foot,<br />
elbow, wrist, hand and finger. Doctors, physical<br />
therapists and athletic trainers report excellent<br />
results treating pain from joint sprains, ligaments<br />
and tendons, osteoarthritis and postoperative pain.<br />
Professional athletes that use <strong>Biowave</strong><strong>PRO</strong> have<br />
reported they experience better pain relief and<br />
functional improvement than with any other<br />
form of electrical stimulation and choose to<br />
be treated with <strong>Biowave</strong><strong>PRO</strong> every time they<br />
return to the training room.<br />
Superior to TENS<br />
Interferential Current (IFC)<br />
IFC uses four surface electrodes (two pairs of<br />
electrodes) that are placed in an “X” pattern<br />
surrounding a painful area. IFC delivers two<br />
separate pulsed, high frequency signals, one<br />
between each pair of electrodes. The two<br />
pulsed signals cross paths on the surface of the<br />
skin. At the point of intersection, an interference<br />
pattern develops resulting in a new low<br />
frequency signal equal to the difference<br />
between the signals. This is called the beat<br />
frequency. Since the two signals intersect on<br />
the surface, the resulting beat frequency produces<br />
mostly a surface effect similar to TENS.<br />
<strong>Biowave</strong><strong>PRO</strong><br />
There are three main differences between<br />
<strong>Biowave</strong><strong>PRO</strong> and IFC or TENS devices:<br />
1<br />
Deep Tissue Signal Technology<br />
<strong>Biowave</strong><strong>PRO</strong> utilizes a proprietary signal<br />
mixing technology that delivers two continuously<br />
summed, high frequency alternating current<br />
signals that pass through the skin into deep<br />
tissue where they interact with tissues to<br />
<strong>Biowave</strong><strong>PRO</strong> ®<br />
System<br />
The <strong>Biowave</strong><strong>PRO</strong> System is a<br />
professional neuromodulation<br />
pain therapy system<br />
for managing pain and<br />
facilitating and accelerating<br />
rehabilitation.<br />
<strong>Biowave</strong><strong>PRO</strong> can utilize<br />
both <strong>Biowave</strong> ®<br />
Noninvasive<br />
Electrodes for pain<br />
management, physical<br />
therapy and athletic training<br />
applications; and <strong>Biowave</strong> ®<br />
Percutaneous Electrodes for<br />
treating severe chronic pain in<br />
a hospital, pain clinic or pain<br />
management setting.<br />
<strong>Biowave</strong> Corporation
produce a range of signals which include a<br />
low frequency electrical field. This field is<br />
optimized for blocking pain and providing<br />
functional improvement including a greater<br />
range of motion and a reduction in stiffness<br />
and muscle spasm. In addition to providing<br />
efficacy, these signals are significantly more<br />
comfortable leading to improved patient<br />
treatment compliance.<br />
2<br />
SmartPad Active Control and<br />
Monitoring Technology<br />
<strong>Biowave</strong><strong>PRO</strong> utilizes SmartPad<br />
technology – active monitoring and control<br />
of the electrical signals to ensure the<br />
accurate and safe delivery of therapeutic<br />
energy into deep tissue. The SmartPad<br />
technology automatically prevents patients<br />
from receiving too much intensity which<br />
could lead to a burn.<br />
3<br />
Electrode Options<br />
There are two types of electrodes<br />
designed to work with <strong>Biowave</strong><strong>PRO</strong>:<br />
<strong>Biowave</strong> ®<br />
Noninvasive Electrodes<br />
<strong>Biowave</strong> Noninvasive Electrodes are reusable<br />
surface electrodes typically used to<br />
reduce pain and facilitate physical therapy<br />
in professional and college sports, in<br />
physical and occupational therapy and in<br />
chiropractic settings.<br />
<strong>Biowave</strong> ®<br />
Percutaneous Electrodes<br />
<strong>Biowave</strong> Percutaneous Electrodes utilize<br />
a patented microneedle technology<br />
to further facilitate the delivery of the<br />
<strong>Biowave</strong><strong>PRO</strong> therapeutic signals through<br />
the skin, directly into deep tissue.<br />
<strong>Biowave</strong> Percutaneous Electrodes are<br />
sterile, single-use electrodes, and are<br />
used under the supervision of a physician<br />
typically in a pain clinic, hospital or<br />
physician office setting to reduce severe<br />
acute, chronic or postoperative acute<br />
pain.<br />
<strong>Biowave</strong> Deep Tissue<br />
Signal Technology<br />
<strong>Biowave</strong>’s signal technology is based on<br />
the discovery that when two sinusoidal high<br />
frequency signals are summed (added)<br />
together in the device and then delivered<br />
into the body through a single electrode,<br />
the signals will pass into deep tissue and<br />
travel to a second opposing electrode. As<br />
the summed signals pass through the body,<br />
polarized structures like the membrane<br />
of the C-fiber, A-delta fiber and muscle<br />
tissue act in a non-linear fashion and force<br />
the further multiplication of these signals,<br />
resulting in a new spectrum of signals.<br />
Multiplication of the high frequency signals<br />
results in the formation of a therapeutic low<br />
frequency electrical field that inhibits action<br />
potential propagation along pain fibers.<br />
This field is focused in a 2-3 inch diameter<br />
hemisphere in the volume of tissue under<br />
and surrounding each electrode. Since the<br />
<strong>Biowave</strong> Percutaneous<br />
Electrode<br />
• Sterile, Single Use Electrode<br />
• 1014 Microneedles 0.75mm<br />
in length<br />
• Feels like Velcro or sandpaper<br />
to the touch<br />
Professional Neuromodulation<br />
Pain Therapy System<br />
nerve fibers and muscle tissue under and<br />
surrounding the electrodes are encompassed<br />
by the low frequency field, at least<br />
one electrode must be placed directly over<br />
the center of the painful area.<br />
“<strong>Biowave</strong><strong>PRO</strong> ®<br />
utilizes a<br />
patented signal mixing<br />
technology to deliver<br />
electrical signals<br />
through the skin into<br />
deep tissue for inhibiting<br />
pain transmission,<br />
improving function and<br />
providing a significant<br />
carryover effect.”<br />
<strong>Biowave</strong><strong>PRO</strong> delivers the two summed<br />
signals to the first electrode; they mix in the<br />
tissue beneath that electrode, then pass<br />
to the second electrode and return to the<br />
device, completing the circuit. Instantaneously,<br />
the summed signals are delivered<br />
to the second electrode; they mix in the<br />
tissue beneath that electrode, then pass<br />
to the first electrode and return to the<br />
device. The device alternates the delivery<br />
of the summed signals so quickly between<br />
the two electrodes that the patient cannot<br />
distinguish that the signals left either<br />
location. The net effect is there are two<br />
active electrodes, each of which can treat<br />
a distinct volume of tissue and there is no<br />
noxious twitching sensation.<br />
Focusing of the <strong>Biowave</strong> Signals<br />
and Electrode Placement<br />
With <strong>Biowave</strong><strong>PRO</strong>, depending on the<br />
nature and location of the painful area, the<br />
electrical signals can be focused to different<br />
parts of the body by pairing electrodes<br />
of different sizes and types with one another.<br />
If two electrodes of equal area are
used, then two distinct volumes of tissue<br />
of similar pain magnitude can be treated<br />
simultaneously. For example, if a patient<br />
has bilateral low back pain, two equal area<br />
electrodes can be placed over the respective<br />
painful areas on each side of the spine.<br />
Two equal area electrodes can also be<br />
used to treat one larger volume of tissue by<br />
placing these electrodes closer together so<br />
that there is only 0.5 to 1.0 inch of space<br />
between them.<br />
With <strong>Biowave</strong> Noninvasive Electrodes, by<br />
pairing an electrode of smaller area with an<br />
electrode of larger area, the density of the<br />
therapeutic low frequency field is greater<br />
in the volume of tissue beneath the smaller<br />
area electrode. Therefore, the smaller electrode<br />
needs to be placed directly over the<br />
primary painful area. The larger electrode<br />
is still active (it is not a grounding pad) and<br />
should be placed over a secondary point of<br />
pain. If there is no secondary point of pain,<br />
then the larger electrode must be placed<br />
over a bony prominence near the treatment<br />
site. Placement of the larger electrode over<br />
a bony prominence allows the patient to<br />
more comfortably increase the intensity of<br />
the signal to higher levels allowing a stronger<br />
electric field to encompass the pain site<br />
under the smaller primary electrode.<br />
With <strong>Biowave</strong> Percutaneous Electrodes,<br />
the electrical signals can be focused by not<br />
only pairing different area electrodes with<br />
each other, but also by combining percutaneous<br />
with noninvasive electrodes. Since<br />
the summed high frequency signals pass<br />
through the skin more easily when using<br />
the percutaneous electrode, the impedance<br />
is lower in this area than at the opposing<br />
surface electrode. By having an impedance<br />
difference between the electrodes,<br />
the therapeutic low frequency field is more<br />
concentrated where the impedance is lower.<br />
Therefore, the electrical field in the volume<br />
of tissue under the percutaneous electrode,<br />
which is placed directly over the painful<br />
area, allows the nerve fibers and muscle tissue<br />
to be more readily encompassed by the<br />
therapeutic low frequency electric field.<br />
Treatment Regimen Protocol<br />
In athletic training applications, because<br />
athletes typically re-aggravate their pain<br />
condition during practices or games, the<br />
recommended regimen is to treat immediately<br />
before a practice or game (which may<br />
be in combination with heat), immediately<br />
after a practice or game (which may be in<br />
combination with cold therapy) and time<br />
permitting, a third treatment 2 – 3 hours<br />
later. Multiple treatments with <strong>Biowave</strong>-<br />
<strong>PRO</strong> may provide cumulative benefit.<br />
In physical therapy applications, <strong>Biowave</strong>-<br />
<strong>PRO</strong> should be used first, in place of heat,<br />
for 8 - 10 minutes. Intensity is then reduced<br />
by about 5% and the patient begins active<br />
or passive range of motion, exercise or<br />
stretching therapy during the remainder of<br />
the 30 minute treatment. Patients can do<br />
more resistance through a greater range of<br />
motion with significantly less pain. <strong>Biowave</strong>-<br />
<strong>PRO</strong> significantly facilitates the ability of the<br />
patient to perform therapy. Additionally, because<br />
of the long carry over effect, patients<br />
have little post exercise soreness.<br />
For chronic pain conditions, <strong>Biowave</strong><strong>PRO</strong><br />
with <strong>Biowave</strong> Percutaneous Electrodes may<br />
be used. The recommended regimen is<br />
6 treatments over 2 weeks, followed by<br />
physician-prescribed <strong>Biowave</strong>HOME<br />
treatments that can be performed on a<br />
daily or as needed basis. <strong>Biowave</strong><strong>PRO</strong> with<br />
<strong>Biowave</strong> Noninvasive Electrodes may also<br />
be used for chronic or acute pain conditions.<br />
Daily treatments are recommended<br />
followed by physician prescribed <strong>Biowave</strong>-<br />
HOME treatments that can be performed<br />
on a daily or as needed basis.<br />
More Comfortable<br />
Because <strong>Biowave</strong> devices deliver the summation<br />
of two high frequency sinusoidal<br />
alternating current signals, the sensation is<br />
smoother, deeper and more comfortable<br />
compared to the noxious electrical twitching<br />
sensation felt on the surface of the skin<br />
that results from IFC and TENS.<br />
Easy to Use<br />
®<br />
biowave<strong>PRO</strong><br />
<strong>Biowave</strong>’s early signal-dose-finding studies<br />
demonstrated that there is an optimal set<br />
of high frequency signals for delivering<br />
energy through the skin into the body<br />
which create an optimal low frequency<br />
signal that inhibits pain transmission along<br />
pain fibers. This resulted in the design of<br />
a very simple to use device from which an<br />
optimized set of signals for blocking pain<br />
is delivered into deep tissue. The device<br />
does not require any programming by the<br />
physician, medical professional or patient.<br />
Thus, <strong>Biowave</strong> devices are far simpler to<br />
use than IFC or TENS devices.<br />
Efficacy<br />
<strong>Biowave</strong> devices provide rapid, long-lasting<br />
efficacy. Responding patients experience<br />
a significant reduction in pain, and often,<br />
an improved range of motion, decrease in<br />
stiffness and reduction of muscle spasm<br />
by the end of the 30 minute treatment<br />
period, which often lasts 24 hours to 48<br />
hours or longer. Clinical studies have also<br />
demonstrated that use of <strong>Biowave</strong> neuromodulation<br />
pain therapy reduces the use<br />
of concomitant pain medications.<br />
Superior Clinical<br />
Outcomes<br />
Published randomized, controlled clinical<br />
studies showing significant pain reduction<br />
with a long carryover effect, functional<br />
improvement and a reduction in pain<br />
medication consumption have been<br />
completed at several prestigious institutions<br />
including:<br />
• Hospital for Special Surgery,<br />
New York, NY<br />
• Rush University Medical Center,<br />
Chicago, IL<br />
• Weill Medical College at Cornell<br />
University/NY Hospital, New York, NY<br />
Clinical studies are available upon request.
®<br />
biowave<strong>PRO</strong><br />
ORDERING INFORMATION<br />
<strong>PRO</strong>DUCT SPECIFICATIONS<br />
Device<br />
BWPD01<br />
Electrodes -<br />
BWEN01-B<br />
BWEP01-B<br />
Electrodes -<br />
BWEN02-E<br />
BWEP02-E<br />
Electrodes -<br />
BWEN03-U<br />
Accessories<br />
BWPL01<br />
BWPTB1<br />
BWPA01<br />
BWPB01<br />
<strong>Biowave</strong><strong>PRO</strong> Neuromodulation Pain Therapy Device<br />
B<br />
Set<br />
<strong>Biowave</strong> Noninvasive B-set: two 2” diameter round equal area<br />
noninvasive electrodes for treating 2 equal points of pain or pain<br />
over a large area.<br />
<strong>Biowave</strong> Percutaneous B-set: two 2.5” diameter round equal area<br />
percutaneous electrodes for treating 2 equal points of pain or pain<br />
over a large area.<br />
B-set electrodes are used for treating:<br />
• bilateral or unilateral pain in the back including,<br />
buttocks, lumbar or thoracic region<br />
• radiculopathies<br />
• pain in two locations in the hip or groin<br />
• bilateral pain in the cervical spine, shoulders or knees<br />
• pain centered directly in the spine<br />
• pain presenting in a large area<br />
E<br />
Set<br />
<strong>Biowave</strong> Noninvasive E-set: one 1.375” diameter small round<br />
electrode for the primary pain site; and one 2” x 4” rectangular<br />
electrode for a secondary pain site or to be placed over the bony<br />
prominence in the region being treated.<br />
<strong>Biowave</strong> Percutaneous E-set: one 2.5” diameter round percutaneous<br />
electrode for the primary pain site; and one 2” x 4” noninvasive<br />
rectangular electrode for a secondary pain site or to be placed<br />
over a bony prominence (comfortable location) in the region being<br />
treated.<br />
E-set electrodes are used for treating:<br />
• pain in the extremities including the knees, ankles, feet, toes,<br />
neck, shoulders, elbows, wrists, hands and fingers<br />
U Set<br />
<strong>Biowave</strong> Noninvasive U-set: one 2” diameter round electrode for<br />
the primary pain site; and one 5” x 8” large rectangular electrode<br />
is placed horizontally across the lumbar region of the lower back.<br />
U-set electrodes are used for:<br />
• unilateral pain in the mid-torso region including, rib, oblique,<br />
hip, buttock, groin, adductor, abductor, gluteus maximus, hamstring<br />
and quadricep locations<br />
<strong>Biowave</strong><strong>PRO</strong> Leadwire Cable<br />
<strong>Biowave</strong><strong>PRO</strong> Padded Travel Bag<br />
<strong>Biowave</strong><strong>PRO</strong> AC Charger and Power Cord - US<br />
<strong>Biowave</strong><strong>PRO</strong> NiMH Rechargeable Battery<br />
Physical Dimensions<br />
Size (H x W x D): 8.80" x 6.31" x 3.07" /<br />
22.35 cm x 16.02 cm x 7.79 cm<br />
Weight: 2.9 lbs / 1.3 kg<br />
Environmental Conditions<br />
Operating Temperature: 0 – 40 °C / 32 – 104 °F<br />
Humidity: 30 – 85% relative humidity,<br />
non-condensing<br />
Signal Output<br />
Feed Frequency 1: 3858 Hz<br />
Feed Frequency 2: 3980 Hz<br />
Output Voltage Range: 0 – 27.5 V rms<br />
Waveform: Sum of 2 sine waves<br />
Power Source<br />
12 V DC, 3850 mAh rechargeable NiMH battery<br />
Provides 8 hours of power at 80% output into<br />
500 Ohms<br />
Leadwire<br />
Rating complies with 21 CFR Part 898<br />
(performance standards for electrode leadwires)<br />
<strong>Biowave</strong> Noninvasive Electrodes<br />
<strong>Biowave</strong> Noninvasive Electrodes are reusable and are of<br />
a silver/carbon construction with a pre-applied hydrogel<br />
and are cleared for marketing under 510(k) numbers<br />
K962332, K900519 and K915333.<br />
<strong>Biowave</strong> Percutaneous Electrodes<br />
<strong>Biowave</strong> Percutaneous Electrodes are sterile, single-use<br />
electrodes comprised of a 1.5 inch diameter microneedle<br />
array within a 2.5 inch diameter hydrogel-based sterile<br />
electrode. The microneedle array is comprised of 1014<br />
microneedles, 0.74 mm in length, made from 316L surgical<br />
stainless steel. Cleared for marketing under 510(k)<br />
number K061166.<br />
biowave<br />
Manufactured by<br />
<strong>Biowave</strong> Corporation<br />
16 Knight Street<br />
Norwalk, CT 06851<br />
Device must only be used with<br />
power supply provided.<br />
1-877-BIOWAVE<br />
info@biowave.com<br />
www.biowave.com<br />
©2012 <strong>Biowave</strong> Corporation<br />
<strong>Biowave</strong> ® , <strong>Biowave</strong><strong>PRO</strong> ® , <strong>Biowave</strong>HOME ® and other trademarks are the property of<br />
<strong>Biowave</strong> Corporation. Due to continual product innovation, system design and specifications<br />
are subject to change without notice.<br />
RevD/111004