NIMENRIX⢠- GlaxoSmithKline
NIMENRIX⢠- GlaxoSmithKline
NIMENRIX⢠- GlaxoSmithKline
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
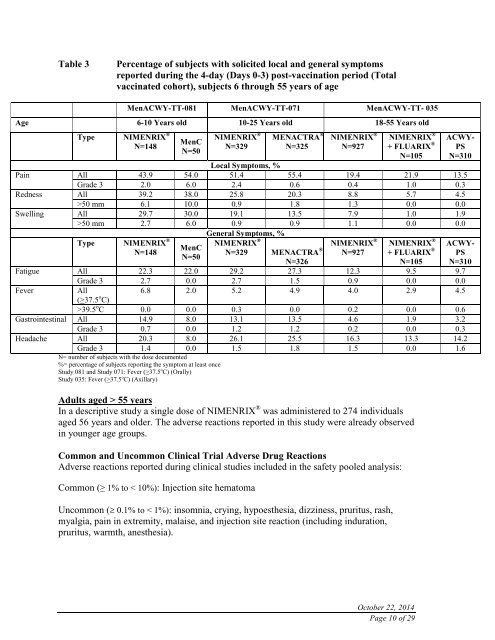
Table 3<br />
Percentage of subjects with solicited local and general symptoms<br />
reported during the 4-day (Days 0-3) post-vaccination period (Total<br />
vaccinated cohort), subjects 6 through 55 years of age<br />
MenACWY-TT-081 MenACWY-TT-071 MenACWY-TT- 035<br />
Age 6-10 Years old 10-25 Years old 18-55 Years old<br />
Type NIMENRIX ®<br />
N=148<br />
MenC<br />
N=50<br />
NIMENRIX ®<br />
N=329<br />
MENACTRA ®<br />
N=325<br />
NIMENRIX ®<br />
N=927<br />
NIMENRIX ®<br />
+ FLUARIX ®<br />
N=105<br />
ACWY-<br />
PS<br />
N=310<br />
Local Symptoms, %<br />
Pain All 43.9 54.0 51.4 55.4 19.4 21.9 13.5<br />
Grade 3 2.0 6.0 2.4 0.6 0.4 1.0 0.3<br />
Redness All 39.2 38.0 25.8 20.3 8.8 5.7 4.5<br />
>50 mm 6.1 10.0 0.9 1.8 1.3 0.0 0.0<br />
Swelling All 29.7 30.0 19.1 13.5 7.9 1.0 1.9<br />
>50 mm 2.7 6.0 0.9 0.9 1.1 0.0 0.0<br />
General Symptoms, %<br />
Type NIMENRIX ®<br />
N=148<br />
MenC<br />
N=50<br />
NIMENRIX ®<br />
N=329 MENACTRA ®<br />
N=326<br />
NIMENRIX ®<br />
N=927<br />
NIMENRIX ®<br />
+ FLUARIX ®<br />
N=105<br />
ACWY-<br />
PS<br />
N=310<br />
Fatigue All 22.3 22.0 29.2 27.3 12.3 9.5 9.7<br />
Grade 3 2.7 0.0 2.7 1.5 0.9 0.0 0.0<br />
Fever<br />
All<br />
6.8 2.0 5.2 4.9 4.0 2.9 4.5<br />
(>37.5 o C)<br />
>39.5 o C 0.0 0.0 0.3 0.0 0.2 0.0 0.6<br />
Gastrointestinal All 14.9 8.0 13.1 13.5 4.6 1.9 3.2<br />
Grade 3 0.7 0.0 1.2 1.2 0.2 0.0 0.3<br />
Headache All 20.3 8.0 26.1 25.5 16.3 13.3 14.2<br />
Grade 3 1.4 0.0 1.5 1.8 1.5 0.0 1.6<br />
N= number of subjects with the dose documented<br />
%= percentage of subjects reporting the symptom at least once<br />
Study 081 and Study 071: Fever (>37.5 o C) (Orally)<br />
Study 035: Fever (>37.5 o C) (Axillary)<br />
Adults aged > 55 years<br />
In a descriptive study a single dose of NIMENRIX ® was administered to 274 individuals<br />
aged 56 years and older. The adverse reactions reported in this study were already observed<br />
in younger age groups.<br />
Common and Uncommon Clinical Trial Adverse Drug Reactions<br />
Adverse reactions reported during clinical studies included in the safety pooled analysis:<br />
Common (≥ 1% to < 10%): Injection site hematoma<br />
Uncommon (≥ 0.1% to < 1%): insomnia, crying, hypoesthesia, dizziness, pruritus, rash,<br />
myalgia, pain in extremity, malaise, and injection site reaction (including induration,<br />
pruritus, warmth, anesthesia).<br />
October 22, 2014<br />
Page 10 of 29