PSTC pre-meeting points and Hy's Law Definition - AASLD
PSTC pre-meeting points and Hy's Law Definition - AASLD
PSTC pre-meeting points and Hy's Law Definition - AASLD
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
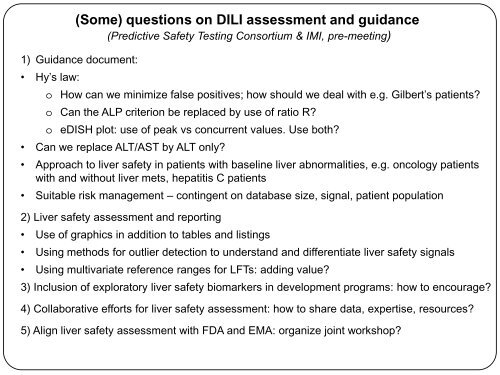
1) Guidance document:<br />
• Hy’s law:<br />
(Some) questions on DILI assessment <strong>and</strong> guidance<br />
(Predictive Safety Testing Consortium & IMI, <strong>pre</strong>-<strong>meeting</strong>)<br />
o How can we minimize false positives; how should we deal with e.g. Gilbert’s patients<br />
o Can the ALP criterion be replaced by use of ratio R<br />
o eDISH plot: use of peak vs concurrent values. Use both<br />
• Can we replace ALT/AST by ALT only<br />
• Approach to liver safety in patients with baseline liver abnormalities, e.g. oncology patients<br />
with <strong>and</strong> without liver mets, hepatitis C patients<br />
• Suitable risk management – contingent on database size, signal, patient population<br />
2) Liver safety assessment <strong>and</strong> reporting<br />
• Use of graphics in addition to tables <strong>and</strong> listings<br />
• Using methods for outlier detection to underst<strong>and</strong> <strong>and</strong> differentiate liver safety signals<br />
• Using multivariate reference ranges for LFTs: adding value<br />
3) Inclusion of exploratory liver safety biomarkers in development programs: how to encourage<br />
4) Collaborative efforts for liver safety assessment: how to share data, expertise, resources<br />
5) Align liver safety assessment with FDA <strong>and</strong> EMA: organize joint workshop
Hy’s <strong>Law</strong> definition<br />
Suggested updates to the July 09 FDA Guidance:<br />
Drug-Induced Liver Injury: Premarketing Clinical<br />
Evaluation<br />
2
Revised definition of Hy’s <strong>Law</strong> suggested<br />
• Hy’s <strong>Law</strong> event is “ominous” – so <strong>pre</strong>cise classification needed:<br />
– hepatocellular injury 1 w/o alk phos restrictions of greatest concern 2<br />
– alk phos >2xULN observed in one third of Hy’s law cases 2<br />
– 96% of liver death/transplant seen with hepatocellular injury 2<br />
• Hy’s <strong>Law</strong> event not met if:<br />
• direct bilirubin normal (or 2xULN<br />
• peak bilirubin elevation <strong>pre</strong>cedes ALT elevation<br />
1<br />
Danan G J Clin Epidemiol, 1993. 46(11): p. 1323-30 [hepatocell. Injury defined as ALT>2xULN & ALT (ULN)/alk phos (ULN) >5]<br />
2<br />
http://www.aasld.org/conferences/Documents/PresentationLibrary/2010Hepatoxicity_SessionIV_Kaplowitz.pdf<br />
3
All Hy’s events should assess hepatocellular injury<br />
w/o alk phos restrictions & bilirubin fractionation<br />
• Hy’s <strong>Law</strong> event is “ominous” – so immediate evaluation requires:<br />
– bilirubin fractionation<br />
– bilirubin concomitant with or following ALT elevation on eDISH<br />
– hepatocellular injury 1 w/o alk phos restrictions of highest concern 2<br />
– alk phos >2xULN observed in one third of Hy’s law cases 2<br />
– 96% of liver death/transplant seen with hepatocellular injury 2<br />
• Gilbert’s syndrome <strong>and</strong>/or drug-induced inhibition of bilirubin<br />
conjugation/transport can be assessed by:<br />
– bilirubin fractionation yielding <strong>pre</strong>dominantly indirect bilirubin<br />
– UGT1A1 genotyping<br />
– <strong>pre</strong>treatment labs revealing asymptomatic hyperbilirubinemia<br />
1<br />
Danan G J Clin Epidemiol, 1993. 46(11): p. 1323-30 [hepatocell. Injury defined as ALT>2xULN & ALT (ULN)/alk phos (ULN) >5]<br />
2<br />
http://www.aasld.org/conferences/Documents/PresentationLibrary/2010Hepatoxicity_SessionIV_Kaplowitz.pdf<br />
4
“Hy’s <strong>Law</strong>”<br />
• 10-50% patients with drug-induced<br />
hepatocellular jaundice will have fatal<br />
liver failure 1<br />
• FDA: “ALT >3xULN <strong>and</strong> bili >2xULN” is<br />
an indicator of significant concern,<br />
termed “Hy’s <strong>Law</strong>” 2<br />
• 9-12% mortality when drug-induced<br />
liver injury accompanied by<br />
ALT>3xULN <strong>and</strong> jaundice in DILI<br />
registries 3,4 Hyman Zimmerman 5<br />
5<br />
1<br />
Zimmerman HJ. Hepatotoxicity (Philadelphia: Lippincott, Williams <strong>and</strong> Wilkins), 1999.<br />
2<br />
FDA http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf<br />
3<br />
Bjornsson E. Hepatol 2005; 42: 481-489.<br />
4<br />
Andrade RJ. Gastroenterol 2005; 129: 512-521.<br />
5<br />
http://jama.ama-assn.org/content/283/6/812.full.pdf+html
Hy’s <strong>Law</strong> cases uncommon in FDA Submissions 1<br />
• FDA retrospective review of 26 NDAs (2004)*, of<br />
which 13 drugs “hepatotoxic” & 13 “nonhepatotoxic”:<br />
– Hy’s <strong>Law</strong> case <strong>pre</strong>sent in only 3 of 26 NDAs:<br />
• Tacrine<br />
• Metformin, topirimate<br />
1<br />
Lana Pauls; FDA website: http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm080526.ppt<br />
6
Bilirubin concom. or following ALT in DILIN 1<br />
• NIH DILI 1 - median duration from DILI recognition:<br />
Peak ALT = 1 (0 –7) days<br />
Peak alkaline phosphatase = 4 (0 –16) days<br />
Peak total bilirubin = 7 (0 –17) days<br />
• DILI with jaundice (total bilirubin 2.5 mg/dL):<br />
median time from peak to 50% decrease: 13 days (4–30)<br />
median time from peak to bili 2.5mg/dl: 26.5 days (3-54)<br />
• Significantly higher mortality: hepatocellular DILI with bili>2.5 mg/dL vs.
Bilirubin rises concomitantly or after ALT in Hy’s <strong>Law</strong> events<br />
90% of Hy’s events have bilirubin elev. within 32days<br />
Age/ Peak ALT Peak Peak alk phos Days from ALT>2xULN<br />
Gender<br />
bilirubin<br />
to bilirubin>2xULN<br />
39M 66xULN 7xULN Alk phos =2xULN 0<br />
F 24xULN 5.5xULN Alk phos
Direct bilirubin increases with liver injury<br />
• Bilirubin is typically measured with two assays to<br />
assess total & “direct reacting” bilirubin 1<br />
• direct bilirubin 0.1mg/dl in normals, 1 10-15% of total 2<br />
• direct bilirubin increased with liver injury & sepsis<br />
• Bilirubin autoanalyzer measurement assoc. with:<br />
– 20% analytic variation<br />
– 30% biologic variation 1<br />
9<br />
1<br />
Dufour DR et al. Clinical Chemistry. 46(12):2050-68, 2000.<br />
2<br />
Bircher J et al. Oxford Textbook of Clinical Hepatology (Oxford University Press, Oxford UK)<br />
Roy-ChowdhuryN et al. Chapter 2.3.5 Bilirubin metabolism p. 168, 1999.
Notable bilirubin biologic variability<br />
• Gilbert's Syndrome affects 3-10% overall 1 , innocuous inherited disorder of<br />
bilirubin conjugation resulting in mild, variable bilirubin elevation:<br />
• affecting 2-5% of Caucasians, 3% of Asians, <strong>and</strong> 36% of Africans 2<br />
• Bilirubin is conjugated through uridine-diphosphate glucuronosyltransferase<br />
(UGT-1A1) to water-soluble bilirubin diglucuronide, secreted in the bile<br />
• In Gilbert’s syndrome, a polymorphism in the promoter region of the UGT-<br />
1A1 gene 2 reduces bilirubin glucuronidation approx. 30%, 1 elevating:<br />
• total bilirubin<br />
• unconjugated (or indirect) bilirubin<br />
10<br />
1<br />
Danoff TM. The Pharmacogenomics Journal 2004;4:49–53.<br />
2<br />
Burchell B. J Gastro Hepatol 1999:14:960-966.
Tranilast inhibits bilirubin conjugation, so<br />
bilirubin>2xULN with Gilbert’s syndrome 1<br />
11 1<br />
Danoff TM. The Pharmacogenomics Journal 2004;4:49–53.
Bilirubin <strong>and</strong> bile salt transport are affected by:<br />
• Decreased by drug-induced or toxic injury 1 , sepsis & inflammation 1<br />
• Bilirubin is:<br />
– taken up in liver cell by OATP1B1 2<br />
– Conjugated by UGT1A1<br />
– Conjugated/direct bilirubin is exported by MDR1 <strong>and</strong> MRP2 into bile 2<br />
• Diverse drugs may affect bilirubin conjugation or transport 1<br />
• So, Gilbert's Syndrome (TA7/TA7 genotype of UGT1A1) subjects are more<br />
likely to develop drug–induced hyperbilirubinemia & Hy’s <strong>Law</strong> events<br />
12<br />
1<br />
Geier A. Biochimica et Biophysica Acta 2007: 1773: 283–308<br />
2<br />
Kamisako M. J Gastroenterol 2000; 35:659–664
Bilirubin <strong>and</strong> Bile Salt Liver Disposition<br />
blood<br />
MRP3<br />
Unconjugated<br />
Bilirubin<br />
hepatocyte<br />
bile<br />
blood<br />
Bilirubin<br />
Bile salts<br />
OATP1B3<br />
OATP1B1<br />
NTCP<br />
UGT1A1<br />
Conjugated<br />
Bilirubin<br />
glucuronide<br />
MDR1 & 3<br />
MDR1<br />
MRP2<br />
Bile salts<br />
BCRP<br />
BSEP<br />
MRP3<br />
MRP2<br />
13<br />
blood<br />
bile<br />
Inhibition may cause<br />
accumulation
14<br />
Re<strong>pre</strong>sentative Hy’s <strong>Law</strong> cases
Concomitant peak ALT & bilirubin<br />
Lab test<br />
ALKALINE PHOSPHATASE<br />
BILIRUBIN TOTAL<br />
SGOT (ASAT)<br />
SGPT (ALAT)
Homozygous wild type UGT1A1 (so no Gilbert’s syndrome)<br />
Oct 10-22<br />
Dashed reference<br />
lines at 1, 2, <strong>and</strong><br />
3xULN<br />
16
Homozygous wild type UGT1A1 (so no Gilbert’s syndrome) with<br />
150 day period between ALT>3xULN <strong>and</strong> bilirubin>2xULN
Are these Hy’s <strong>Law</strong> cases
Adjudicated as possible DILI - is this a Hy’s <strong>Law</strong> case<br />
Hyperbilirubinemia <strong>pre</strong>ceded & followed ALT elevations<br />
Lab test<br />
ALKALINE PHOSPHATASE<br />
BILIRUBIN TOTAL<br />
SGOT (ASAT)<br />
SGPT (ALAT)<br />
19
Gilbert’s syndrome <strong>and</strong> peak bilirubin <strong>pre</strong>ceding peak ALT<br />
adjudicated as probable drug-induced liver injury<br />
Lab test<br />
ALKALINE PHOSPHATASE<br />
BILIRUBIN TOTAL<br />
SGOT (ASAT)<br />
SGPT (ALAT)<br />
Dashed reference<br />
lines at 1, 2, <strong>and</strong><br />
3xULN