An assessment of the Silt Density Index based on RO membrane ...
An assessment of the Silt Density Index based on RO membrane ...
An assessment of the Silt Density Index based on RO membrane ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
234 S. G. Yiantsios. A.J. Karabelas /Desalinati<strong>on</strong> 151 (2002) 229-238<br />
-y = lOO- 0.56x R= 0,79<br />
y-100-3,84x R=O,99<br />
+ 0,61x R= 0.98<br />
1<br />
I<br />
,_,’<br />
_,’<br />
_,_’<br />
,.<br />
_,.’<br />
_,”<br />
,/<br />
,I<br />
70<br />
0 2 4 6 8 10 12 14<br />
105<br />
t(h)<br />
-y=lOO-0,22x<br />
R=O.89<br />
y= 100-2.02x R=0,99<br />
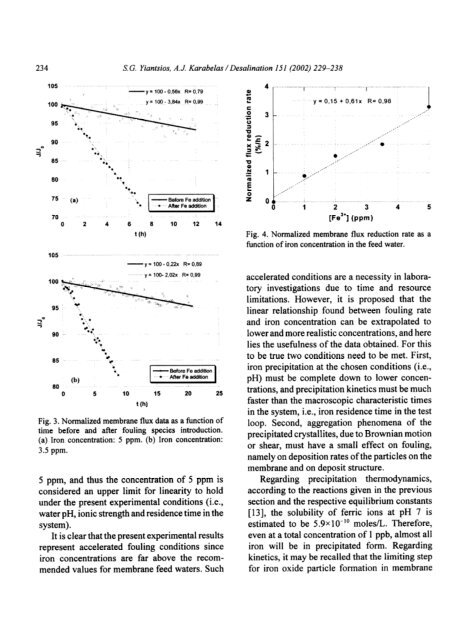
Fig. 3. Normalized <strong>membrane</strong> flux data as a functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
time before and after fouling species introducti<strong>on</strong>.<br />
(a) Ir<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong>: 5 ppm. (b) Ir<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong>:<br />
3.5 ppm.<br />
5 ppm, and thus <str<strong>on</strong>g>the</str<strong>on</strong>g> c<strong>on</strong>centrati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 5 ppm is<br />
c<strong>on</strong>sidered an upper limit for linearity to hold<br />
under <str<strong>on</strong>g>the</str<strong>on</strong>g> present experimental c<strong>on</strong>diti<strong>on</strong>s (i.e.,<br />
water pH, i<strong>on</strong>ic strength and residence time in <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
system).<br />
It is clear that <str<strong>on</strong>g>the</str<strong>on</strong>g> present experimental results<br />
represent accelerated fouling c<strong>on</strong>diti<strong>on</strong>s since<br />
ir<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong>s are far above <str<strong>on</strong>g>the</str<strong>on</strong>g> recommended<br />
values for <strong>membrane</strong> feed waters. Such<br />
[Fe”1(ppm)<br />
3 4<br />
Fig. 4. Normalized <strong>membrane</strong> flux reducti<strong>on</strong> rate as a<br />
functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> ir<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong> in <str<strong>on</strong>g>the</str<strong>on</strong>g> feed water.<br />
accelerated c<strong>on</strong>diti<strong>on</strong>s are a necessity in laboratory<br />
investigati<strong>on</strong>s due to time and resource<br />
limitati<strong>on</strong>s. However, it is proposed that <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
linear relati<strong>on</strong>ship found between fouling rate<br />
and ir<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong> can be extrapolated to<br />
lower and more realistic c<strong>on</strong>centrati<strong>on</strong>s, and here<br />
lies <str<strong>on</strong>g>the</str<strong>on</strong>g> usefulness <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> data obtained. For this<br />
to be true two c<strong>on</strong>diti<strong>on</strong>s need to be met. First,<br />
ir<strong>on</strong> precipitati<strong>on</strong> at <str<strong>on</strong>g>the</str<strong>on</strong>g> chosen c<strong>on</strong>diti<strong>on</strong>s (i.e.,<br />
pH) must be complete down to lower c<strong>on</strong>centrati<strong>on</strong>s,<br />
and precipitati<strong>on</strong> kinetics must be much<br />
faster than <str<strong>on</strong>g>the</str<strong>on</strong>g> macroscopic characteristic times<br />
in <str<strong>on</strong>g>the</str<strong>on</strong>g> system, i.e., ir<strong>on</strong> residence time in <str<strong>on</strong>g>the</str<strong>on</strong>g> test<br />
loop. Sec<strong>on</strong>d, aggregati<strong>on</strong> phenomena <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
precipitated crystallites, due to Brownian moti<strong>on</strong><br />
or shear, must have a small effect <strong>on</strong> fouling,<br />
namely <strong>on</strong> depositi<strong>on</strong> rates <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> particles <strong>on</strong> <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
<strong>membrane</strong> and <strong>on</strong> deposit structure.<br />
Regarding precipitati<strong>on</strong> <str<strong>on</strong>g>the</str<strong>on</strong>g>rmodynamics,<br />
according to <str<strong>on</strong>g>the</str<strong>on</strong>g> reacti<strong>on</strong>s given in <str<strong>on</strong>g>the</str<strong>on</strong>g> previous<br />
secti<strong>on</strong> and <str<strong>on</strong>g>the</str<strong>on</strong>g> respective equilibrium c<strong>on</strong>stants<br />
[13], <str<strong>on</strong>g>the</str<strong>on</strong>g> solubility <str<strong>on</strong>g>of</str<strong>on</strong>g> ferric i<strong>on</strong>s at pH 7 is<br />
estimated to be 5.9~10-‘~ moles/L. Therefore,<br />
even at a total c<strong>on</strong>centrati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> 1 ppb, almost all<br />
ir<strong>on</strong> will be in precipitated form. Regarding<br />
kinetics, it may be recalled that <str<strong>on</strong>g>the</str<strong>on</strong>g> limiting step<br />
for ir<strong>on</strong> oxide particle formati<strong>on</strong> in <strong>membrane</strong><br />
5